Synergistic Action of fMLP-boanmycin Combination on the Growth of Mouse Colon Carcinoma and Its Action Mechanisms
Zhong-dong Li, Yan Gao
Department of Pharmacology, General Hospital of PLA Air Force, Beijing 100142, China
Synergistic Action of fMLP-boanmycin Combination on the Growth of Mouse Colon Carcinoma and Its Action Mechanisms
Zhong-dong Li*, Yan Gao
Department of Pharmacology, General Hospital of PLA Air Force, Beijing 100142, China
Objective:The inhibitory action of fMLP-boanmycin (BAM) combination on the growth of mouse colon carcinoma and its action mechanisms were observed in order to provide experimental proof for probing novel regimen of chemotactic modulation in combination with chemotherapy in the treatment of cancer.
Methods:Cytotoxicity of BAM-fMLP combination to tumor cells was determined by MTT assayin vitro. Antitumor activity of BAM-fMLP combination was assessed in mice subcutaneously transplanted colon carcinoma 26. The amount of superoxide anion (O2¯‧) released from fMLP stimulated macrophages was determined by NBT assay. The amount of nitric oxide (NO) was indirectly determined by Griess method.
Results:BAM-fMLP combination had no synergistic effect on tumor cells(CDI>0.85), but BAM at the doses of 10μg/ml, 30μg/ml and 100μg/ml in combination with fMLP at the concentration 20μg / ml exhibited synergistic effect on tumor cells in the presence of macrophages(CDI<0.75). fMLP inhibited the growth of colon carcinoma 26 by 50.0% when it at dose of 1 mg/mouse was administered peritumorally. BAM (1 mg/kg, intraperitoneally, three times) alone and BAM - fMLP combination inhibited the growth of colon carcinoma 26 by 38.6% and 78.4%, respectively (CDI=0.71) on day 12. The amount of O2¯‧ released from fMLP 4.6×10-7mol/L (0.2μg/ml) stimulated macrophages which were treated by BAMin vitroincreased significantly(P<0.01). fMLP 2.3×10-6mol/L (1μg/ml) could not stimulate macrophages to release NO, but may stimulate macrophages treated with BAM 10μg/ml and 100μg/ml to release NO significantly(P<0.01).
Conclusion:The inhibitory action of fMLP-boanmycin combination on the growth of mouse colon carcinoma have synergism, which may associate with the increase of O2¯‧ and NO released by macrophages. Chemotactic modulation in combination with chemotherapy may be a novel regimen in the treatment of cancer.
Chemotactic peptide; fMLP; Boanmycin; Colon carcinoma
Chemotactic peptide can attract and activate leukocytes including macrophages, which can interfere the process of tumor growth, invasion and metastasis[1-3]. Bacterial chemotactic peptide fMLP (CHO-Met-Ile-Phe, fMLP) can induce the adhesion of leukocytes to endothelial cells, and promote them to migrate through endothelial cell space residing outside blood vessels[4]. It was reported that fMLP induced immune inflammation of mucus membranewhen it was injected into mouse colon[5]. Zhang L et al[6]discovered that when fMLP was administered peritumorally in mice 48 h after tumor inoculation once every 2 days for 21 days, tumor growth delayed significantly. Ottonello L et al[7-8]reported that monoclonal antibody (Lym-1) identified antigen HLA-DR on B type lymphoma cells could inhibit the proliferation of tumor cells by activating peripheral blood neutraphils only when fMLP was applied at the same time.
Boanmycin (BAM), a single A6 component of bleomycin, was isolated fromstreptomycesverticillusmetabolites of China. It can inhibit the growth of subcutaneously transplanted human hepatoma Bel-7402[9], human colon carcinoma HT-29 and cecum carcinoma Hce-8693[10]in nude mice. It can also inhibit liver metastasis of subcutaneous, intracecal wall, intra-hepatic and intra-splenic transplanted colon carcinoma 26[11]. It was reported that bleomycin at lower dose may restore the tumoricidal activity of macrophages from rats bearing KDH-8 hepatoma[12].
This paper was set to investigate the synergistic action of fMLP-BAM combination on the growth of mouse colon cancer and its action mechanisms in order to provide experimental proof for probing novel regimen of chemotactic modulation in combination with chemotherapy in the treatment of cancer.
MATERIALS AND METHODS
Reagents
fMLP was purchased from Sigma Company. It was dissolved with tiny amount of DMSO and then diluted with PBS 0.05 mol/L (pH 8.5) so that DMSO content was less than 5%. BAM, was dissolved with 0.9% NaCl before experiment.
Animals
The animals used in the experiment were Balb/C mice (Female, 18-22 g, Grade Ⅱ, certificate No. SCXK (Army) 2002-001, from Experimental Animal Institute, Chinese Military Medical Academy, Beijing, China)
Tumor cells
Mouse colon carcinoma 26 cell suspension of 0.2 ml (107cells) stored in liquid nitrogen was placed in room temperature for some time, then centrifuged at 400×g for 5 min. The pellete was diluted with RPMI-1640 medium supplemented with 10% inactivated fetal calf serum, and then the dilution was transferred into culture flask and cultivated in 5% CO2incubator at 37°C for 3 days. Cells in the phase of exponential growth were collected and resuspended at 1×105cells/ml for next use.
Macrophages
Peritoneal macrophages were collected from peritoneal lavage of Balb/c mice injected intraperitoneally with 2 ml RPMI-1640 medium. The macrophage suspension was centrifuged at 400×g for 5 min, and then the macrophage pellet diluted with about 10 ml RPMI-1640 medium was transferred into culture dishes to cultivate for 2 h in 5% CO2incubator at 37°C. After the supernatant was dislodged, the cells were collected by scraping with scraper and cell suspension at 5~6×105cells/ml in RPMI-1640 medium was prepared for next use.
Evaluation of Cytotoxicity of BAM in Combination with fMLP to Tumor Cells by MTT Assay
Mouse colon carcinoma 26 cell suspension (104cells/100 µl) was seeded into each well of 96-well plate. Each well was further supplied with an equal volume of RPMI-1640 culture media. Vehicle control group, BAM group, fMLP group and BAM+ fMLP group were designed in the experiment. The plates were incubated in 5% CO2incubator at 37°C for 4 h after drugs of various concentrations were added into each well of drug groups. Stock MTT solution (2 g/L) of 50 µl was added into each well and the plates were continued to incubate for 4 h in the same incubator. Supernatant from each well was drawn out carefully and 150 µl DMSO was added. The plate was shaked strongly for 15 min. Absorbance was measured at 560 nm. To observe the cytotoxicity of the drugs to tumor cells in the presence of macrophages, 100 μl tumor cell suspension (1×104cells) and 100 μl macrophage cell suspension (5×104cells) were mixed, and then seeded into 96 well plate and cultivated for 4 h in 5% CO2incubator at 37°C. The inhibitory rate was calculated as follows∶
Inhibitory rate=[(A0-A1)/A0] × 100%,
A0represents total absorbance of tumor cell control,
A1represents the absorbance of drug treatment group.
Animal Experiment
Mouse colon carcinoma 26 cell suspension (106cells) were injected subcutaneously into each mouse’s right lateral chest wall near the axilla. The cells were passed every 13 days and the second passage was used for the experiment. At the time of animal experiment, mouse subcutaneous tumor was removed, weighed, cut up with eye scissors and transferred into homogenizer. After homogenization, the cell suspension was sedimented for 5 min and upper cell suspension was collected for next use.
The above cell suspension(0.2 ml) was injected subcutaneously into each mouse’s right lateral chest wall near the axilla. Twenty four animals were averagely divided into vehicle control group, BAMgroup, BAM + fMLP group and fMLP group. BAM was administered intraperitoneally and fMLP peritumorally once every 3 days for three times beginning at day 1. Tumor diameters were measured with a caliper. Tumor size was calculated by the formula “a×b2/2”, where “a” represents long diameter and “b” short diameter. Inhibitory rate was calculated as follows∶ inhibitory rate (%)=[(C-T)/C]×100%, where “C” represents tumor size of control group and“T” tumor size of treatment group.
Superoxide Anion Free Radical (O2¯· ) Determination[13]
The macrophages were pretreated by BAM at concetrations of 1μg/ml, 10μg/ml and 100μg/ml for 30 min, followed by incubation for 30 min after addition of NBT(1mg/ml) 500 μl/well and fMLP 4.6×10-7mol/L(0.2μg/ml). The macrophages were stained with Giemsa for 10 min, washed with distilled water and air-dried. NBT positive macrophages contained over 3 blue-black particles were counted under microscope. Positive percentage was calculated.
Qualification of Nitric Oxide(NO)[14]
The macrophage cell suspension at 1×106cells/0.2 ml and BAM at various concentrations were added into each well of 96-well plate. The plate was incubated in 5% CO2incubator at 37°C for 30 min. After fMLP at final concentration of 2.3×10-6mol/L (1μg/ml) was added into the wells, the plate was continuously incubated for another 24 h. One hundred μl supernatants were mixed with an equal volume of Griess’s reagent (1% sulfanilamine/0.1% naohthylethylenediamine dihydrochloride, Sigma Chemical Co.) and incubated at room temperature for 15 min. The absorbance at 560 nm was measured in an ELISA reader. The NO concentration was determined using sodium nitrite as a standard and fresh medium as a blank.
Data Analysis
The synergism of drug combination was evaluated as follows[15]∶ CDI(combination drug index)=AB/A×B, where AB was the ratio of the data of combination groups to that of control group and A or B was the ratio of the data of drug group to that of control group. CDI<0.85 represents synergism and CDI<0.75 indicates significant synergism in the experiment.
Data were expressed as mean±standard deviation (¯x±s). The significance of the results was determined by “F” test using SAS 6.12 & 8.0 software.
RESULTS
Cytotoxicity of fMLP-BAM Combination to Tumor Cells
fMLP at the concentration of 20 µg/ml showed no cytotoxicity to mouse colon carcinoma 26 cells . CDI of cytotoxicity of fMLP at the concentration of 20 µg/ml in combination with BAM at the concentrations of 10 µg/ml and 100 µg/ml to mouse colon carcinoma 26 cells was more than 0.85 (Table 1). These experiments indicated that fMLP-BAM combination had no synergism in cytotoxicity to mouse colon carcinoma 26 cells.
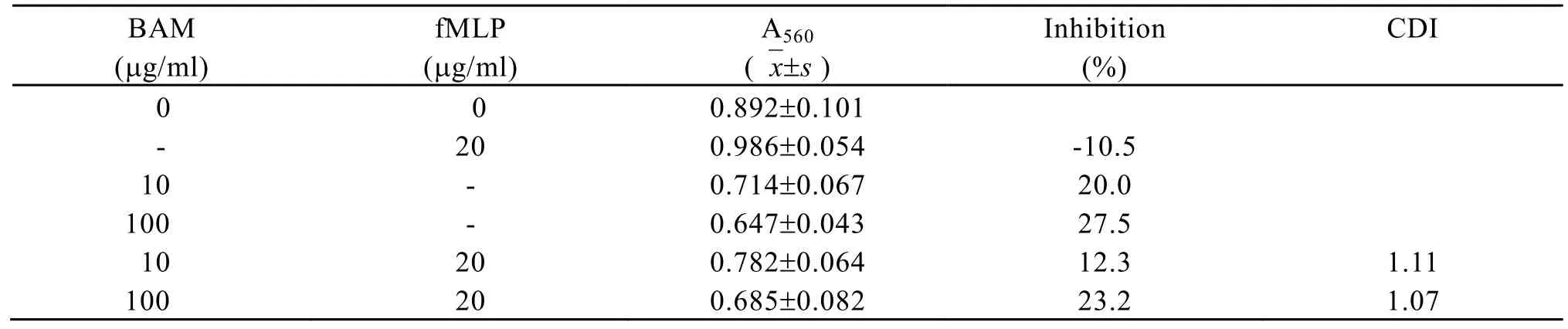
Table 1. Cytotoxicity of BAM in combination with fMLP to colon carcinoma 26 cells
Cytotoxicity of fMLP-BAM Combination to Tumor Cells in the Presence of Macrophages
CDI of cytotoxicity of fMLP at the concentration of 20 µg/ml in combination with BAM at the concentrations of 10 µg/ml, 30 µg/ml and 100 µg/ml, in the presence of macrophages, was all lower than 0.75 (Table 2). These experiments indicated that fMLP- BAM combination had synergism in cytotoxicity to mouse colon carcinoma 26 cells in the presence of macrophages.
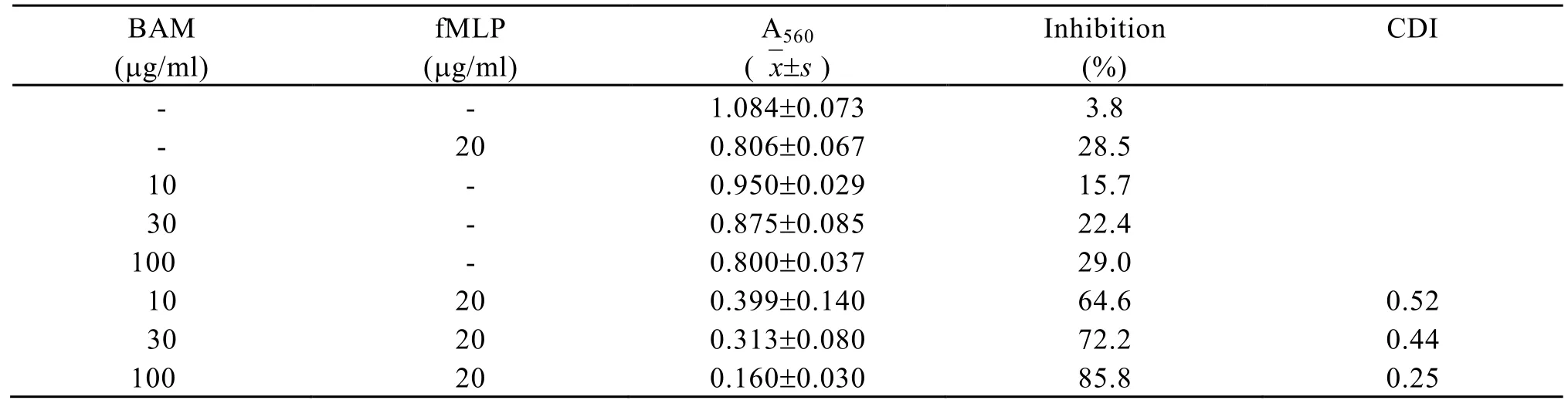
Table 2. Cytotoxicity of BAM in combination with fMLP on colon carcinoma26 cells in the presence of macrophages
Tumor Growth Inhibition of fMLP and BAM Applied 24 h after Tumor Inoculation
Mouse colon carcinoma 26 cells were injected s.c into each mouse at right lateral chest wall near the axilla 24 h before first administration. fMLP ( peritumorally) and BAM (intraperitoneally) were all administered for three times. On day 12, fMLP 1 mg/kg inhibited tumor growth by 50.0%. BAM 1mg/kg alone and BAM-fMLP combination suppressed the tumor growth by 38.6% and 78.4% (P<0.05, CDI=0.71), respectively. This result showed fMLP-BAM combination had synergism (Table 3).
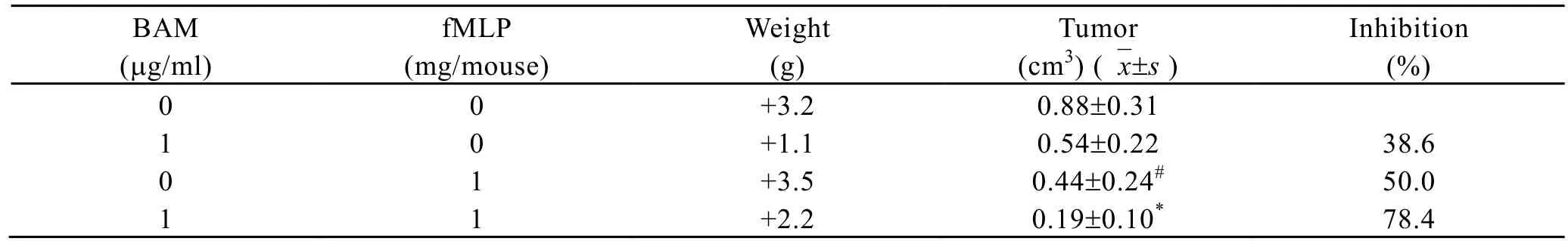
Table 3. The effect of BAM in combination with fMLP on the growth of coloncarcinoma in mice
O2¯Production of Macrophages
The amount of O2released from fMLP stimulated macrophages was determined by NBT assay. The result showed that the amount of O2¯‧ released from fMLP (4.6×10-7mol/L, 0.2μg/ml)stimulated macrophages which were treated by BAM in vitro increased significantly. Positive rate(%) ascended from 24.4±6.6 in fMLP group to 62.0±9.4, 57.2±7.4 and 48.8±10 in those treated with BAM 1μg/ml, 10μg/ml and 100μg/ml, respectively (P<0.01) (Table 4).
Nitric oxide (NO) becames nitrite (NO2) soon in vitro. The amount of NO2¯‧ determined by Griess method can represent that of NO indirectly. When murine peritoneal macrophages were incubated with drugs for 24h, fMLP 2.3×10-6mol/L(1 μg/ml) could not stimulate macrophages to release NO, but did stimulate macrophages treated with BAM 10μg/ml and 100μg/ml to release NO significantly. The amount of NO was increased from 18.8±2.1 μmol/L per 106cells in fMLP group to 30.8±3.9 μmol/L per 106cells and 46.6±3.4 μmol/L per 106cells in groups treated with BAM 10μg/ml and 100 μg/ml, respectively (P<0.01) (Figure 1).
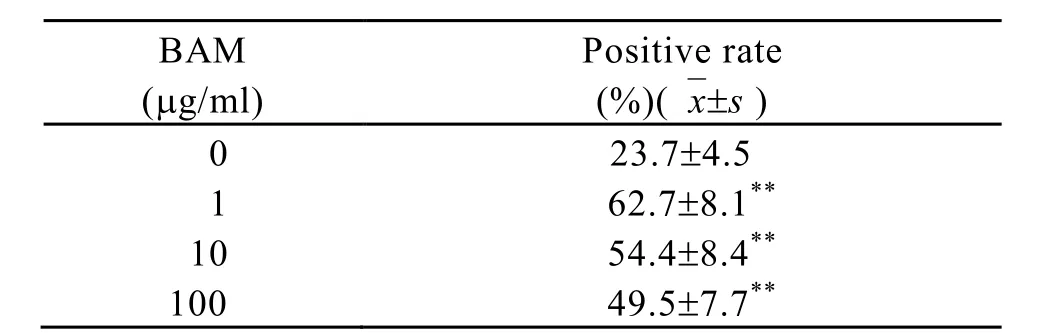
Table 4. The effect of fMLP on the release of O2¯‧ from macrophages treated by BAM, in vitro
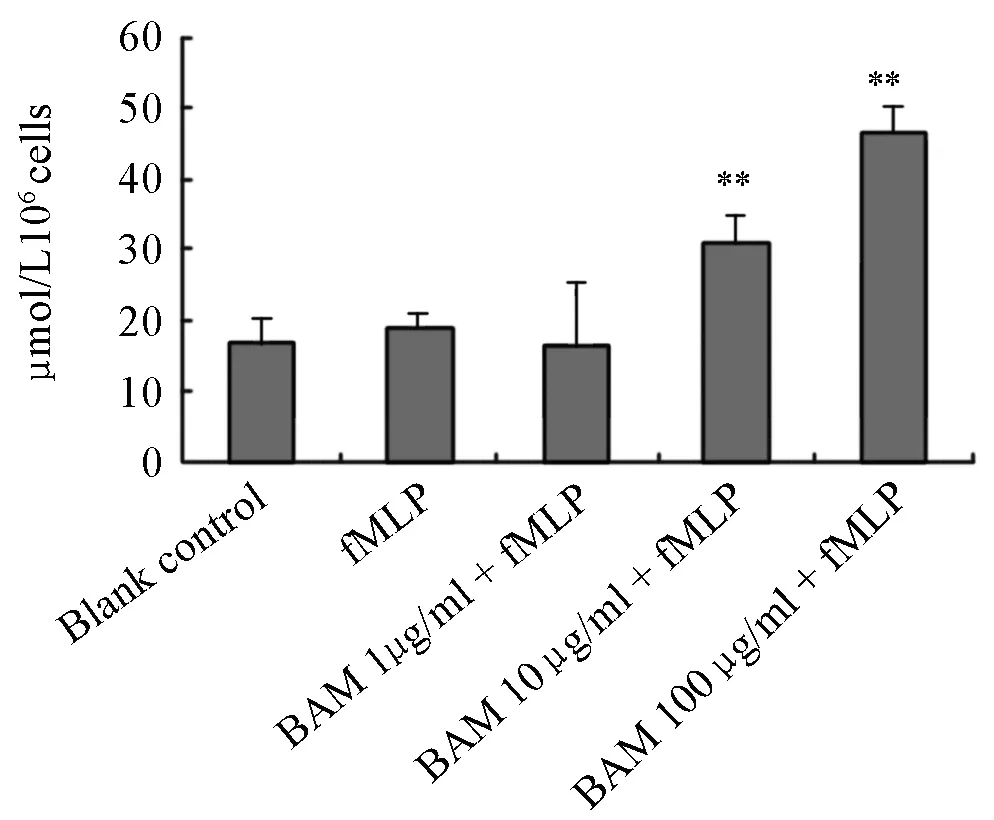
Figure 1. The effect of fMLP on NO release of macrohages treated by BAM.**P<0.01, as compared with fMLP group. n=3, ¯x±s. BAM∶ µg/ml. fMLP∶ 2.3×106mol/L (1µg/ml).
DISCUSSION
Tumor treatment by chemotactic modulation is to attract immune cells of peripheral blood into tumor tissue and to activate the cells to inhibit tumor growth. Chemotactic peptide fMLP has the function of attracting and activating peripheral leukocyte including macrophages. It may induce immune inflammation reaction of intestinal mucous membrane when it is injected into colon[5]. fMLP may stimulate human colon carcinoma HT29 - MTX cell line to release IL-8 which may attract peripheral leukocyte further[16]. So, mouse colon carcinoma 26 transplanted model was selected in this experiment to observe the effect of fMLP or fMLP-BAM combination on tumor growth. Our resultsin vivoshowed that fMLP inhibited tumor growth by 50%, similar to the results reported by Zhang L et al[6], and fMLP-BAM combination had synergism in inhibiting the growth of tumor. This indicated that chemotactic modulation in combination with chemotherapy may be a new regimen in tumor treatment.
Our experimentin vitroshowed that the cytotoxicity of BAM alone to mouse colon carcinoma cells increased with concentration and the cytotoxicity of fMLP-BAM combination did not show further increase, but in the presence of macrophages, cytotoxicity of fMLP-BAM combination had synergism, which indicated that the antitumor action of fMLP-BAM combination need the participation of macrophages.
The mechanism of the synergism of BAM-fMLP combination was studied. The experiment was set on the basis that fMLP may stimulate macrophages to release free radicals. The results showed that fMLP at 4.6×10-7mol/Lin vitrostimulated BAM-pretreated macrophages to release O2¯‧ significantly and fMLP at 2.3×10-6mol/L could not stimulate macrophages to release nitric oxide (NO), but could stimulate macrophages pretreated with BAM of 10μg/ml and 100μg/ml to release NO significantlyin vitro, similar to the result reported by Yuan L et al[12]that lipopolysaccharide, one of bacterial immunomodulators, stimulated macrophages from rats bearing KDH-8 hepatoma treated by bleomycinin vivoto release NO. Above results indicated that one of the mechanisms of antitumor action of fMLP-BAM combination may be related to the production of O2¯‧and NO.
It is well known[17]that NO is formed enzymatically from the terminal guanidine -nitrogen of L-arginine by the Ca2+-independent, inducible NO synthase (iNOS). There are regulatory sites in iNOS gene promoter region for the transcription factor, nuclear factor-B(NF-κB). NF-κB and Iκ-B, an inhibitory protein, coexist in the cytoplasm in their inactive complex form. Activation of signaling cascade in response to external stimuli such as fMLP induces phosphorylation of Iκ-B and its dissociation from the NF-κB complex; proteolytic degradation of Iκ-B by Iκ-B protease; and translocation of the activated NF-κB and its binding to the relevant gene promoter. It is reported that the fMLP-induced signal transduction for macrophage NO production is involved in NF-κB[17]and BLM induced the transcriptional activation of NF-κB signaling in bronchial epithelial cells[18]. These results suggested that fMLP-BAM combination may synergistically activate NF-κB signaling pathway to cause NO release from fMLP-stimulated BAM-pretreated macrophages.
In conclusion, Besides cytotoxic action of BAM on tumor cells, chemotactic peptide fMLP may stimulate leukocyte including macrophages pretreated with BAM to produce active oxygen (O2¯‧ and NO) to suppress the growth of tumor further. Chemotactic modulation in combination with chemotherapy may be a novel regimen in tumor treatment.
Acknowledgement
We thank Prof. Yong-su Zhen (Institute of Medicinal and Biotech, China) for presenting compound of Boanmyein and mouse colon carcinoma 26 cells to us. We also thank Prof. Liang-ping Hu (Military Medicine Academy, China) for providing SAS6.12 8L8.0 software.
REFERENCES
[1] Melani C, Pupa SM, Stoppacciaro A, et al. Anin vivomodel to compare human leukocyte infiltration incarcinoma xenografts producing different chemokines [J]. Int J Cancer 1995; 62∶ 572-8.
[2] Opdenakker G, Van Damme J. Chemotactic factors, passive invasion and metastasis of cancer cells[J]. Immunol Today 1992; 13∶ 463-4.
[3] Wang JM, Chertov O, Proost P, et al. Purification and identification of chemokines potentially involved in kidney-specific metastasis by a murine lymphoma variant∶ induction of migration and NFκB activation[J]. Int J Cancer 1998; 75∶ 900-7.
[4] Gebhard B, Gnant M, Schutz G, et al. Different transendothelial migration behaviour pattern of blood monocytes derived from patients with benign and malignant disease of the breast[J]. Anticancer Res 2000; 20(6B)∶ 4599-604.
[5] Chester JF, Ross JS, Malt RA, et al. Acute colitis produced by chemotactic peptides in rats and mice[J]. Am J Pathol 1985; 121∶ 284-90.
[6] Zhang L, Khayat A, Cheng H, et al. The pattern of monocyte recruitment in tumors is modulated by MCP-1 expression and influences the rate of tumor growth[J]. Lab Invest 1997; 76∶ 579-90.
[7] Ottonello L, Morone P, Mancini M, et al. fMLP-and TNF-stimulated monoclonal Lym-1 antibodydependent lysis of B lymphoblastoid tumor targets by neutrophils[J]. Br J Cancer 1999; 80∶ 331-7.
[8] Ottonello L, Morone P, Dapino P, et al. Monoclonal Lym-1 antibody dependent lysis of B-lymphoblastoid tumor targets by human complement and cytokinineexposed mono-nuclear and neutrophilic polymorphonuclear leukocytes[J]. Blood 1996; 87∶ 5171-8.
[9] Jiang M, Zhen YS. Antitumor activity of bleomycin A6 against human liver cancer in cell culture and in nude mice[J]. Yao Xue Xue Bao(in Chinese) 1987; 22∶ 881-5.
[10] Deng YC, Zhen YS, Zheng S, et al. Inhibitory effect of bleomycin A6 on human colon cancer xenografts in nude mice[J]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao(in Chinese) 1990; 12∶ 335-40.
[11] Liu XJ, Li Y, Zhen YS. Inhibitory effect of boanmycin on the growth of colon carcinoma and hepatic metastasis in mice[J]. Yao Xue Xue Bao(in Chinese) 2001; 36∶ 14-8.
[12] Yuan L, Kobayashi M, Kuramitsu Y. Restoration of macrophage tumoricidal activity by bleomycin correlates with the decreased production of transforming growth factor β in rats bearing KDH-8 hepatoma cells[J]. Cancer Immunol Immunother 1997; 45∶ 71-6.
[13] Bryant SM, Lynch RE, Hill HR. Kinetic analysis of superoxide anion production by activated and resident murine peritoneal macrophages[J]. Cell Immunol 1982; 69∶ 46-58.
[14] Nielsen H. Antibiotics and human monocyte function I. Chemotaxis[J].Acta Pathol Microbiol Immunol Scand B 1987; 95∶ 293-6.
[15] Cao SS, Zhen YS. Potentiation of antimetabolite antitumor activityin vivoby dipyridamole and amphotericin B[J]. Cancer Chemother Pharmacol 1989; 24∶ 181-6.
[16] Leiper K, Campbell BJ, Jenkinson MD, et al. Interaction between bacterial peptides, neutrophils and goblet cells∶ a possible mechanism for neutrophil recruitment and goblet cell depletion in colitis[J]. Clin Sci (Lond) 2001; 101∶ 395-402.
[17] Sodhi A, Biswas SK. fMLP-inducedin vitronitric oxide production and its regulation in murine peritoneal macrophages[J]. J Leukoc Biol 2002; 71∶262-70.
[18] Ma Y, Wang M, Li N, et al. Bleomycin-induced nuclear factor-kappaB activation in human bronchial epithelial cells involves the phosphorylation of glycogen synthase kinase 3 beta[J]. Toxicol Lett 2009; 187∶194-200.
R735.3+5 Document code: A Article ID: 1000-9604(2010)02-0135-06
10.1007/s11670-010-0135-3
2009−10−19; Accepted 2010−02−23
*Corresponding author.
E-mail∶ zhd1009@126.com
© Chinese Anti-Cancer Association and Springer-Verlag Berlin Heidelberg 2010
 Chinese Journal of Cancer Research2010年2期
Chinese Journal of Cancer Research2010年2期
- Chinese Journal of Cancer Research的其它文章
- Triptolide Inhibits Cell Growth and Induces G0- G1 Arrest by Regulating P21wap1/cip1 and P27 kip1 in Human Multiple Myeloma RPMI-8226 Cells
- Different Outcome of Myeloid Sarcoma with Spinal Cord Compression Preceding Acute Myeloid Leukemia: Report of Two Cases and Review of Literatures
- Effects of Triptolide on Histone Acetylation and HDAC8 Expression in Multiple Myeloma in vitro
- CGI-100 Specific shRNA Inhibits Proliferation and Induces Differentiation in Leukemia K562 Cells
- Expression of Embryonic Stem Cell Marker Oct-4 and Its Prognostic Significance in Rectal Adenocarcinoma
- Differential Diagnosis of Warthin's Tumor Complicated with Lung Adenocarcinoma by 18F- FDG PET/CT Imaging and Radioisotope Scanning with Tc-99m Pertechnetate: A Case Report and Literature Review
