Antifeedant and Deterrent Activity of Extracts from Different Parts of Xanthium Sibiricum(Compositae) against Crucifer Pests
ZHOU Qiong, WEI Mei-cai, YAN Yu-chen, WANG Wen-xue
(1.Collegy of Life Science, Hunan Normal University, Changsha 410081, China;2.Laboratory of Insect Systematics and Evolutionary Biology, Central South University of Forestry & Technology, Changsha 410004, China)
Because of increasing problems associated with the use of acutely toxic synthetic insecticides, including resistance and the impacts on both humans and non-target biological systems[1-3], there is a pressing need for the development of safe, alternative crop protectants. Among the most effective insect pest control measures, a botanical regulator of insect pest behaviors, such as antifeedants and oviposition deterrents, may be one of the most preferred options. Botanical products are useful and desirable tools in most pest management programs because they can be effective and often complement the actions of natural enemies[4-5]. Plant secondary compounds have been the subject of thorough investigations in an effort to discover new sources of botanical insect antifeedants and oviposition deterrents.
The diamondback moth (Plutellaxylostella(Linnaeus)), cabbage white butterfly (Pierisrapae(Linnaeus)), peach aphid (Myzuspersicae(Sulzer)), and mustard aphid (Lipaphiserysimi(Kaltenbach)) are destructive insects of cruciferous vegetables in China and throughout the world[6-8]. Some of them, especiallyP.xylostella, have been difficult to control, and have developed a considerable resistance to a range of insecticides, including organophosphorus, carbamates, pyrethroids,Bacillusthuringiensis, and benzoylphenyl ureas[1,6,9-13]. There is an urgent need to find new types of safe, alternative crop protectants against these insect pests of crucifers.
Siberian cocklebur (XanthiumsibiricumPatrin) (Compositae) is an annual dicotyledonous weed that is extremely competitive with soybean and other agronomic crops in China[14-16]. Its fruit is used as a traditional herbal medicine in China for the effective treatment of sinusitis, urticaria, and arthritis, and to regulate immunity and restrain bacteria[17-18]. It has been used as a traditional pesticide in China since ancient times[19]. The ethanol extracts fromX.sibiricumproved highly effective growth inhibitors and produced stomach poison inP.rapae[20]. Among them, the alcohol extracts fromX.sibiricumproved to have a highly effective antifeedant effect on laboratory populations ofL.erysimiandM.persicae, but safe for natural enemies of aphids such as ladybird beetles (Menochilussexmaculata(Fabricius) andCoccinellatrasversalis(Fabricius)), and parasitoids (AphidiusgifuensisandDiaeretiellarapae)[21-23]. The purpose of the current study is to examine and compare which part extracts fromX.sibiricumplant have more antifeedant, oviposition and inhabiting deterrent effects on insect pests of cruciferous crops, and to serve as the foundation for more detailed investigations of those parts that prove to be most active on the feeding deterrent and oviposition deterrent behaviours of phytophagous insects.
1 Materials and Methods
PlantextractsExtracts from the fruit, stem, leaf, and root ofX.sibiricumwere pulped from fresh plant material collected from the suburb of Xiangtan (112°56′E, 27°50′N), P. R. China, in October-November 2005. The four parts ofX.sibiricumwere air-dried, then further dried at 50 ℃ in an oven with air circulation for 24~48 h, and ground in an electric blender into a powder, respectively. Each of the four powders, leaf, fruit, stem, and root, were extracted by methanol for 24 h in a Soxhlet extractor, and the extracted solutions were filtered. The filtered solutions were evaporated in a rotary evaporator under reduced pressure to obtain the crude extracts. The crude extracts were stored in a refrigerator and temporarily diluted with distilled water before testing.
HostplantPakchoi cabbages (Brassicachinensis) were grown in 500 mm×500 mm×500 mm plastic pots containing a mixture of sandy loam soil and peatmoss to the six to seven fully extended true leaf stage. The pots were housed in an alnico cage, which was placed outdoors, with an ambient temperature and humidity, enough natural light for bioassays, and to maintainP.xylostella,P.rapae,M.persicae,L.erysimicolonies on their leaves.
TestinsectsLaboratoryP.xylostella,P.rapae,M.persicae, andL.erysimicultureswere established from field collections on a cabbage farm in a suburb of Changsha (111°53′E, 27°58′N), P. R. China, and maintained on individually potted Pakchoi cabbages in the cage, in a room temperature-controlled at 25℃, 60%~80%RH and nature photoperiod.
AntifeedantchoicebioassayonP.rapaeandP.xylostellaThe antifeedant activity of leaf, fruit, stem, and root extracts fromX.sibiricumwas assayed by using a leaf disc choice assay. Fresh leaves ofB.chinensiswere excised into 20 mm×20 mm square discs, and then dipped in one of the diluted crude extracts (0.05 g/mL, dried powder weight) for about five seconds. The leaf discs that served as controls were dipped in distilled water and 5% methanol. After air-drying for 1 h, four pakchoi leaf discs were placed in a 125 mm diameter Petri dish, with two treatments and two controls, arranged alternately, in each dish and underlaying with a damp filter paper to keep leaf discs fresh for 24 h. Five 3rdinstar larvae which pre-starved for 3 h, were placed at the center of each Petri dish, and allowed to feed for 24 h at 25 ℃. The leaf discs were replenished if needed. Each treatment was replicated six times. A clear graph paper was used to measure the leaf area eaten by larvae ofP.rapaeandP.xylostella, and the proportion of eaten leaf to the total leaf area was calculated.
OvipositionpreferenceofP.xylostellaIn the choice oviposition deterrent assays, an improved method following[24]was used. The leaf discs ofB.chinensisexcised into 18 mm diameter were treated as same as in the choice feedant deterrent assays, and adhibited to the bottom of 80mm diameter Petri dishes with 2% agar gel. The leaf surface faced the bottom of the Petri dish, so as to keep the leaf disc fresh for several days. The two treatments(0.05 g/mL, dried powder weight) and two controls were alternately arranged in the Petri dish. The Petri dish was then placed upside down to cover the top of a plastic cylinder (diam 75 mm), and the bottom was wrapped by a piece of gauze to maintain ventilation. Three pairs of adults, male and female, emerged for two or three days, were confined in each cylinder for three days at 25 ℃. Adults were supplied with 10% honey-water solution by a soaked small cotton ball in the fundus of the cylinder. The number of eggs laid on the leaf disc was counted daily. Each treatment was replicated twelve times.
SettlingdeterrentchoicebioassayonM.persicaeandL.erysimiAn improved half-leaf disc bioassay[21,25]was applied. The leaves ofB.chinensiswere excised to form round pieces (diam 40 mm) with vein in the certer. Then the leaf discs were adhibited on 2% agar gel as mentioned above. The diluted crude extracts(0.05 g/mL, dried powder weight) were spread uniformly on one side of the back of the leaf as treatment, and the isodose alcohol and distilled water was spread on the other side as the control. Following the spread extract leaf discs being air-dried,16~22 3rdinstar aphids were carefully placed onto the each leaf-disc back in equal number on both sides of the leaf vein. The Petri dish was sealed by a parafilm pricked with air holes by an insect needle to keep it ventilated, turned upside down and held at 25 ℃. The numbers of aphids that settled on either the treated or control sides of every leaf disc were recorded at 24 h, 48 h, and 72 h after treatment. Each treatment was replicated three times.
DataanalysesA non-choice / choice feeding (oviposition) deterrence rate was calculated using the formula:
Choice feeding (or inhabiting/oviposition) deterrence rate(%)=100% [(C-T)/(C+T)], whereCandTare the control and treated leaf areas consumed by the insects (Ismanetal. 1990), or numbers of aphids settled in the control and treated leaf-half disc[21], or numbers of eggs deposited on the control and treated leaf disc by the diamondback moth[25].
Data analyses were carried out using SPSS (Statistical Product and Service Solutions, Statistics Package for the Social Sciences) on the basis of actual numbers observed. Paired-samplesTTest and Duncan’s Multiple Range Test ( DMRT) were used to determine the significant differences between the control groups and treated groups.
2 Results
AntifeedantchoiceassayonP.rapaelarvaeThe antifeedant effect of crude extracts from different parts ofX.sibiricumonP.rapaewas shown in Table 1. The Pakchoi cabbage leaf areas eaten byP.rapaelarvae for the control and the treatment spreaded by leaf and ftruit crude extracts showed a significant difference. The proportion of Pakchoi cabbage leaf area eaten byP.rapaelarvae for the control sample was 99.53%, whereas the leaf extract treated sample was 0.47%. For the fruit extract experiment, the figures were 98.06% for the control and 1.94% for the treatment. The highest antifeedant rate of 99.07% was achieved from the leaf extract ofX.sibiricum. The fruit extract followed with antifeedant rate of 96.13%. The remaining stem and root extracts produced an antifeedant rate of 45.60% and 32.57%, respectively, but the treated pakchoi cabbage leaf areas eaten byP.rapaelarvae did not show any significant difference from the control groups.
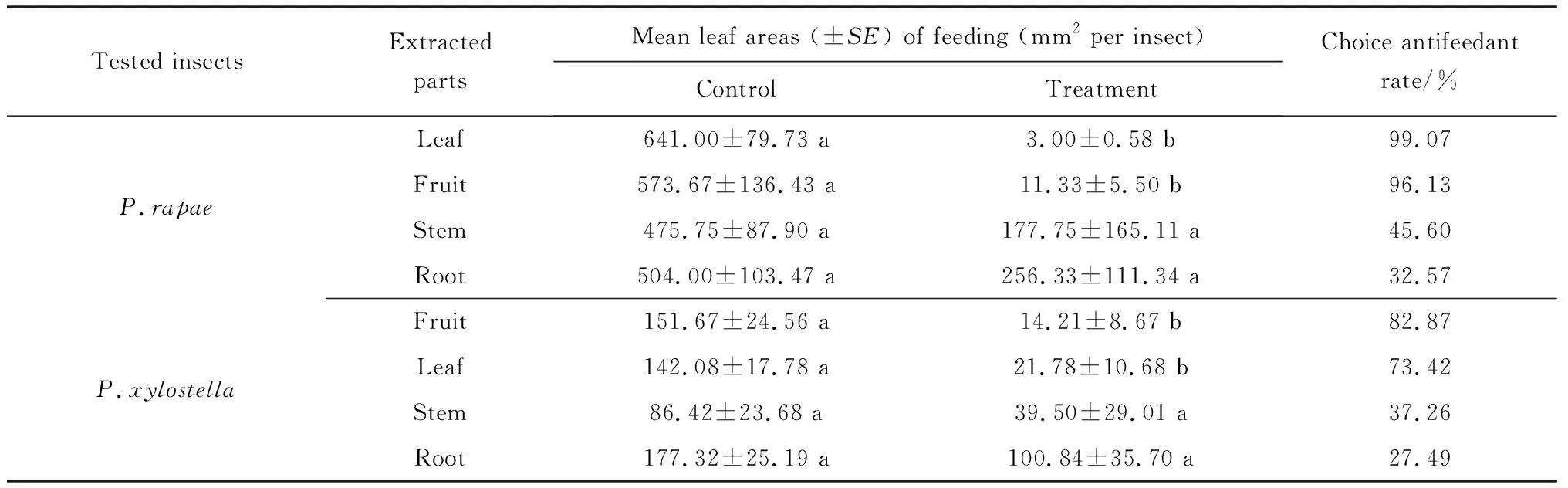
Tab.1 Antifeedant effect of methanol extracts from four parts of X.sibiricum on P.rapae and P.xylostella in leaf disc choice bioassays
Note: Means followed by common letters in a row are not significantly different atP=0.05 by Paired-samplesTTest.
AntifeedantchoiceassayonP.xylostellaIn the antifeedant choice assay onP.xylostella, there was also a significant difference in the control and fruit and leaf extract treatments on the pakchoi cabbage leaf areas eaten byP.xylostellalarvae (Table 1). The proportion of Pakchoi cabbage leaf areas eaten byP.xylostellalarvae on the control sample was 91.43%, whereas the fruit extract treatment produced 8.75%. The corresponding measures for the leaf extract spread experiment were 86.71% for the control and 13.29% for the treatment. The fruit extract exhibited a strong antifeedant capability, with antifeedant rate of 82.87%, followed by the leaf extract treatment with antifeedant rate of 73.42%. The antifeedant rate from the stem and root extracts indicated a lower antifeedant effect than either fruit or leaf extracts, with a rate of 37.26% and 27.49% respectively. Once again, the stem and root extract assays showed no significant difference between control and treatment for the treated pakchoi cabbage leaf areas eaten byP.xylostellalarvae.
DeterrentchoicebioassayThe crude extracts ofX.sibiricumshowed an obvious oviposition deterrent effect onP.xylostella, and an inhabiting deterrent effect onL.erysimiandM.persicaein the deterrent choice bioassay. The oviposition deterrent activity for the four extracts was calculated by oviposition deterrent rate values. The inhabiting deterrent effect on aphids was also varied for the four extracts and was calculated by inhabiting (settling) deterrent rate values.
OvipositiondeterrentchoiceassayonP.xylostellaThe crude extracts from the leaf, fruit, and stem ofX.sibiricumshowed obvious oviposition deterrent effects onP.xylostella(Table 2).P.xylostellafemales deposited an average of 22.77 eggs (94.29%) on the controls and 1.38 eggs (5.71%) on the leaf extract treatments 24 h after spread. The rate was followed by 43.69 eggs (81.25%) on the controls and 10.08 eggs (18.75%) on leaf discs treated with fruit extract. The stem extract experiment resulted in females depositing, an average, 34.67 eggs (72.06%) on the controls and 13.44 eggs (27.94%) on the treatment spread with stem extract. As a result, the crude extract with highest oviposition deterrent activity onP.xylostellawas leaf extract fromX.sibiricum, with the oviposition deterrent rates of 88.57%(24 h), 78.91%(48 h), and 69.88%(72 h) after spread, followed by the fruit extract with a deterrent rate of 62.51% (24 h), 50.67% (48 h), and 42.70% (72 h) after spread. The stem extract treatment oviposition deterrent rate was 44.13% (24 h), 29.19% (48 h), and 32.46% (72 h) after spread. The root extract experiment showed no oviposition deterrent effect on the diamondback moth. The accumulative oviposition deterrent rate reduced as time elapsed.
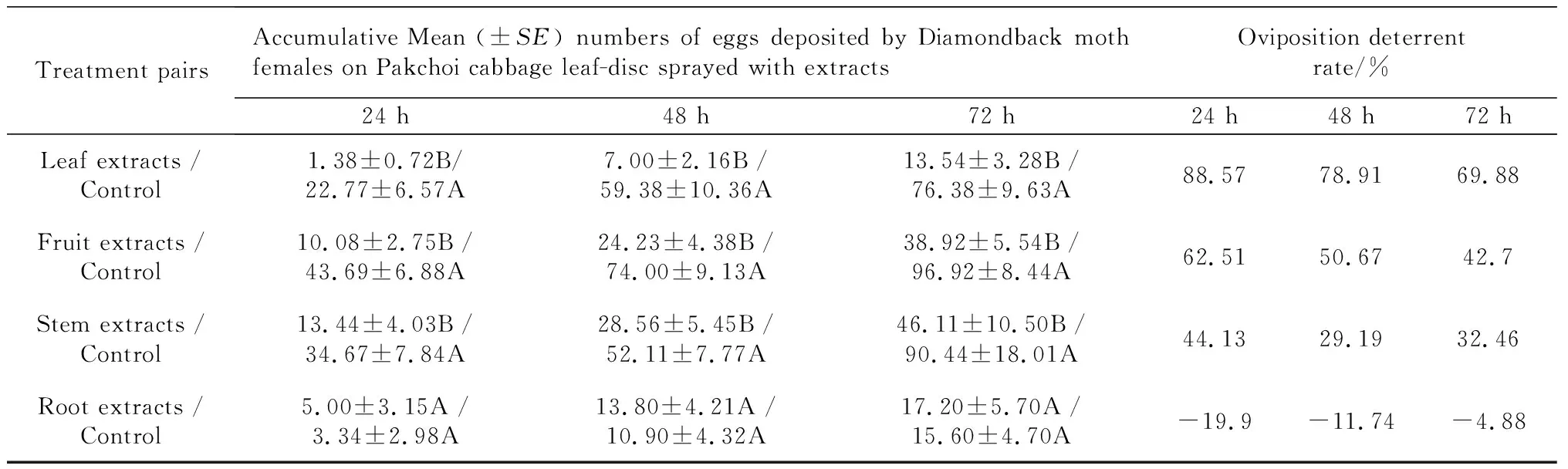
Tab.2 Oviposition deterrent effect of extracts from four parts of X.sibiricum on P.xylostella in leaf disc choice bioassays
Note: Means followed by common letters in a pair are not significantly different atP=0.01 by Paired-samplesTTest.
InhabitingdeterrentchoiceassayonL.erysimiIn the inhabiting deterrent choice assay, the inhabiting deterrent activity onL.erysimivaried according to the extract ofX.sibiricumapplied (Table 3). The average numbers ofL.erysimiinhabiting on the Pakchoi cabbage leaf were significantly different between the control section and the section treated with leaf, fruit, or stem crude extracts 24 h later. The most active crude extract was from the leaves ofX.sibiricumwith a choice deterrent rate of 83.01% (24 h), 54.99% (48 h), and 43.54% (72 h) after treatment. The fruit extract treatment returned a choice deterrent rate of 53.52% (24 h), 44.98 % (48 h), and 27.83% (72 h) after treatment. The stem extracts proved to be most active 24 h after treatment with an inhabiting deterrent rate of 62.50%, but there was no deterrent effect 48 h and 72 h after treatment. The root extract activity was the lowest at 24 h (17.33 %), and no deterrent effect was shown after 48 h or 72 h. The inhabiting deterrent effect of all extracts fromX.sibiricumwas reduced as time elapsed, but the degree varied according to the particular extract. The stem extract decreased fastest from 62.50% at 24 h to -5.91% at 48 h after treatment.
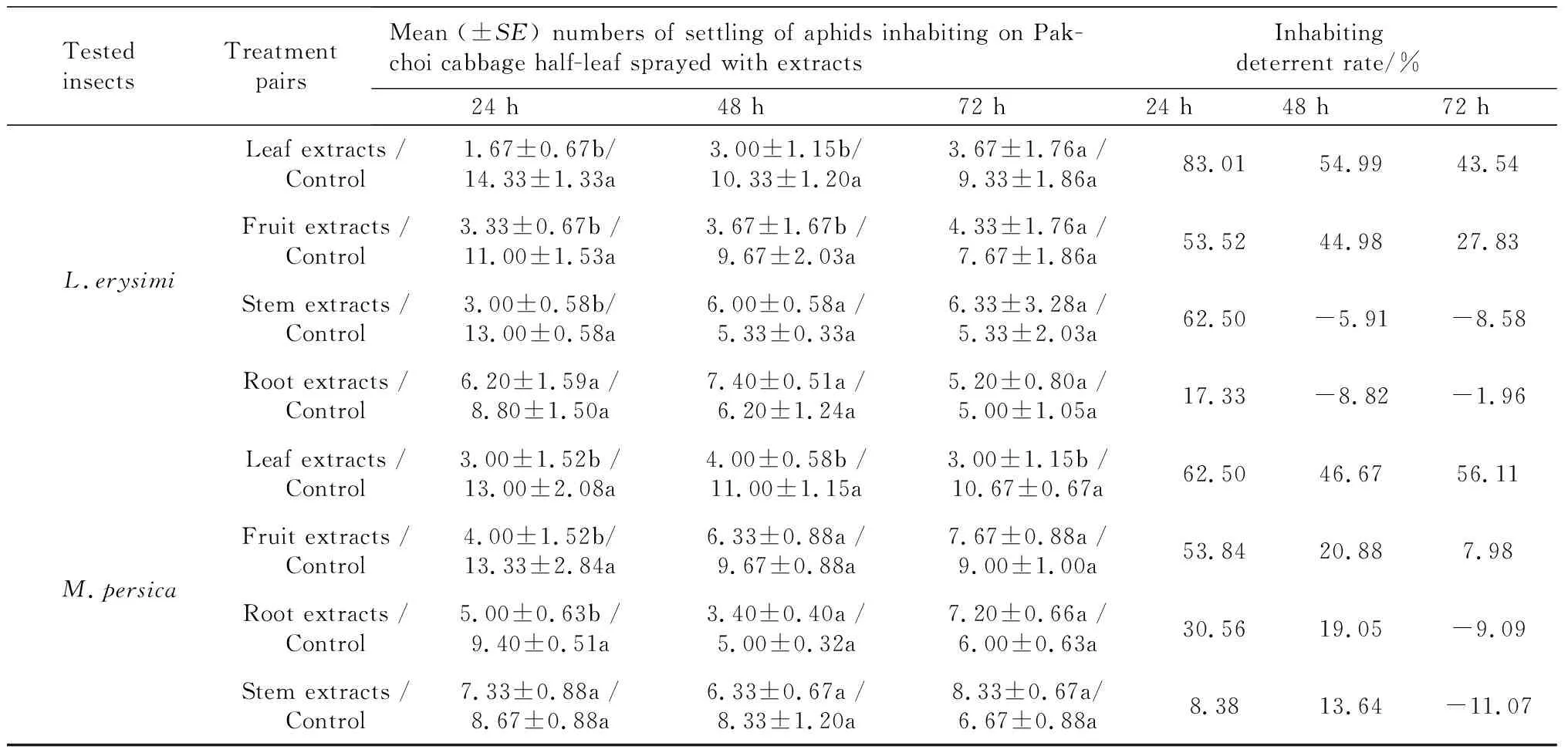
Tab.3 Inhabiting deterrent effect of extracts from four parts of X.sibiricum on L.erysimi and M.persicae in half-leaf choice bioassays
Note: Means followed by a common letter in a pair mean there are not significantly difference atP=0.05 by Paired-samplesTTest.
InhabitingdeterrentchoiceassayonM.persicaeThe inhabiting deterrent activity varied according to the particular extract ofX.sibiricumagainstM.persicae(Table 3). The average number ofM.persicaeinhabiting on the pakchoi cabbage leaf 24 h after treatment was significantly different between the control section and the section treated by leaf extract. However it showed a smaller or no difference between the control and the section treated with the fruit, stem, and root extracts. The most active extract was from the leaf ofX.sibiricumwith a choice inhabiting deterrent rate of 62.50% (24 h), 46.67% (48 h), and 56.11% (72 h) after treatment, followed by the fruit extract with 53.84% (24 h), 20.88% (48 h), and 7.98% (72 h) after treatment. The root and stem extracts showed an even smaller inhabiting deterrent activity rate at 24 h and 48 h after treatment, and no deterrent effect at 72 h.
ComparativeactivityofthefourextractsofX.sibiricumThe results of the leaf disc choice bioassays onP.xylostellaandP.rapaesuggest that the highest antifeedant active components principally exist in the leaf and fruit extracts ofX.sibiricum(especially onP.rapaewith a choice antifeedant rate over 96% at the tested concentration), followed by the stem extract treatment. The lowest antifeedant activity was returned for the root extract solution. In the oviposition / inhabiting deterrent bioassays onP.xylostella,L.erysimi, andM.persicae, the most active components were also extracted from the leaves and fruits, in particular the leaves, followed by the stem extract, and the root extract last of all. However, the root extract did show a higher inhabiting activity than the stem extract againstM.persicae.
3 Discussion
X.sibiricumis a non-host plant for the four herbivores tested. Our results demonstrated that the effect of antifeedant and oviposition / inhabiting deterrents on the test insects varied according to the particular extract of the plant. The most active treatments were from leaves, fruits and stem extracts, which indicates that the insect-susceptible active components existed mostly in the aboveground parts ofX.sibiricum. The leaves and fruits contained more antifeedant and oviposition/inhabiting deterrent active components than either the stems or roots. The active chemicals in the aboveground parts of the plant could interfere with the host-location of phytophagous insects, and deter them from inhabiting or depositing eggs on the plant leaf. The results also indicate that the extracts prevent the insects from ingesting the plant and thus impede these pests building-up their populations onX.sibiricum. Moreover, the treatments from the leaf and fruit extracts might possess intensive insecticidal activity to larvaP.rapaeby inducing raised larval mortality, abnormal pupae or pupa mortality, or crimpled wings of emerged adults in this experiment. Based on their antifeedant and oviposition / inhabiting deterrent properties, the effective active components fromX.sibiricumhave potential for use as alternative crop protectant against a number of pest species.
It was reported thatXanthiumspecies plants could produce variant combinations of sesquiterpene lactones in their leaves and fruits, such as xanthinin, xanthanol, xanthatin, xanthumin, xanthumanol and deacetoxylxanthumin[26-29]. Xanthanolides, isolated fromXanthiumspecies, were used as an anti-attaching repellent against Blue mussel[30], and acted as insect development inhibitors[31]. Further, more detailed studies are warranted to determine whether or not the antifeedant and oviposition / inhabiting deterrent active components are sesquiterpene lactones, whether one or more ingredients are most active, and how insects are affected. Further investigation may also determine how the extracts from different stages of the development ofX.sibiricummight protect plants from some herbivore insects and how it might vary with the seasons.
The effect of plant defensive compounds is diverse with different insects, even though their food preferences are similar. The effect of defensive compounds showed more activity for specialists than for generalists. In this study, among the four tested vegetable insect pests,M.persicaeis a generalist, with host range of more than 400 plant species from more than 40 families[32]. The other three insects,P.xylostella,P.rapae, andL.erysimiare specialist herbivores with limited feeding choices of the family Cruciferae[6]. Our results found that the leaf and fruit extracts fromX.sibiricumhad the highest antifeedant effect onP.rapae, with an antifeedant rate of 99.07% and 96.13% respectively. The effect onP.xylostellawas 73.42% (leaf) and 82.87% (fruit). The leaf extract had higher inhabiting deterrent effect onL.erysimi(83.01%) than onM.persicae(62.50%). This result is consistent with Bernays (2000) who concluded that specialists have greater sensitivity to deterrents than generalists[33]. The high ability ofP.xylostellato develop resistance to almost all groups of insecticides[6]might be one reason why the feeding deterrent effect ofX.sibiricumextracts was less sensitive onP.xylostellathan onP.rapae.
The earliest forms of land plants and insects have coexisted for as long as 350 million years[34]. On the basis of this long-standing relationship, the strategies employed by plants to resist or evade their insect herbivores are very diverse. Accumulating high levels of compounds that function as biochemical defenses through their toxicity is one of the important strategies that plants seek to minimise herbivore damage. As a result, an insect herbivore only feeds on a limited range of plant species, and damage are limited by the number insect herbivores attracted to a particular species[35]. Moreover, the plant secondary compounds are genus-or species-specific[36]. The sensitivity of a range of phytophagous insects with similar host preference is diverse. This restricts the excess ingesting of host plants by the insect herbivores. Consequently, the plant population avoids being wiped out, and the phytophagous insect populations also survive, which accounts for the defensive compounds found in plants playing a significant role in regulating the relationship between insects and plants, and for the survival and evolution of plants.
Isman (2006) suggested that to produce a botanical insecticide on a commercial scale, the source plant biomass must be obtainable on an agricultural scale, and preferably, not on a seasonal basis. Unless the plant in question is extremely abundant in nature, or already grown for another purpose, it must be amenable to cultivation[37].X.sibiricummeets this criterion because of its highly adaptable to a range of environments, and easily spreading and planting[14]. It may serve as a potential new type of botanical behavior regulative protectant against insect pests, and it is safe for natural enemies, human beings, and the environment. The antifeedant and ovoposition/inhabiting active compounds inX.sibiricumis effective against insect herbivores, although the type and strength of the defensive compounds from different stages of growth, and from various parts of the plant need to be investigated further in order to help us to understand the natural mechanisms of non-host plants against insect herbivores. Such elucidation may lead to the development of new types of behavior regulators in insect pest management.
AcknowledgementsWe express our appreciation to our Australian colleague, Mr.Brian Hillyar, for reviewing earlier drafts of the manuscript.
:
[1] SUN C N. Insecticide resistance in diamondback moth [C]// Talekar N S. Management of Diamondback moth and other crucifer pests. Proceedings of the Second International Workshop. Shanhua, Taiwan: Asia Vegetable Research and Development Center, 1992:419-426.
[2] FORGET G, GOODMAN T, VILLIERS A. Impact of pesticide use on health in developing countries[M]. Ottawa: Int Dev Res Centre, 1993.
[3] PERRY A S, YAMAMOTO I, ISHAAYA I,etal. Insecticides in agriculture and environment: Retrospects and Prospects[M]. Berlin: Springer-Verlag, 1998.
[4] ASCHER K R S. Nonconventional insecticidal effects of pesticides available from the neem tree,Azadirachataindica[J]. Arch Insect Biochem, 1993, 22: 433-449.
[5] SCHMUTTER H. Properties and potential of natural pesticides from the neem tree[J]. Ann Rev Entomol, 1990, 35: 271-298.
[6] TALEKAR N S, SHELTON A M. Biology, ecology and management of the diamondback moth[J]. Ann Rev Entomol, 1993, 38: 275-301.
[7] DIXON A F G. Structure of aphid populations[J]. Ann Rev Entomol,1985, 30: 155-174.
[8] RENWICK J A A, CHEW F S. Oviposition bahavior in Lepidoptera[J]. Annu Rev Entomol, 1994, 39: 377-400.
[9] SHELTON A M, WYMAN J A, CUSHING N L. Insecticide resistance of diamondback moth (Lepidoptera:Pluntellidae) in North America[J]. J Econ Entom, 1993, 86:11-19.
[10] TABASHNIK B E, CUSHING N L, FINSON N,etal. Field development of resistance toBacillusthuringiensisin diamondback moth (Lepidoptere: Plutellidae)[J]. J Econ Entom, 1990, 83: 1 671-1 676.
[11] ZHOU C A, WANG X P, CHEN Z F,etal. Insecticide resistance of diamondback moth in Changsha and effects of synergist on insecticides[J]. J Hunan Agri Univ (Natural Sci), 2000, 26: 358-362.
[12] WU Q J, ZHU G R, ZHAO J Z,etal. Studies on biochemical mechanisms of Chlorfluazuron resistance in diamondback moth,Plutellaxylostella(L.)[J]. Acta Entom Sinica, 1998, 41: 42-48.
[13] ZHAO J Z, WU S C, GU Y Z,etal. Strategy of insecticide resistance management in the diamondback moth[J]. Agr Sci China, 1996, 29: 8-14.
[14] QIAN X. The preliminary studies on habits of growth and damage of cocklebur and its chemical control[J]. Soybean Sci, 1988, 7: 61-68.
[15] CHEN T B, ZHANG Z Y, WANG Y M,etal. Research on application of harmony for controlling weeds in soybean fields[J]. J Weed Sci, 1991, 5: 31-32.
[16] ZHANG Z P. Development of chemical weed control and integrated weed management in China[J]. Weed Biology and Management, 2003, 3: 197-203.
[17] Editorial Board of Pharmacopoeia of the People’s Republic of China. Pharmacopoeia of P.R. China (English Edition): Book Ⅰ[M]. Beijing: Chemical Industry Press, 2000.
[18] HOU H G, LU Y T, SU Y H,etal. Textual studies onXanthiumsibiriumin ancient Chinese medicinal literatures[J]. Chinese Traditional and Herbal Drugs,2002,33: 1 128-1 130.
[19] Editor Committee of China Traditionary Insecticides. China traditionery insecticides[M]. Beijing: China Sci Press, 1959.
[20] GAO H M, WANG Z L, ZHANG B,etal. Insecticidal activity of some plant extracts againstPierisrapae[J]. Jiangsu Agr Res, 1999, 20: 32-34.
[21] ZHOU Q, LIANG G W, ZENG L,etal. The control efficiency of plant alcohol extracts on the laboratory populations ofMyzuspersicae(Sulzer) andLipaphiserysimi(Kaltenbach)[J]. Agr Sci China, 2002, 1: 1 199-1 203.
[22] ZHOU Q, LIANG G, ZENG L,etal. Effect of plant extracts and some biorational insecticides on egg hatch, survival and predation rates ofMenochilussexmaculata(Fabricius) andCoccinellatrasversalis(Fabricius): the key natural enemies of cruciferous vegetable aphids in south China[J]. Acta Ecol Sinica, 2003, 23: 2 736-2 740.
[23] ZHOU Q, LIANG G W, ZENG L,etal. Effect of plant extracts and pesticides on survival, emergence and parasitism of the aphid parasitoidsAphidiusgifuensisAshmead andDiaeretiellarapaeM’Intosh[J]. Acta Ecol Sinica, 2005, 25: 1 357-1 361.
[24] CUI D J, CHIU F S, LIU X Q. Study on the crude extracts from marigold against the oviposition of Diamonback moth[J]. Pesticides, 1998, 37: 31-35.
[25] LIU S S. Introduce a method of tearing aphids—a new method of leaf disc[J]. Entomol Knowl, 1987, 25: 79-80.
[26] ABDEI-MOGIB M, DAVIDAR A M, METWAUY M A,etal. Xanthanolides fromXanthiumspinosum[J]. Phytochemistry, 1991, 30:3 461-3 462.
[27] AHMED A A, JAKUPOVIC J, BOHLMANN F,etal. Sesquiterpene lactones fromXanthiumpungens[J]. Phytochemistry, 1990, 29: 2 211-2 215.
[28] CUMANDA J, MARINONI G, BERNARDI M. New sesquiterpenes fromXanthiumcatharticum[J]. J Nat Prod, 1991, 54: 460-465.
[29] MAHMOUD A A. Xanthanolides and xanthane epoxide derivatives fromXanthiumstrumarium[J]. Planta med, 1998, 64: 724-727.
[30] HARADA A, SAKATA K, INA H. Isolation and identification of xanthatin as an antiattaching repellent against Blue mussel[J]. Agric Biol Chem, 1985, 49: 1 887-1 888.
[31] KAWAZU K, NAKAJIMA S, ARIWA M. Xanthumin and 8-epi-xanthantin as insect development inhibitors fromXanthiumcanadenseMill[J]. Cellular and Molecular Life Sciences, 1979, 35: 1 294-1 295.
[32] VANGAS R R, TRONCOSO A J, TAPIA D H,etal. Behavioural differences during host selection between alate virginoparae of generalist and tobacco-specialistMyzuspersicae[J]. Entomol Exp Appl, 2005, 116: 43-53.
[33] BERNAYS E A, OPPENHEIM S, CHAPMAN R F,etal. Taste sensitivity of insect herbivores to deterrents is greater in specialists than in generalists: a behavioral test of the Hypothesis with two closely related caterpillars[J]. J Chem Ecol, 2000, 26: 547-563.
[34] GATEHOUSE J A. Plant resistance towards insect herbivores: a dynamic interaction[J]. New Phytol, 2002, 156: 145-169.
[35] PANG X F. Plant protectants and plant immune engineering against insect pests[J]. World Sci-Tech R & D, 1999,21: 24-28.
[36] BALANDRIN M F, KLOCKE J A, WURTELE E S,etal. Natural plant chemicals: sources of industrial and medicinal materials[J]. Sci, 1985, 228:1 154-1 160.
[37] ISMAN M B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasing regulated world[J]. Annu Review Entom, 2006, 51: 45-66.

