云南马尾杉的化学成分研究
谢小燕, 蒋金和, 陈业高
(云南师范大学 化学化工学院,云南 昆明 650092)
云南马尾杉的化学成分研究
谢小燕, 蒋金和, 陈业高*
(云南师范大学 化学化工学院,云南 昆明 650092)
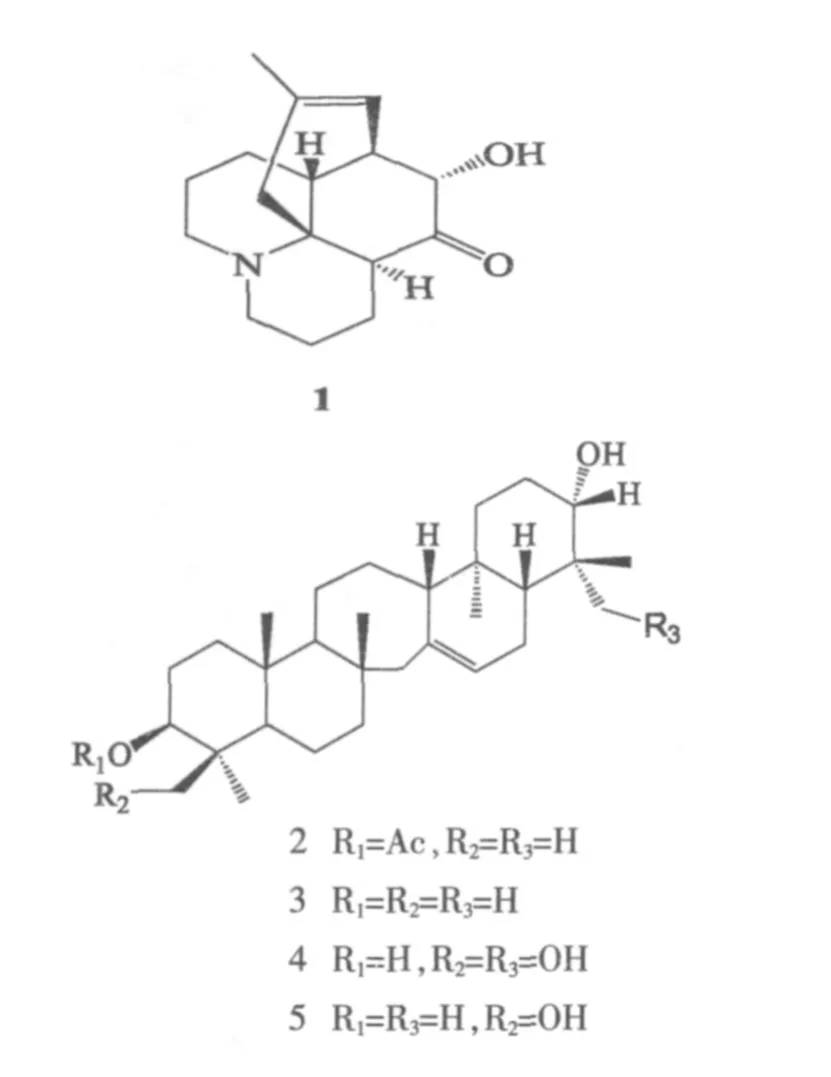
从蕨类植物云南马尾杉(Phlegmariurus yunnanensis)地上部分通过溶剂提取、硅胶柱层析、Sephadex LH-20柱层析和反相柱层析等方法分得5个化合物.利用现代波谱法结合理化分析对提纯的化合物进行结构鉴定.首次从云南马尾杉中分离得到1个生物碱lycoposerramine H、4 个 serratene 型三萜:serratenediol-3-acetate、serratenediol、lycocryptol和 serratriol..
云南马尾杉;化学成分;生物碱;三萜;结构鉴定
石杉科植物分为石杉属(Huperzia)和马尾杉属(Phlegmariurus)[1].上世纪八十年代,我国科学家从蛇足石杉(Huperzia serrata)中分得石杉碱甲,该化合物具有很强的抑制乙酰胆碱酯酶 (AChE)活性,临床试验研究表明其对治疗重症肌无力和早老性痴呆有显著疗效,已被国际上列为第二代乙酰胆碱酯酶抑制剂之一,是近年来广为瞩目的天然药物成分[2-5].从蛇足石杉和类似植物中发现新的活性成分一直受到国内外天然药物化学家的重视,得到了许多结构奇特且具有较好活性的生物碱[6-8].云南马尾杉(Phlegmariurus yunnanensisChing)系云南西北部特有植物.薄层层析可检测出其中含有五种生物碱,即石杉碱甲、石杉碱乙、lycodoline、lucid ioline和 lycopodine,但未见单体化合物的分离纯化[9].为探索云南马尾杉的活性成分,我们对产于云南贡山的云南马尾杉化学成分进行了研究,采用各种分离方法包括常压硅胶柱层析、减压硅胶柱层析、凝胶层析和反相柱层析等方法,共分得5个化合物.经过波谱分析(核磁共振氢谱、碳谱)确定它们的结构分别为1个生物碱lycoposerramine H(1)、4 个 serratene 型三萜;serratenediol-3-acetate(2)、serratenediol(3)、lycocryptol(4)和 serratriol(5).化合物1-5均为首次从本植物中分到,结构式为:
1 仪器、试剂与材料
1.1 仪器
ZF-II型紫外分析仪 (上海顾村中实仪器厂);Varian Mat-711质谱仪(美国Finnigan公司);核磁共振仪(Bruker DRX-500型,德国布鲁克公司);旋转蒸发仪(SB-2000系列,日本东京理化).
1.2 试剂
层析硅胶(青岛海洋化工厂),Sephadex LH-20(20~80 mm,Pharmacia Fine Chemical Co,Ltd.),高效薄层层析硅胶G板(烟台化工研究院),显色剂为体积分数10%硫酸-乙醇溶液(乙醇为工业纯,使用前重蒸),其它试剂为化学纯或分析纯.
1.3 材料
云南马尾杉采自云南贡山,由云南大学生命科学学院陆树刚教授鉴定为Phlegmarium yunnanensis Ching的地上部分.
2 试验方法
云南马尾杉干燥地上部分(419 g),用工业乙醇室温浸提7次.浸出液减压浓缩得乙醇提取物.提取物加入1 mol/L H2SO4酸化至pH 2后,用乙酸乙酯萃取得到非碱性脂溶性成分(22 g),萃取后的酸水液用浓氨水碱化至pH 10,然后用氯仿萃取,减压浓缩后得到碱性脂溶性成分(2.1 g).氯仿萃取物上硅胶柱,以氯仿∶甲醇(1∶0→0∶1)进行梯度洗脱,薄层层析检测共分成五部分.其中氯仿∶甲醇10∶1 部分(60 mg)经反相柱层析(甲醇∶水 6∶4→1∶0)和凝胶层析(氯仿∶甲醇 3∶2)得到化合物 1(15 mg).乙酸乙酯萃取物进行硅胶柱层析,以氯仿∶丙酮(1∶0→0∶1)进行梯度洗脱,薄层层析检测共分成五个部分.其中氯仿部分(200 mg)和氯仿∶丙酮 80∶1 部分(150 mg),分别进行反复重结晶得到化合物2(20 mg)和化合物 3(15 mg).氯仿∶丙酮 20∶1 部分(200 mg)经反复硅胶柱层析(氯仿∶甲醇 50∶1→0∶1)和凝胶层析(氯仿∶甲醇 3∶2)得到化合物 4(30 mg)和化合物 5(12 mg).
3 结果与讨论
首次从云南马尾杉地上部分分离得到5个成分,利用现代核磁共振波谱法,结合理化分析进行鉴定,5个化合物的结构分别为lycoposerramine H(1)、serratenediol-3-acetate (2)、serratenediol(3)、lycocryptol(4)和 serratriol(5).
化合物1,无色针状晶体,易溶于甲醇,碘化铋甲显色呈红色.13C-NMR和DEPT谱显示该化合物有16个碳,包括1个CH3,7个CH2,5个 CH 和3个季碳.δ 213.2处的季碳说明连有羰基,δ 77.2处的连氧 CH 说明连有羟基.δ 1.52 (3H,s,H-16)处的甲基和三取代双键[δ 123.2(d,C-8),135.5(s,C-15)]表明为石松类生物碱.1H-NMR(C5D5N,500 MHz) ∶δ 3.88(1H,dd,J=3.1,11.8 Hz,H-4),4.16(1H,s,H-6),5.42 (1H,d,J=5.0 Hz,H-8),1.71(1H,m,H-11),2.46 (1H,dd,J=1.7 Hz,H-11),2.79(1H,d,J=18.0 Hz,H-14)1.52(3H,s,H-16);13C-NMR(C5D5N,125MHz):δ 47.3(t,C-1),18.4 (t,C-2),19.8(t,C-3),38.8(d,C-4),213.2(s,C-5),77.2(d,C-6),43.1(d,C-7),123.2(d,C-8),47.7(t,C-9),27.4(t,C-10),26.7(t,C-11),43.2(d,C-12),61.2(s,C-13),41.4(t,C-14),135.5(s,C-15),23.1(q,C-16).数据与文献报道的化合物 lycoposerramine H[10]数据完全一致,结构鉴定为lycoposerramine H.
化合物 2,白色片状结晶,溶于氯仿.5%浓硫酸乙醇溶液显色呈红色.13C-NMR和DEPT谱显示该化合物有 32个碳,其中8个为 CH3[δ 28.0(q,C-23),19.2(q,C-24),16.2 (q,C-25),21.7 (q,C-26),13.8(q,C-28),15.0(q,C-29),28.0(q,C-30)],有 1个甲基为乙酰氧基[δ 171.0,27.6 和 2.05(3H,s)].2 个连氧碳信号[δ 80.8(d,C-3),79.1(d,C-21)]及 δ 55.9(t,C-27)处的亚甲基显示了 serratene 型五环三萜型特征,推断为三萜类化合物.1H-NMR(CDCl3,500 MHz)∶δ 5.32 (1H,s,H-15),4.47(1H,dd,J=5.2,11.3 Hz,H-3),3.22(1H,dd,J=4.3,11.3 Hz,H-21),2.05(3H,s,-OCOCH3),0.96 (3H,s,H-30),0.79-0.84 (15H,s,H-24,25,26,28,29),0.66(3H,s,H-23);13C-NMR(CDCl3,125 MHz) ∶δ 27.5(t,C-1),27.2(t,C-2),80.8(d,C-3),38.0(s,C-4),44.9(d,C-5),18.7(t,C-6),49.4(t,C-7),38.1(s,C-8),62.6(d,C-9),37.8(s,C-10),24.0(t,C-11),25.2(t,C-12),57.1(d,C-13),138.1(s,C-14),122.1(d,C-15),23.8 (t,C-16),55.7 (d,C-17),36.0 (s,C-18),38.8(t,C-19),27.2(t,C-20),79.1(d,C-21),37.0 (s,C-22),28.0 (q,C-23)19.2 (q,C-24),16.2(q,C-25),21.7 (q,C-26),55.9 (t,C-27),13.8(q,C-28),15.0 (q,C-29),28.0 (q,C-30),171.0 (s,CH3COO-),27.6(q,CH3COO-).以上数据与文献报道的serratenediol-3-acetate数据一致[11],故鉴定该化合物为serratenediol-3-acetate.
化合物3,片状晶体,易溶于石油醚、氯仿、苯、热甲醇,不溶于水,硫酸乙醇显色呈红色.13C-NMR和DEPT谱显示该化合物有30个碳,其中7个为CH3碳.核磁共振谱与化合物2非常相似,仅少了一个乙酰基.1H-NMR (C5D5N,500 MHz)∶δ 5.51(1H,brs,H-15),3.56(2H,m,H-3a,21b),1.26(3H,s,H-30),1.20(3H,s,H-28),1.08(3H,s,H-26),0.95 (3H,s,H-29),0.88(3H,s,H-24),0.85(3H,s,H-25),0.79(3H,s,H-23);13C-NMR (C5D5N,125MHz):δ 38.9(t,C-1),28.0(t,C-2),79.0(d,C-3),39.5(s,C-4),56.5(d,C-5),19.8(t,C-6),46.0(t,C-7),37.9(s,C-8),63.4(d,C-9),38.0(s,C-10),25.1(t,C-11),28.6(t,C-12),58.1(d,C-13),39.2(s,C-14),123.2(d,C-15),25.1 (t,C-16),50.5 (d,C-17),39.9 (s,C-18),36.9(t,C-19),28.7(t,C-20),78.6(d,C-21),40.0(s,C-22),29.2(q,C-23),16.0(q,C-24),16.6(q,C-25),20.6 (q,C-26),57.0 (t,C-27),14.2(q,C-28),16.9(q,C-29),28.7(q,C-30).以上数据与文献报道的serratenediol对照完全一致[11-13],故鉴定化合物3为serratenediol.
化合物 4,白色片状结晶,溶于氯仿.浓硫酸乙醇显色呈红色,13C-NMR和DEPT谱显示该化合物有 30 个碳,其中有 5 个 CH3[δ 23.7(q,C-23),14.5(q,C-25),23.1(q,C-26),16.6(q,C-28),20.1 (q,C-29)]和 2 个连氧碳信号[δ 80.5(d,C-3),80.0 (d,C-21)].此外,δ 64.6(t,C-24),64.0(t,C-30)处的 2个羟甲基,以及serratene型五环三萜的特征信号δ 56.3(t,C-27),说明化合物 4 为 serratene 型三萜化合物.1H-NMR (C5D5N,500 MHz)∶δ 5.46(1H,m,H-15),4.57(1H,d,J=10.9,H-24a),4.50(1H,d,J=10.1,H-30a),3.82 (1H,d,J=10.9,H-24b),3.70(1H,d,J=10.1,H-30b),3.63 (1H,m,H-3),3.59(1H,s,H-21),1.53 (3H,s,H-23),1.49 (3H,s,H-26),0.86 (3H,s,H-29),0.80 (3H,s,H-28),0.72(3H,s,H-25);13C-NMR (C5D5N,125 MHz)∶δ 37.5(t,C-1),28.8(t,C-2),80.5(d,C-3),42.7(s,C-4),50.9 (d,C-5),19.7(t,C-6),45.7(t,C-7),38.2 (s,C-8),62.9(d,C-9),36.2(s,C-10),25.7(t,C-11),27.7 (t,C-12),57.6 (d,C-13),138.6 (s,C-14),122.7(d,C-15),24.7(t,C-16),56.6(d,C-17),37.6(s,C-18),38.9(t,C-19),29.0(t,C-20),80.0(d,C-21),43.4(s,C-22),23.7(q,C-23),64.6(t,C-24),14.5(q,C-25),23.1(q,C-26),56.3(t,C-27),16.6(q,C-28),20.1(q,C-29),64.0(t,C-30).以上数据与文献报道的lycocryptol对照一致[14-15],故鉴定化合物4为lycocryptol.
化合物 5,白色片状结晶,溶于氯仿.浓硫酸乙醇显色呈红色,13C-NMR和DEPT谱显示该化合物有 30 个碳,其中有 6 个 CH3[δ 28.3(q,C-23),15.5(q,C-25)23.7(q,C-26)16.6(q,C-28)20.1(q,C-29),13.8(q,C-30)]和 2 个连氧碳信号 [δ 80.0(d,C-3)78.3(d,C-21)].此外,还有一个羟甲基[δ 64.5(t,C-24)],以及 serratene 型三萜的特征信号[δ56.4(t,C-27)],由此可推断为 serratene 类三萜化合物.1H-NMR(C5D5N,500 MHz)∶δ 5.39(1H,br s,H-15),4.52(1H,d,J=10.9,H-24a),3.73(1H,d,J=10.9,H-24b),3.67 (1H,m,H-3),3.52 (1H,m,H-21),1.55 (3H,s,H-23),1.20 (3H,s,H-25),1.10(3H,s,H-26),0.84 (3H,s,H-28),0.79 (3H,s,H-29),0.76(3H,s,H-30);13C-NMR (C5D5N,125 MHz)∶δ 37.6(t,C-1),28.7(t,C-2),80.0(d,C-3),43.4 (s,C-4),50.1(d,C-5),20.0 (t,C-6),45.8 (t,C-7),38.2(s,C-8),62.9(d,C-9),36.5(s,C-10),25.6(t,C-11),27.7(t,C-12),57.6(d,C-13),138.6(s,C-14),122.9(d,C-15),24.7(t,C-16),56.7(d,C-17),37.5 (s,C-18),39.0 (t,C-19),28.8 (t,C-20),78.3(d,C-21),39.5(s,C-22),28.3(q,C-23),64.5(t,C-24),15.5(q,C-25),23.7(q,C-26),56.4(t,C-27),16.6(q,C-28),20.1(q,C-29),13.8(q,C-30).以上数据与文献报道的serratriol对照一致[16],故鉴定化合物5为serratriol.
[1]中国科学院中国植物志编辑委员会.中国植物志第六卷(第3分册)[M].北京:科学技术出版社,2004.
[2]Liu J S,Zhu Y L,Yu C M,et al.The structures of huperzine A and B,two new alkaloids exhibiting marked anticholinesterase activity[J].Can J Chem,1986,64(4):837-839.
[3]Wang R,Yan H,Tang X C.Progress in studies of huperzine A,a natural cholinesterase inhibitor from Chinese herbal medicine[J].Acta Pharmacol Sin,2006,27(1):1-26.
[4]Tang X C,Han Y F.Pharmacological profile of huperzine A,a novel acetylcholinesterase inhibitor from Chinese herb[J].CNS Drug Rev,1999,5(3):281-300.
[5]Carlier P R,Du D M,Han Y F,et al.Dimerization of an inactive fragment of huperzine A produces a drug with twice the potency of natural product[J].Angew Chem Int Ed,2000,39(10):1775-1777.
[6]Kubota T,Yahata H,Yamamoto S,et al.Serratezomines D and E,new lycopodium alkaloids from Lycopodium serratum var.serratum[J].Bioorg Med Chem,2009,19(13):3577-3580.
[7]Morita H,Ishiuchi K,Haganuma A,et al.Complanadine B,obscurumines A and B,new alkaloids from two species of Lycopodium[J].Tetrahedron,2005,61(8):1955-1960.
[8]Hirasawa H,Kobayashi J,Morita H,Lycoperine A,A novel C27N3-type pentacyclic alkaloid from Lycopodium hamiltonii,inhibiting acetylcholinesterase[J].Org Lett,2006,8(1):123-126.
[9]Ma X Q,Jiang S H,Zhu D Y.Alkaloid patterns in Huperzia and some related genera of Lycopodiaceae Sensu Lato occurring in China and their contribution to classification[J].Biochem Syst Ecol,1998,26(7):723-728.
[10]Takayama H,Katakawa K,Kitajima M,et al.Ten new lycopodium alkaloids having the lycopodane skeleton isolated from Lycopodium serratum thunb[J].Chem Pharm Bull,2003,51(10):1163-1169.
[11]裴刚,周朴华,何桂霞,等.皱边石杉脂溶性化学成分的研究[J].天然产物研究与开发,2004,16(3):213-214.
[12]Sano T,Tsuda Y.Strutures of tohogenol triterpenoids of Lycopodium serratum[J].Tetrahedron,1970,26(12):2984-2986.
[13]Haruo S,Kazuo F,Xu GY,et al.Assignments of the1H-and13C-NMR spectra of four Lycopodium triterpenoids by the application of a new two-dimensional technique,heteronuclear multiple bond connectivity (HMBC)[J].Agric Biol Chem,1988,52(7):1797-1801.
[14]Tsuda Y,Fujimoto T,Isobe K,et al.Chemotaxonomical studies on the triterpenoids of Lycopodium plants[J].Yakugaku Zasshi,1974,94(8):970-990.
[15]Tsuda Y,Isobe K,Sano T.Triterpenoid chemistry.IX.Lycopodium triterpenoid.(6).The structures of three new tetraols,lycocryptol,21-epilycocryptol,and diepilycocryptol,and two new acids,lycernuic acid-A and-B[J].Chem Pharm Bull,1975,23(2):264-271.
[16]Yoshisuke T,Takehiro S,Akira M,et al.Triterpenoid chemistry.VI.Lycopodium triterpenoid.Structures and stereochemistry of serratriol,21-episerratriol,and lycoclavanol[J].Chem Pharm Bull,1974,22(10):2383-2395.
Study on the Chemical Constituents fromPhlegmariurus yunnanensis
XIE Xiaoyan,JIANG Jinhe,CHEN Yegao*(School of Chemistry and Chemical Engineering,Yunnan Normal University,Kunming650092,China)
Five compounds were obtained by silica gel chromatography,Sephadex LH-20 column chromatography and reverse phase C-18 column chromatography from the aerial parts ofPhlegmariurus yunnanensis,and the chemical structures of these compounds were identified by modern spectroscopy as well as physico-chemical analysis.These compounds were identified to be lycoposerramine H,serratenediol-3-acetate,serratenediol,lycocryptol and serratriol.They were obtained from P.yunnanensis for the first time.
Phlegmariurus yunnanensis;chemical constituents;alkaloid;triterpenoids;structural identification
R 248.1
A
1674-4942(2010)01-0068-04
2009-10-27
中国科学院西部之光人才培养项目(2005)
*通讯作者
毕和平

