Increased susceptibility to experimental steatohepatitis induced by methionine-choline deficiency in HBs-Tg mice
Miao-Miao Fu, Rui Sun, Zhi-Gang Tian and Hai-Ming Wei
Hefei, China
Increased susceptibility to experimental steatohepatitis induced by methionine-choline deficiency in HBs-Tg mice
Miao-Miao Fu, Rui Sun, Zhi-Gang Tian and Hai-Ming Wei
Hefei, China
BACKGROUND:Worldwide, about 25% of individuals with chronic hepatitis B have fatty liver disease. Lipogenic diets that are completely devoid of methionine and choline induce nonalcoholic fatty liver disease. However, no animal model of nonalcoholic steatohepatitis associated with HBV infection is available, and the influence of viral infection on nutritional hepatic steatosis is unclear.
METHODS:We used HBV surface antigen transgenic mice (HBs-Tg mice), which mimic healthy human carriers with hepatitis B surface antigen. The mice were fed with a high-fat methionine-choline-deficient diet (MCD) to build a reliable rodent nutritional model of nonalcoholic steatohepatitis associated with HBV infection, and the changes in body weight and serum triglycerides were measured. Hepatocyte ballooning changes were determined by hematoxylin and eosin staining. The extent of hepatic fat accumulation was evaluated by oil red O staining. Immunohistochemical assays were performed to detect proliferating cell nuclear antigen as an index of cell proliferation.
RESULTS:MCD feeding provoked systemic weight loss and liver injury. MCD feeding caused more macrovesicular fat droplets and fat accumulation in the livers of HBs-Tg mice than in wild-type C57BL/6 mice. In addition, within 30 days of MCD exposure, more PCNA-positive nuclei were found in the livers of HBs-Tg mice.
CONCLUSIONS:HBs-Tg mice fed with a lipogenic MCD form more macrovesicular fat droplets earlier, coincident with more hepatocyte proliferation, resulting in the appearance of increased susceptibility to experimental steatohepatitis in these mice.
(Hepatobiliary Pancreat Dis Int 2010; 9: 513-519)
high-fat methionine-choline-deficient diet; HBV surface antigen transgenic mice; steatohepatitis, experimental
Introduction
With the global increase in risk factors including obesity, insulin resistance, type II diabetes, and other components of metabolic syndrome, nonalcoholic fatty liver disease (NAFLD) is one of the common forms of liver disease and the most common cause of abnormal liver chemistry tests worldwide.[1-3]In a number of cases, patients go on to develop nonalcoholic steatohepatitis (NASH), a more severe disease associated with obesity, insulin resistance,[4]and mitochondrial dysfunction.[5]In recent years, an upsurge of the risk for NAFLD has been witnessed not only in Western countries but also in the Asia-Pacific region.[6]As known to all, more than half a billion of the world's population is chronically infected with HBV, especially in the Asian region. According to a recent study,[7]at least one-quarter of individuals with chronic hepatitis B (CHB) have hepatic steatosis. Although many studies focus on the relation between HCV infection and steatosis, little is known about the influence of chronic HBV infection on the prevalence of hepatic steatosis. In addition, animal models have greatly contributed to the understanding of NASH. While several models of steatosis exist,[8]no model of NASH associated with HBV infection is available. Significant work is needed to understand the relations between HBV, the accumulation of fat in hepatocytes, and the initiation and propagation of inflammation.
In the present study, a reliable rodent nutritional model of NASH associated with HBV infection was created by feeding HBV surface antigen transgenic (HBs-Tg) mice a high-fat methionine-choline-deficient diet (MCD), and the HBs-Tg mice were sensitive to experimental steatohepatitis. We found that the HBs-Tg mice were more inclined to form macrovesicular fat droplets and accumulate fat in the liver. In addition, exuberant hepatocyte proliferation in the HBs-Tg mice fed with MCD indicated that the liver in these mice is more sensitive to the hepatic injury caused by steatohepatitis.
Methods
Animals and diet
Male wild-type C57BL/6 (B6) and B6 HBs-Tg mice were used in all experiments. Male HBs-Tg mice, C57BL/6J-TgN (AlblHBV) 44Bri, which harbor the genes encoding the S, PreS1, PreS2, and HBx domains of HBV and express HBsAg in the serum, liver, and kidney, although without virus replication,[9,10]were purchased from the Department of Laboratory Animal Science of Peking University, which obtained them from the Jackson Laboratory (Bar Harbor, ME) and bred them for us. All HBs-Tg mouse sera were HBsAgpositive, and the HBsAg OD values were 1.5-2.4. The wild-type C57BL/6J mice were obtained from the same source and served as a control group. The animals were allowed to acclimatize to their new conditions for 1 week prior to the commencement of the study. Two study groups were HBs-Tg mice and wild-type mice fed on a diet deficient in methionine and choline (MCD; MP Bio. Cat 296043910) (n=20 per group) for 15 days, whereas control groups (n=20 per group) were HBs-Tg and wild-type mice receiving standard rodent chow. Two additional groups of HBs-Tg and wild-type mice fed with MCD (n=5 per group) for 30 days were studied to examine the proliferation and apoptosis of hepatocytes. The weight of the mice was monitored, and all had free access to their corresponding diet and waterad libitumfor the duration of the study. All animals were 10 weeks of age at the start of the study and were maintained under specific pathogen-free and controlled conditions (22 ℃, 55% humidity, and 12-hour day/night rhythm) and received humane care in compliance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals. All protocols and procedures were approved by the Animal Experimentation Ethics Committee of the University of Science and Technology of China.
Histological examination
After being fed with MCD for 7, 10, and 15 days, the mice were sacrificed, and liver tissues were isolated for histological analysis. One-third of the left posterior lobe of the liver was fixed in 10% neutral-buffered formalin for at least 24 hours and embedded in paraffin (50% ethanol 15 minutes, 70% ethanol 15 minutes ×2, 80% ethanol 30 minutes, 95% ethanol 30 minutes, 100% ethanol 15 minutes ×2, xylene 60 minutes ×2, paraffin 60 ℃ 60 minutes ×3). Sections of 7-μm thickness were affixed to the slides, deparaffinized (xylene 15 minutes × 2), rehydrated (100% ethanol 3 minutes, 95% ethanol 3 minutes, 80% ethanol 3 minutes, 70% ethanol 3 minutes, and H2O 5 minutes), and then stained with hematoxylin and eosin (HE) using routine methods. To detect fat deposition in the liver, 8-μm frozen sections of the left posterior lobe were rinsed with distilled water, stained with 0.18% oil red O (Sigma-Aldrich) with 60% 2-propanol (Sigma-Aldrich) for 20 minutes at 37 ℃, and then stained with hematoxylin. The fat accumulation stained with oil red O was quantified using an Image-Pro Plus Analyzer (Media Cybernetics, Inc., Bethesda, MD).
Immunohistochemical assay for proliferating cell nuclear antigen (PCNA)
Immunohistochemical assays were performed to detect PCNA as an index of cell proliferation according to the manufacturer's instructions (SP-9002; Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China). In brief, the sections were deparaffinized and hydrated. The slides were washed with Tris buffer, and peroxidase blocking was performed for 5 minutes. After rewashing, the mouse monoclonal anti-PCNA antibody (dilution: 1∶100, ZM-0213; Zhongshan Golden Bridge) was applied for 30 minutes at 4 ℃. The slides were rinsed, and the specimens were incubated with biotin-labeled goat anti-mouse IgG secondary antibody for 30 minutes at room temperature, and then sections were incubated with peroxidase-labeled streptavidin under the same conditions. The DAB substrate was added as the visualization reagent. Finally, the sections were stained with peroxidase substrate 3, 3-diamino-benzidine (DAB; DAKO Envision System, DAKO, Carpinteria, CA) and counterstained with hematoxylin for 10 seconds.
Biochemical assays
Serum alanine aminotransferase (ALT) levels were determined using spectrophotometric assay kits (Shanghai Rongsheng Biotechnology Co., Ltd., Shanghai, China). Serum triglycerides (TGs) were estimated using commercial detection kits (Changchun HuiliBiotechnology Co., Ltd., Chuangchun, China) according to the manufacturer's instructions.
Statistical analysis
Data from each group were expressed as mean± SD. A nonparametric Mann-WhitneyUtest (two-tailed) was used for group comparisons using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). APvalue of less than 0.05 was considered to be statistically significant.
Results
Weight loss and liver injury in HBs-Tg mice after MCD feeding
HBs-Tg mice fed with MCD lost more body weight than those fed with the standard rodent diet: the latter gained body weight day by day, whereas the former showed a steady decrease throughout the experimental period of 20 days (Fig. 1). The HBs-Tg mice lost about 7.5 g (34% body weight) after being fed with MCD for 20 days. Apart from weight loss, the mice still appeared to be active and with smooth fur, and no abnormal behavior was detected during MCD feeding. We also found that wild-type C57BL/6 mice fed with MCD had the same body weight loss as reported previously.[11,12]
To determine whether MCD caused liver injury in the HBs-Tg mice, plasma ALT activity, a marker for hepatic injury, was measured. Serum ALT was significantly elevated in the MCD-fed HBs-Tg mice (Fig. 1C), indicating that MCD did cause liver injury in these mice. An ALT increase was also found in wild-type mice after MCD feeding. We also evaluated the effect of MCD on serum TGs. MCD also provoked a decline in serum TGs in both HBs-Tg and wild-type mice (Table 1).
Early macrosteatosis in HBs-Tg mice after MCD feeding
Although no significant difference was seen in the body weight changes between the two types of mice after MCD feeding, but different types of hepatic steatosis were formed. As shown by HE staining, the mice fed with the standard diet had normal liver histology (Fig. 2A, E); however, the liver of mice fed with MCD had a light yellow and greasy appearance and developed steatohepatitis with hepatocyte ballooning changes.Scattered lobular inflammatory cell infiltration and vesicular fat droplets were also found in the liver of mice fed with MCD (Fig. 2 B-D, F-H). All MCD-fed mice had histologic evidence of both significant hepatic steatosis and mild inflammation. The liver in HBs-Tg mice fed with MCD for 7 days was found to have slight vesicular fat droplets; but no vesicular fat droplets were found in the liver of the wild-type mice fed with standard chow for 7 days. The HBs-Tg mice fed with MCD developed more macrovesicular fat droplets (diameter >25 μm) at day 10 than wild-type mice (Table 2), and the livers of both types of mice fed with MCD for 15 days had more macrovesicular fat droplets than those fed with MCD for 10 days. Clearly, the HBs-Tg mice developed macrosteatosis after 10 days of MCD feeding, whereas the wild-type mice developed microsteatosis.
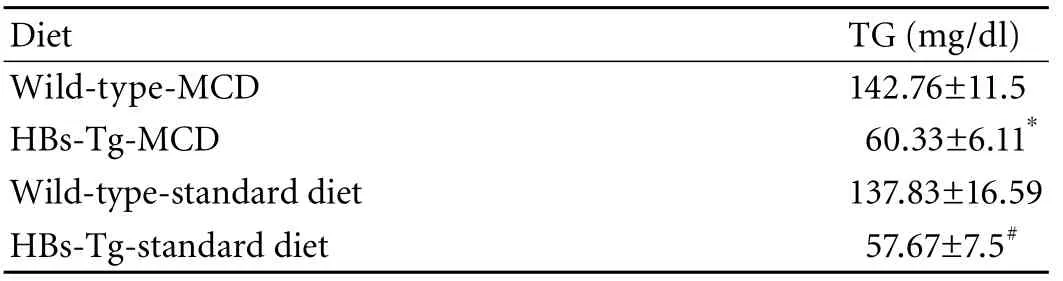
Table 1. Serum TG level of mice fed with MCD for 15 days
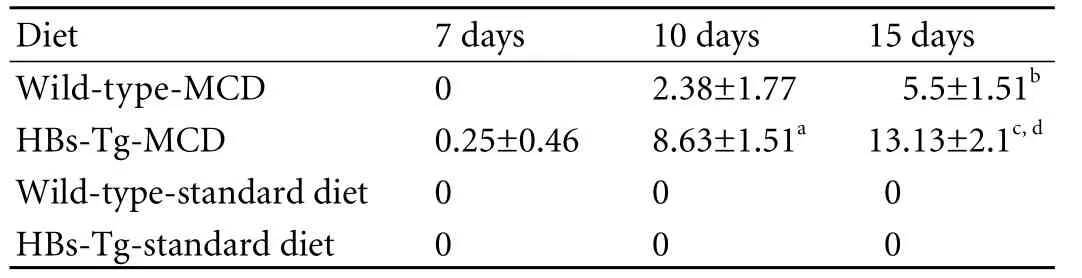
Table 2. Number of hepatic fat droplets (diameter >25 μm)

Fig. 1. Both HBs-Tg mice and wild-type C57BL/6 mice lost weight after MCD feeding. A: Body weights of mice fed with standard diet and MCD. B: Changes in body weight when both types of mice were fed with MCD and standard diet. Data were expressed as the mean weight of 5 animals per group. C: Serum ALT significantly increased in MCD-fed mice. Both HBs-Tg mice and wild-type mice had the same ALT level after feeding with MCD. #: P<0.005 mice fed with standard diet vs. mice fed with MCD (#1: t=75.74, P<0.0001; #2: t=9.7, P<0.001; #3: t=18.65, P<0.0001; #4: t=29.82, P<0.0001; #5: t=8.29, P<0.005; #6: t=11.23, P<0.001; #7: t=23.41, P<0.0001; #8: t=7.74, P<0.005; #9: t=15.97, P<0.0001; #10: t=11.69, P<0.001).
Accumulation of fat in the liver of HBs-Tg mice after MCD feeding
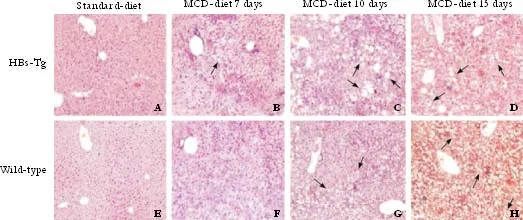
Fig. 2. Hematoxylin-eosin stained liver sections from mice fed MCD (original magnification ×100). A, E: standard diet-fed mice. B, F: MCD-fed mice for 7 days. Livers of HBs-Tg and wild-type C57BL/6 mice showed foci of necroinflammation and vesicular fat droplets. C, G and D, H: mice fed with MCD for 10 and 15 days. HBs-Tg mice showed more macrovesicular fat droplets (diameter >25 μm; arrows) than wild-type mice when fed with MCD. The group data for macrosteatosis are shown in Table. Sections are representative of 5 separate experiments (n=5/group).
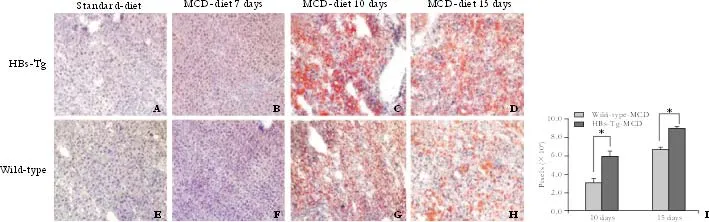
Fig. 3. Time-course change in fat accumulation (oil red O staining) in the liver of mice fed with MCD (original magnification ×100). Red indicates lipid droplets. A, E and B, F: mice fed with standard diet showed no fat deposition, and no lipid accumulation was found when both types of mice were fed with MCD for 7 days. C, G and D, H: HBs-Tg mice fed with MCD for 10 and 15 days had more fat deposition in the liver tissue than wild-type C57BL/6 mice. Representative sections from 5 mice per group. I: Liver fat stained with Oil Red O was greater in HBs-Tg mice fed with MCD for 10 and 15 days. The number of pixels shows the total area of fat droplet in each images. Data were expressed as the mean number of pixels. *: P<0.05, t=3.13, P=0.0021.
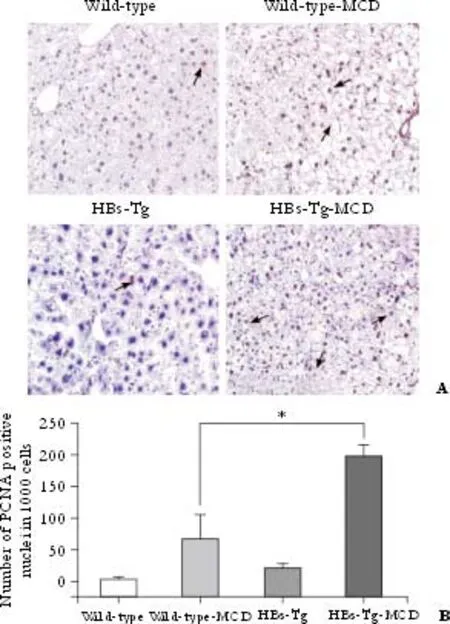
Fig. 4. Hepatocyte proliferation in HBs-Tg mice after feeding with MCD. A: HBs-Tg mice had more PCNA-positive cells in the liver than wild-type C57BL/6 mice after feeding with MCD for 30 days. Only a few PCNA-positive cells were found in the liver of mice fed with the standard diet. Arrows show PCNA-positive nuclei (original magnification ×200). B: Increased numbers of PCNA-positive cells in MCD-fed HBs-Tg mice. Representative sections from 4 mice per group. *: P<0.05, MCD HBs-Tg mice compared with MCD wild-type mice (t=6.18, P=0.0016).
The livers of the HBs-Tg and wild-type mice fed with MCD for 15 days accumulated more fat droplets than those fed with MCD for 10 days, but a number of larger lipid droplets were clearly observed in the HBs-Tg mice after feeding MCD for 10 and 15 days (Fig. 3C, D). The livers of the wild-type mice fed with MCD only had smaller fat droplets (Fig. 3G, H). Oil red O staining confirmed that the lipid droplet-like structures seen in HE staining were indeed lipid droplets containing TG. Standard-diet mice showed a normal appearance (Fig. 3A, E), and no lipid deposition was detected in the livers of mice fed with MCD for just 7 days (Fig. 3B, F). Total fat accumulation, assessed by oil red O and by an Image-Pro Plus Analyzer, was greater (P<0.05,t=3.13,P=0.0021) in the HBs-Tg than in wild-type mice after exposure to MCD for 10 and 15 days (Fig. 3I).
Increased proliferation of hepatocytes in HBs-Tg mice fed with MCD
PCNA, a cofactor of DNA synthase and an indicator of cell cycle progression at the G1/S transition, is an index of hepatocyte proliferation. Numerous PCNA-positive cells, especially in the HBs-Tg mice, were seen in the liver tissue of mice fed with MCD for 30 days (t=6.18,P=0.0016) (Fig. 4), indicating that more hepatocyte proliferation occurred in HBs-Tg mouse liver tissue. There were about 20% PCNA-positive cells in the liver of the wide-typemice mice after MCD feeding; however, only 7.5% PCNA-positive cells were found in the liver of the HBs-Tg mice after being fed with MCD. On the contrary, PCNA staining was minimal in the standard diet-fed mice (Fig. 4A).
Discussion
The prevalence of fatty liver in CHB patients has attracted attention from researchers in recent years, and hepatic steatosis is seen in many individuals with CHB. The recent data on experimental NAFLD have involved mouse models. The MCD dietary model of steatohepatitis and MCD feeding has become popular in recent years as a model of hepatic steatosis and nutritional steatohepatitis.[13-15]HBs-Tg mice are used to mimic healthy human carriers with hepatitis B surface antigen in CHB research.[16-18]In the current study using HBs-Tg mice, HBV transgenic mice formed more macrovesicular fat droplets and accumulated more fat in hepatocytes than wild-type mice after being fed with MCD. In addition, MCD-fed HBs-Tg mice had more hepatocyte proliferation. Thus HBs-Tg mice are more susceptible to experimental steatohepatitis than wildtype mice. Consistent with previous studies, male mice fed with MCD lost weight, whereas controls showed a steady increase in body weight and a decline in serum TGs.[13,19,20]The decreased serum TGs might be a result of impaired very-low-density lipoprotein (VLDL). VLDL plays an important role in transporting TGs and free fatty acids from the liver to serum. Methionine and choline are important precursors of phosphatidylcholine, the principal phospholipid comprising the outer coat of VLDL particles.[21]When these nutrients are in short supply, VLDL production is impaired, and TGs accumulate in hepatocytes instead of being transported by VLDL to serum. Therefore, abnormal VLDL production might be a reason for the decline of serum TGs after mice were fed with MCD. In this study, male HBs-Tg mice also lost body weight and experienced a decline in serum TGs when fed with MCD, while their general condition remained satisfactory. It has been reported that MCD causes hypermetabolism and hepatic steatosis.[13]MCD feeding also induced metabolic derangements with significant weight loss in combination with hepatic steatosis in HBs-Tg mice, which might be a form of lipodystrophy. Besides, untreated HBs-Tg mice were a little heavier than wildtype mice at the same age (Fig. 1A). This phenomenon might be the result of alteration of the expression ofseveral protein molecules in HBs-Tg mouse liver.[22]These protein molecules have been identified, including enzymes protective against oxidative stress and regulatory proteins related to lipid metabolism. Thus this might be one of the reasons why HBs-Tg mice were heavier.
Hepatic steatosis can be characterized quantitatively (mild, moderate, or severe) and qualitatively (macrovesicular or microvesicular) with various methods of histopathologic examination. Although the effect of macrovesicular or microvesicular hepatic steatosis on liver injury remains controversial, in this study HBs-Tg mice developed macrovesicular fat droplets (diameter >25 μm) more easily; with time, microvesicular fat droplets in wild-type mice fed with MCD became macrovesicular (Fig. 2), suggesting that microvesicular hepatic steatosis is a primary form of hepatic steatosis. It was reported that macrosteatosis might induce reperfusion injury and microcirculatory failure during liver transplantation. Macrosteatosis is known as a risk factor for primary nonfunctional liver; and grafts with severe macrosteatosis are no longer recommended for use.[23]Whether the type of liver steatosis, such as microsteatosis and macrosteatosis, affects the postoperative outcome in patients undergoing liver resection is unknown. Experimental data suggest that both types of steatosis have deleterious effects on ischemic injury and regeneration,[24]although in one study, macrosteatosis caused more severe injury.[25]In this study, the model was established by feeding HBs-Tg mice with MCD to develop macrosteatosis, and it was expected to contribute to the understanding of the role of macrosteatosis in liver transplant programs.
In addition, after oil red O staining, more accumulated fat droplets were observed in the liver of HBs-Tg mice fed with MCD, indicating that the virus-affected liver is more sensitive to diet-induced experimental steatohepatitis. Reports also demonstrated that the viral protein HBV X (HBx), the product of the HBV X gene, binds directly to host liver cells to upregulate lipogenic transcription factors, and the HBx has been implicated in abnormal lipid metabolism in HBV-associated hepatic steatosis.[26-28]Because HBs-Tg mice harbor the coding regions for HBV X antigens, these mice could accumulate more fat than wild-type mice, suggesting a role of HBx in the process of hepatic steatosis induced by MCD. Hence, HBV might have an effect on fat accumulation and fat metabolism in the liver. We also found that HBs-Tg mice fed with MCD showed more hepatocyte proliferation than wild-type mice. Hepatocyte proliferation might be the result of hepatic injury, and serum ALT clearly rose after animals were fed with MCD. HBs-Tg and wild-type mice had the same degree of serum ALT rise after being fed with MCD, which was presumably attributable to the MCD-induced injury model. Unlike lipopolysaccharide/ D-galactosamine- or polyinosinic-polycytidylic acidinduced hepatitis, which leads to an ALT rise in a short time,[29,30]MCD caused liver injury by hypermetabolism and increased internal oxidative stress, a prolonged process that might last for a few months. Therefore, there may not be significant differences in ALT at the very beginning. Although there is no significant difference in serum ALT, hepatic injury still plays an important role in the course of hepatocyte proliferation. This is because hepatocyte proliferation might reflect compensation for hepatic injury caused by MCD. A higher proliferation rate implies more severe injury, and thus viral hepatitis might make the liver vulnerable to diet-induced experimental steatohepatitis. In addition, unlimited hepatocyte proliferation might be involved in hepatocellular carcinoma caused by NAFLD and other factors,[31-33]and a previous study found that HBV also induces hepatocyte proliferation.[34]Perhaps HBV infection exacerbates the proliferation and leads to a worse result, i.e., HBs-Tg mice, and even healthy human HBsAg carriers, may develop hepatocellular carcinoma more easily and earlier when they face the threat of NAFLD.
In conclusion, our current rodent model of NASH associated with HBV infection provides a new one for the study of NALFD. This model successfully induced typical steatohepatitis in only 10 days as well as hepatic proliferation, and thus may contribute to better understanding the effect of CHB viral hepatitis on NAFLD.
Funding:This work was supported by grants from the National Natural Science Foundation of China (30730084 and 30721002) and the National Key Basic Research Program of China (973 Program) (2009CB522403, 2007CB512405, and 2007CB512807).Ethical approval:Not needed.
Contributors:FMM designed and performed all the experiments, analyzed and interpreted the data. SR established techniques of immunohistochemistry. TZG provided strategic planning and conceived the project. WHM supervised the project, provided crucial ideas and helped with data interpretation. FMM wrote the manuscript with WHM. WHM is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Byron D, Minuk GY. Clinical hepatology: profile of an urban, hospital-based practice. Hepatology 1996;24:813-815.
2 Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology 2002;122:1649-1657.
3 Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis 2001;21:3-16.
4 Luyckx FH, Lefebvre PJ, Scheen AJ. Non-alcoholic steatohepatitis: association with obesity and insulin resistance, and influence of weight loss. Diabetes Metab 2000;26:98-106.
5 Solís Herruzo JA, García Ruiz I, Pérez Carreras M, Munoz Yagüe MT. Non-alcoholic fatty liver disease. From insulin resistance to mitochondrial dysfunction. Rev Esp Enferm Dig 2006;98:844-874.
6 Fan JG, Peng YD. Metabolic syndrome and non-alcoholic fatty liver disease: Asian definitions and Asian studies. Hepatobiliary Pancreat Dis Int 2007;6:572-578.
7 Fan JG, Chitturi S. Hepatitis B and fatty liver: causal or coincidental? J Gastroenterol Hepatol 2008;23:679-681.
8 Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis 2001;21:89-104.
9 Chen Y, Wei H, Sun R, Tian Z. Impaired function of hepatic natural killer cells from murine chronic HBsAg carriers. Int Immunopharmacol 2005;5:1839-1852.
10 Chisari FV, Pinkert CA, Milich DR, Filippi P, McLachlan A, Palmiter RD, et al. A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science 1985;230: 1157-1160.
11 Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, Leclercq I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology 2003;38:123-132.
12 Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest 2000;105:1067-1075.
13 Rizki G, Arnaboldi L, Gabrielli B, Yan J, Lee GS, Ng RK, et al. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res 2006;47:2280-2290.
14 Nagasawa T, Inada Y, Nakano S, Tamura T, Takahashi T, Maruyama K, et al. Effects of bezafibrate, PPAR panagonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and cholinedeficient diet. Eur J Pharmacol 2006;536:182-191.
15 Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol 2009;51:168-175.
16 Chen Y, Wei H, Sun R, Dong Z, Zhang J, Tian Z. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology 2007;46:706-715.
17 Zheng BJ, Ng MH, He LF, Yao X, Chan KW, Yuen KY, et al. Therapeutic efficacy of hepatitis B surface antigen-antibodiesrecombinant DNA composite in HBsAg transgenic mice. Vaccine 2001;19:4219-4225.
18 Dong Z, Zhang J, Sun R, Wei H, Tian Z. Impairment of liver regeneration correlates with activated hepatic NKT cells in HBV transgenic mice. Hepatology 2007;45:1400-1412.
19 Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology 1996;111:1645-1653.
20 Kirsch R, Clarkson V, Shephard EG, Marais DA, Jaffer MA, Woodburne VE, et al. Rodent nutritional model of nonalcoholic steatohepatitis: species, strain and sex difference studies. J Gastroenterol Hepatol 2003;18:1272-1282.
21 Vance JE, Vance DE. The role of phosphatidylcholine biosynthesis in the secretion of lipoproteins from hepatocytes. Can J Biochem Cell Biol 1985;63:870-881.
22 Yang F, Yan S, He Y, Wang F, Song S, Guo Y, et al. Expression of hepatitis B virus proteins in transgenic mice alters lipid metabolism and induces oxidative stress in the liver. J Hepatol 2008;48:12-19.
23 Urena MA, Ruiz-Delgado FC, González EM, Segurola CL, Romero CJ, García IG, et al. Assessing risk of the use of livers with macro and microsteatosis in a liver transplant program. Transplant Proc 1998;30:3288-3291.
24 Selzner M, Rüdiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology 2000;32:1280-1288.
25 Selzner N, Selzner M, Jochum W, Amann-Vesti B, Graf R, Clavien PA. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. J Hepatol 2006;44:694-701.
26 Kim K, Kim KH, Kim HH, Cheong J. Hepatitis B virus X protein induces lipogenic transcription factor SREBP1 and fatty acid synthase through the activation of nuclear receptor LXRalpha. Biochem J 2008;416:219-230.
27 Na TY, Shin YK, Roh KJ, Kang SA, Hong I, Oh SJ, et al. Liver X receptor mediates hepatitis B virus X protein-induced lipogenesis in hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2009;49:1122-1131.
28 Kim KH, Shin HJ, Kim K, Choi HM, Rhee SH, Moon HB, et al. Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SREBP1 and PPARgamma. Gastroenterology 2007;132:1955-1967.
29 Jiang W, Sun R, Wei H, Tian Z. Toll-like receptor 3 ligand attenuates LPS-induced liver injury by down-regulation of toll-like receptor 4 expression on macrophages. Proc Natl Acad Sci U S A 2005;102:17077-17082.
30 Xiong Q, Hase K, Tezuka Y, Namba T, Kadota S. Acteoside inhibits apoptosis in D-galactosamine and lipopolysaccharideinduced liver injury. Life Sci 1999;65:421-430.
31 Yang S, Lin HZ, Hwang J, Chacko VP, Diehl AM. Hepatic hyperplasia in noncirrhotic fatty livers: is obesity-related hepatic steatosis a premalignant condition? Cancer Res 2001; 61:5016-5023.
32 Nakae D, Uematsu F, Kishida H, Kusuoka O, Katsuda S, Yoshida M, et al. Inhibition of the development of hepatocellular carcinomas by phenyl N-tert-butyl nitrone in rats fed with a choline-deficient, L-amino acid-defined diet. Cancer Lett 2004;206:1-13.
33 Weber MM, Fottner C, Liu SB, Jung MC, Engelhardt D, Baretton GB. Overexpression of the insulin-like growth factorireceptor in human colon carcinomas. Cancer 2002; 95:2086-2095.
34 Zhang JL, Zhao WG, Wu KL, Wang K, Zhang X, Gu CF, et al. Human hepatitis B virus X protein promotes cell proliferation and inhibits cell apoptosis through interacting with a serine protease Hepsin. Arch Virol 2005;150:721-741.
September 16, 2009
Accepted after revision April 26, 2010
Author Affiliations: Institute of Immunology, Hefei National Laboratory for Physical Sciences at Microscale, School of Life Sciences, University of Science and Technology of China, Hefei 230027, China (Fu MM, Sun R, Tian ZG and Wei HM)
Hai-Ming Wei, MD, Institute of Immunology, School of Life Sciences, University of Science and Technology of China, Hefei 230027, China (Tel: 86-551-3607379; Fax: 86-551-3606783; Email: ustcwhm@ustc.edu.cn)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
 Hepatobiliary & Pancreatic Diseases International2010年5期
Hepatobiliary & Pancreatic Diseases International2010年5期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- News
- Advances in prognostic factors in acute pancreatitis: a mini-review
- Hepatocellular carcinoma metastatic to the kidney mimicking renal oncocytoma
- Simultaneous breast and ovarian metastasis from gallbladder carcinoma
- An eight-year journey of Hepatobiliary & Pancreatic Diseases International
- Interferon and lamivudine combination therapy versus lamivudine monotherapy for hepatitis B e antigen-negative hepatitis B treatment: a meta-analysis of randomized controlled trials
