Life made easy: simplifying reconstruction for dual portal veins in adult right lobe live donor liver transplantation
Albert C.Y. Chan, Chung Mau Lo, Kenneth S.H. Chok, See Ching Chan and Sheung Tat Fan
Hong Kong, China
New Techniques
Life made easy: simplifying reconstruction for dual portal veins in adult right lobe live donor liver transplantation
Albert C.Y. Chan, Chung Mau Lo, Kenneth S.H. Chok, See Ching Chan and Sheung Tat Fan
Hong Kong, China
In live donor liver transplantation, anatomical anomalies of the portal vein are more frequently encountered in right lobe than left lobe grafts. Of these, a dual portal vein is one of the most common anatomical anomalies encountered. We hereby report our method of using a recipient portal vein patch after venoplasty for reconstruction in a right lobe graft with separate anterior and posterior portal vein branches.
live donor liver transplantation; portal vein reconstruction; dual portal vein
Introduction
Live donor liver transplantation (LDLT) is an effective treatment for end-stage liver diseases, especially in areas where cadaveric grafts are in short supply. Using the right lobe for LDLT has been popularized by most centers due to the larger graft size for adult recipients. However, anatomical anomalies of the portal vein are more frequently encountered in right than left lobe grafts.[1]We report our method of using a recipient portal vein patch after portal venoplasty for vascular reconstruction in a right lobe graft with dual portal vein branches.
Surgical technique
A 48-year-old female with hepatitis B-related liver cirrhosis and recurrent hepatocellular carcinoma was referred to our center for consideration of salvage liver transplantation. She received percutaneous radiofrequency ablation for the primary tumor in July 2008. The initial tumor measured 13×9×13 mm. Surveillance computed tomography in December 2009 showed a recurrent tumor measuring 24×21×31 mm in the subcapsular area of segments VI/VII of the liver. Serum alpha-fetoprotein was raised to 18 103 ng/ml. Her baseline liver function test revealed hyperbilrubinemia (42 μmol/L) and severe thrombocytopenia (39 μmol/L) that precluded her from other curative surgical treatments. Her model for endstage liver disease score was 12. Distant metastasis was excluded after various scans including computer tomography of the thorax, bone scan and whole body positron emission tomography. LDLT using the right lobe with inclusion of the middle hepatic vein was performed in March 2010. The liver was donated by her younger sister. The liver graft weighed 585 gm and the estimated size of liver mass of the recipient was 55.5%. Early bifurcation of the right portal vein was noted intraoperatively. The diameters of the anterior and posterior branches of the portal vein were 13 mm and 10 mm respectively (Fig. 1). The hepatic artery anatomy was normal and there was one bile duct opening.
For portal vein reconstruction, the two branches of the portal vein were first joined together by venoplasty at the backtable using 6-0 polypropylene sutures to form a common orifice measuring 16 mm in the longitudinal axis (Fig. 2). Because the portal vein wall was found to be very thin such that it could be easily torn by suturing if the portal vein anastomosis was performed within the deep abdominal cavity in this patient, a 2-cmlong portal vein patch was transected just distal to the recipient portal bifurcation and was anastomosed to the graft venoplasty using continuous 6-0 polypropylenesutures for both anterior and posterior layers at the backtable (Figs. 3 and 4). A leakage test was then performed by pulsatile injection of heparin-saline into the portal vein orifice.

Fig. 1. Dual right portal veins in the graft liver with thin and delicate walls.
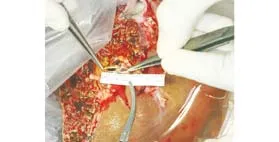
Fig. 2. Anterior and posterior branches of the portal vein were anastomosed together by venoplasty using 6-0 polypropylene sutures to form a single rhomboid-shaped orifice.
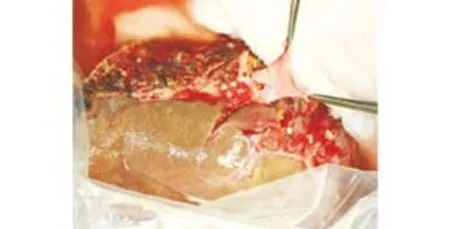
Fig. 3. Side-view of the anastomosis between the graft portal vein and recipient portal vein patch. Performing a portal anastomosis at the backtable facilitates precise and meticulous suturing in thin and delicate vein walls in the liver graft. The anastomosis was performed with continuous 6-0 polypropylene sutures for both anterior and posterior layers.
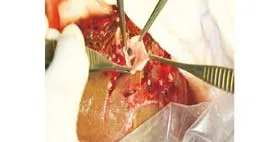
Fig. 4. End-view of the anastomosis between the graft portal vein and recipient portal vein patch. An interposition portal vein patch facilitates in situ anastomosis between the graft and recipient portal vein with different wall thickness and consistency. This method is particularly useful for recipients with a deep abdominal cavity.
After placement of the graft into the right subphrenic space and re-anastomosis of the hepatic vein with the inferior vena cava, anastomosis between the interposition portal vein patch and recipient portal vein was done using 6-0 polypropylene for the anterior and posterior layers. Hepatic artery anastomosis was performed with the microvascular technique and a duct-to-duct anastomosis was performed for bile duct reconstruction.
Routine postoperative Doppler ultrasonography confirmed patency of the portal vein with a portal flow measuring 0.7-0.9 m/s. Computed tomography of the liver on postoperative days 3 and 10 showed portal vein anastomosis with no evidence of thrombosis. The graft function was satisfactory at the time of writing this report.
Discussion
The frequency of portal vein anomalies in right lobe LDLT range between 10% and 35%.[1-3]Various techniques for portal vein reconstruction have been reported ranging from the use of interposition graft, either from the great saphenous vein,[4]superficial femoral vein,[5]or recipient portal bifurcation as a Y-shaped graft,[2]to the use of a cryopreserved iliac vein graft from cadaveric donors. However, all these methods have their pitfalls. Procurement of autologous veins from other sources prolongs the operating time and incurs potential morbidity to the donor site. The suboptimal longterm result of cryopreserved vein grafts has limited its widespread application in vascular reconstruction in LDLT. On the other hand, combining the technique of venoplasty and using recipient portal vein tissue for vascular reconstruction in dual portal veins hasadvantages.
First, venoplasty entails spatulation of two veins into one common orifice which is a more desirable anastomosis between the recipient and graft portal veins than two separate anastomoses in using interposition Y-graft. Second, anin situportal vein anastomosis is more cumbersome than anex situone, especially in patients with a deep abdominal cavity. In anex situsituation, the anastomosis is not interfered by any surgical instruments or by neighboring structures in the hilum that could be an obstacle for suturing. A good alignment between the graft venoplasty and recipient portal vein is also easily achievable as the portal vein patch can rotate freely and the orientation of the anastomosis can be adjusted accordingly. Third, the portal reconstruction is done under cold ischemic time in a controlled environment, and the sense of urgency to complete the anastomosis is somewhat alleviated. This also enables the operating surgeon to deal with more difficult anastomosis, especially when meticulous skills in suturing are required for reconstruction of the thin and delicate portal vein walls. Moreover, the anastomosis can be checked for leaks and it is easier to insert additional sutures in a bloodless field than after graft reperfusion inside the recipient abdominal cavity. Together with the ready availability of portal vein tissue, portal venoplasty followed by interposition portal vein patch is an ideal method for vascular reconstruction in dual right portal vein branches.
Funding:None.
Ethical approval:Not needed.
Contributors:LCM proposed the study and contributed to further drafts. CACY wrote the first draft. All authors were involved in the procedure. LCM is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Marcos A, Orloff M, Mieles L, Olzinski A, Sitzmann J. Reconstruction of double hepatic arterial and portal venous branches for right-lobe living donor liver transplantation. Liver Transpl 2001;7:673-679.
2 Thayer WP, Claridge JA, Pelletier SJ, Oh CK, Sanfey HA, Sawyer RG, et al. Portal vein reconstruction in right lobe livingdonor liver transplantation. J Am Coll Surg 2002;194:96-98.
3 Xu MQ, Yan LN, Li B, Zeng Y, Wen TF, Zhao JC, et al. Surgical procedures for management of right portal venous branching in right lobe living donor liver transplantation. Transplant Proc 2008;40:1529-1533.
4 Chen CL, Concejero AM, Wang CC, Wang SH, Liu YW, Yong CC, et al. Remodeled saphenous vein as interposition graft for portal vein reconstruction in living donor liver transplantation. Liver Transpl 2007;13:1472-1475.
5 Sato K, Sekiguchi S, Watanabe T, Enomoto Y, Akamastu Y, Kawagishi N, et al. The use of recipient superficial femoral vein as a venous graft for portal vein reconstruction in right lobe living donor liver transplantation. Transplant Proc 2009;41:195-197.
(Hepatobiliary Pancreat Dis Int 2010; 9: 547-549)
June 1, 2010
Accepted after revision August 20, 2010
Author Affiliations: Department of Surgery, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong, China (Chan ACY, Lo CM, Chok KSH, Chan SC and Fan ST)
Chung Mau Lo, FRCS, MS, Department of Surgery, The University of Hong Kong, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong, China (Tel: 852-22554748; Fax: 852-28175475; Email: chungmlo@hkucc.hku.hk)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
 Hepatobiliary & Pancreatic Diseases International2010年5期
Hepatobiliary & Pancreatic Diseases International2010年5期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- News
- Advances in prognostic factors in acute pancreatitis: a mini-review
- Hepatocellular carcinoma metastatic to the kidney mimicking renal oncocytoma
- Simultaneous breast and ovarian metastasis from gallbladder carcinoma
- An eight-year journey of Hepatobiliary & Pancreatic Diseases International
- Interferon and lamivudine combination therapy versus lamivudine monotherapy for hepatitis B e antigen-negative hepatitis B treatment: a meta-analysis of randomized controlled trials
