Deactivation Kinetics of Nitrile Hydratase in Free Resting Cells*
SUN Xudong (孙旭东), YU Huimin (于慧敏)** and SHEN Zhongyao (沈忠耀)
Deactivation Kinetics of Nitrile Hydratase in Free Resting Cells*
SUN Xudong (孙旭东), YU Huimin (于慧敏)** and SHEN Zhongyao (沈忠耀)
Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
Nitrile hydratase (NHase) is an important industrial enzyme used for acrylamide production from acrylonitrile. The deactivation kinetics of NHases in free resting cells ofsp. was presented based on a bi-steady state assumption. Effects of hydration temperature, product concentration and substrate concentration on NHase deactivation were investigated experimentally and correlated with a first order deactivation kinetics. The results showed that the hydration temperature and product concentration were major factors governing the deactivation of NHases under substrate-feeding conditions. When acrylamide concentration was higher than 250 g·L-1, the deactivation of NHases became serious and the bi-steady state assumption was not applicable. When the hydration temperature was controlled at a relatively higher level such as 28°C, the total deactivation rate constant was about 2.8-fold of that at 20°C.
nitrile hydratase, deactivation kinetics, free resting cell, bi-steady state assumption, total deactivation rate constant
1 INTRODUCTION
Industrial production of acrylamide can be implemented by either chemical or enzymatic approaches. Compared to chemical catalysis based on copper or vitriol catalysts, the enzymatic bioconversion of acrylamide from acrylonitrile catalyzed by nitrile hydratase (NHase) is receiving more attention due to its high yield, mild condition, clean process but low production cost [1-3]. Since the development of the first, second and third generation catalysts of Fe-type or Co-type NHases existed in cells ofsp. N-774,B23 orJ1, NHases were usually prepared in the form of immobilized cells when used for acrylamide production [4-6]. In recent years, a new process using free cells as biocatalysts with a membrane bioreactor has been developed and applied for hydration of acrylonitirle to acrylamide [7]. Deactivation of NHases during the hydration reaction is a key problem for both free and immobilized cell-biocatalysts, because both substrate acrylonitrile and product acrylamide are organic solvents, which are prone to denaturize enzymes. Additionally, thermal inactivation of NHases is also significant since nitrile-hydration is an exothermic reaction. Therefore, many researchers focused on exploring the structure and catalytic characteristics of NHases [8-11], and improved their stability and activity by genetic modifications [12-17].
Some studies have been carried out on deactivation kinetics of enzymes since the deactivation performance plays an important role in industrial biocatalysis. For example, Icier. [18] proposed a deactivation kinetic model for polyphenoloxidase with ohmic heating; Illeová. [19] investigated the thermal inactivation of jack bean urease; Figueroa-Soto and Valenzuela-Soto [20] found that the kinetic behavior of the porcine kidney betaine aldehyde dehydrogenase was consistent with an iso-ordered bi-bi steady-state mechanism. In addition, Chen. [21] experimentally studied the effects of cell concentration, pH, temperature, acrylonitrile/acrylamide concentration on the NHase activity. Cantarella. [22] investigated the activity and stability characteristics of the NHase in resting cells fromCBS 498-74 for propionamide production and found a diffusion controlled process with strong substrate inhibition.
In this work, we study the deactivation kinetics of NHases in free resting cells ofused for acrylamide bioproduction based on a new bi-steady state model. The effects of three key factors, temperature, substrate concentration and product concentration, are investigated.
2 Experimental
2.1 Cell culture and preparation of free resting cells harboring NHases
The bacterial strain used in this study was asp. and the culture medium was prepared as reported previously [7]. Flask seed culture was carried out in 500 ml flasks with 50 ml of broth in a rotary shaker (220 r·min-1) for 48 h. Then 10% inoculums of the seed were inoculated into 1000 ml flasks with 100 ml broth and cultured at 28°C for another 48 h. Cells were harvested by centrifugation (8000 r·min-1, 5 min) then resuspended in 50 mmol·L-1phosphate buffered saline (PBS buffer, pH 7.0), and free resting cells harboring NHases were obtained.
2.2 NHase deactivation at different temperatures
The effect of temperature on deactivation of NHases in free resting cells was investigated in a 1 L jacketed bioreactor coupled with a hollow-fiber membrane apparatus [7]. The hydration condition was controlled at pH 7.0, temperature 5, 10, 15, 20 and 28°C (by cold alcohol). The concentrations of substrate acrylonitrile and product acrylamide were controlled at 8 and 100 g·L-1, respectively, by feeding substrate and water or separating product through the membrane.Appropriate volume of cell samples were taken out every 60 min, rinsed once with 50 mmol·L-1PBS buffer, and the remaining NHase activity was measured.
2.3 NHases deactivation by product acrylamide
The effect of acrylamide on reaction deactivation during the hydration reaction was investigated in the bioreactor as described in Ref. [7]. Around 12 g·L-1cells were loaded into the reactor in a water solution. Acrylonitrile was fed into the reaction and its concentration was controlled at about zero. When acrylamide accumulated to the final concentration, such as 50, 100, 150, 200, 250, 300 and 350 g·L-1, at 20°C and pH 7.0, cell samples were taken out to measure the remaining NHases activity every 60 min after one-time rinse.
The immersion deactivation effect caused by acrylamide incubation without hydration reaction was evaluated by dipping a fixed volume of free resting cells harboring enzymes in the acrylamide solution of 50, 100, 150, 200, 250, 300 and 350 g·L-1separately at 20°C and pH 7.0 for a period of time. Appropriate volume of cell samples were taken out every 60 min, washed once with 50 mmol·L-1PBS buffer, and the remaining NHase activity was measured.
2.4 NHases deactivation by substrate acrylonitrile
The deactivation effect of acrylonitrile on NHases in free resting cells was also investigated in the bioreactor. The concentration of acrylonitrile was controlled at 0.8, 4, 8, 24, 40, and 56 g·L-1separately by feeding the substrate, and the concentration of acrylamide was controlled at 100 g·L-1by continuously separating the product from the reaction system with the membrane. Consequently, the remaining NHase activity was measured every 60 min after one-time rinse of cells.
2.5 Gas chromatography analysis for acrylamide and acrylonitrile
The concentrations of acrylamide and acrylonitrile in the reaction mixture were measured by gas chromatography (GC) using a GC-2010 (SHIMADZU Japan) equipped with a polyethylene glycol polymer capillary column (30 m×0.25 mm×2 μm). Column temperature was set at 160°C, injection and detection temperature were set at 260°C. Nitrogen was used as the carrier gas at the flow rate of 25 cm·min-1and acetamide was served as the internal standard.
2.6 NHase activity assay
The activity of NHase in cells was measured by GC method as stated above. The NHase reaction was carried out as follows. 1 ml acrylonitrile was added into 5 ml of PBS buffer (50 mmol·L-1, pH 7.0) in a 50 ml conical flask with stopple at constant 28°C in a water bath. Then 0.5 ml cells diluted by the same PBS buffer were added to start the reaction. After 5 min, the reaction was terminated by adding 0.5 ml HCl (6 mol·L-1), then the reaction solution was mixed with 0.4% acetamide at the volume ratio of 1︰1. The concentration of acrylamide was measured to calculate the activity of NHases. One activity unit (U) was defined as the formation of acrylamide (μmol) per-minute.
3 RESULTS AND DISCUSSION
3.1 Reaction system and deactivation factor
A hydration reaction system for acrylonitrile conversion to acrylamide catalyzed by free resting cells harboring NHases is described in Fig. 1. Cells with NHases are suspended in a water solution. Substrate acrylonitrile is fed into the system in such a way to keep its low concentration. Product acrylamide accumulates fast once the reaction starts, and its final concentration is usually 250-320 g·L-1for one batch of reaction. To improve the cell-usage efficiency and process productivity, the cells are expected to be re-used at least 4-5 batches. The hydration temperature is usually controlled at 15-20°C to prevent the thermal inactivation of NHases and formation of by-products. In the mean time, the hydration pH is controlled at about 7.0-7.5. Since the hydration of acrylonitrile is an exothermic reaction and the NHases are unstable at high temperature, the hydration temperature is one of the key factors affecting the deactivation of NHases. Certainly, both the substrate and product concentrations are also dominant factors for deactivation of NHase, because they are organic solvents prone to inactivate enzymes.

Figure 1 Schematic reaction system of a free cell harboring nitrile hydratases converting acrylonitrile to acrylamide in a water solution
As shown in Fig. 1, the NHases harbored in cells can be regarded as special immobilized enzymes with cell-microcapsule, which exhibit some unique character relative to conventional immobilized ones. At first, the NHases are freely distributed in the cytoplasm of the cell, so that the conformation does not change, and the activity does not decrease during the immobilization by the “cell carrier”. Secondly, both acrylonitrile and acrylamide are small molecules, so there is little resistance for substrate and product to transfer across cell membrane and cell wall. Therefore, the diffusion effect and the distribution effect of the substrate and product can be reasonably ignored. Collectively, the NHases in free resting cells can be regarded as the “ideal immobilized enzymes” in the following mathematical simulations.

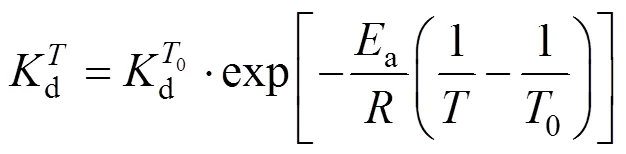
To better understand the deactivation progress of NHases in free resting cells, we investigated the deactivation kinetics of the NHase hydration system mathematically and experimentally.
3.2 Effect of temperature on deactivation of NHases in free resting cells

From Fig. 3, we have

Figure 2 Experimental results and linear fitting at 293K


Introducing the base-temperature (0) of 293 K,

This equation describes the effect of temperature on deactivation of NHases at the fixed concentrations of substrate and product. The deactivation energyaof the NHases in free resting cells at 293 K is calculated as 94.565 kJ×mol-1.
3.3 The bi-steady state model
In an industrial process of acrylamide production using NHases as biocatalyst, the molar concentrations of both substrate and product are usually much higher than that of enzyme in cells except at the initial time. Therefore, we presented a “bi-steady state” model for thecatalytic process of NHases according to the Michaelis- Menten equation [23] and the concept of “steady state” presented by Briggs and Haldane [24]. In the “bi-steadystate” model, the NHase would form an enzyme-substrate complex (ES) and an enzyme-product complex (EP) during the reaction, and the concentration of ES/EP complex is not a function of concentrations of substrate and product. We assume that the complex EP does not reversibly generate the complex ES, and the reactions involved in the overall conversion from substrate S to product P are as follows,
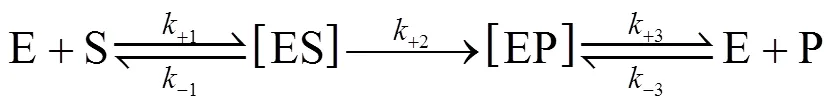

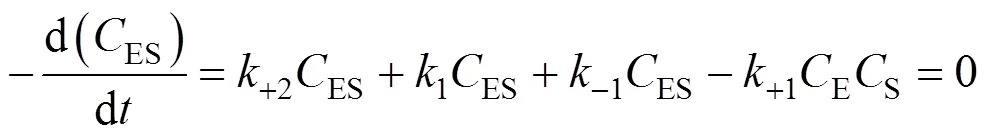

Similarly, the decomposition rate of active free enzymes is

SubstitutingESandEPcalculated from Eqs. (4) and (5) into Eq. (6), we obtain
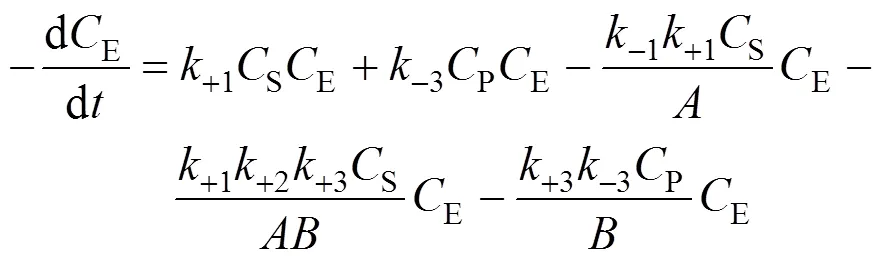


According to the bi-steady state assumption,

Combining Eqs. (7), (8), and (9), we obtain
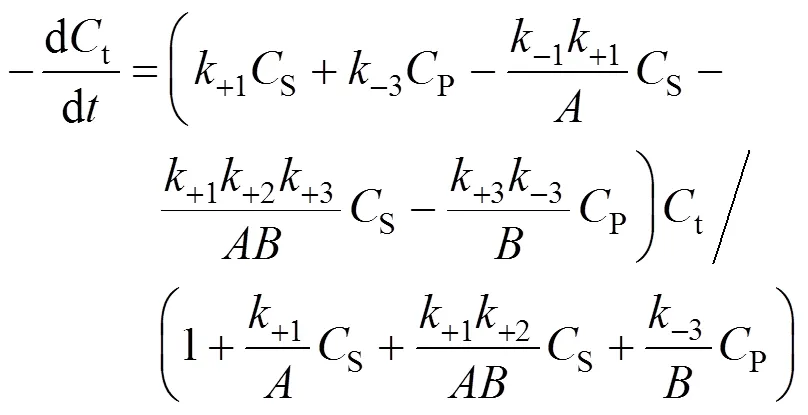





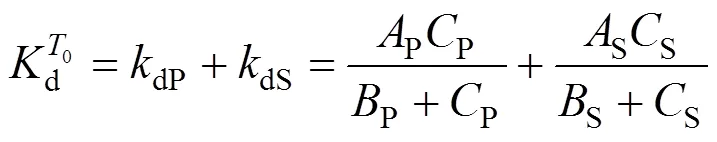
3.4 Determination of the product deactivation rate constant kdP
The deactivation rate constant of product,dP, can be expressed as a function of 1/P,

As described in Section 2, we measured the activity changes of NHases with respect to different levels of accumulated-acrylamide when acrylonitrile was controlled at a very low concentration. Consequently, we obtained the deactivation rate constantdPfrom the first order deactivation kinetics, as shown in Fig. 4.
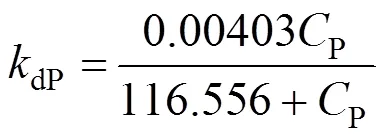
However, whenP>250 g·L-1(1/P>0.004), the intercept is negative, so that the assumption of bi-steady state is not applicable. The relationship betweendPandPis

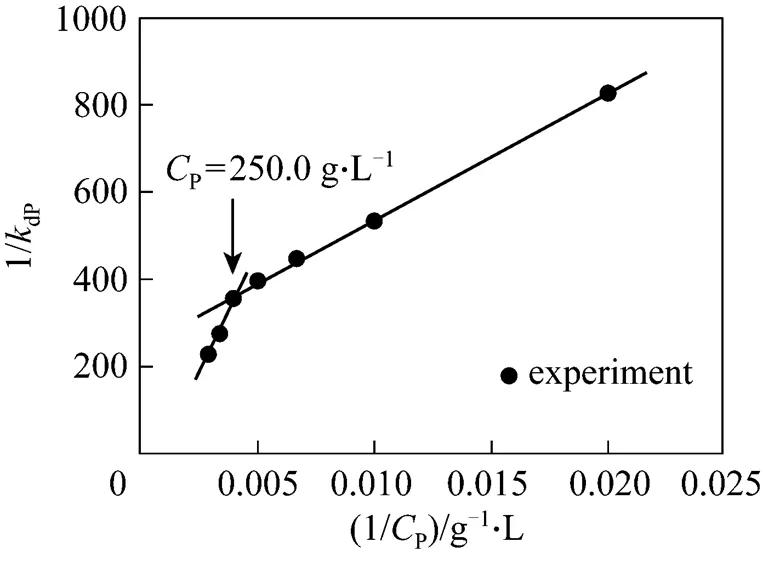
Figure 4 Two-segment linear correlation of 1/dPwith 1/Pin thePrange of 50-350 g·L-1
To further investigate the deactivation behavior of NHases in free resting cells at higher acrylamide concentrations (P), we carried out the experiments with in-cell NHases immersed in various concentrations of acrylamide in the absence of substrate and each experiment was repeated twice. The results are shown in Fig. 5. When the concentration of acrylamidePis higher than 150 g·L-1, the activity of the immersed-NHases in free resting cells is significantly reduced. WhenPis higher than 250 g·L-1, the deactivation is more serious and the half-life of the NHases is less than 120 min. Without the substrate added in this system, the serious deactivation can not be described by the assumption of bi-steady state reaction. Since acrylamide is an organic solvent, its high concentration would result in significant changes of enzyme structure, which may be the major reason for the poor-applicability of the bi-steady state assumption.

Figure 5 Deactivation curves of NHases in free resting cells immersed in acrylamide solutions (20°C)
acrylamide concentration/g·L-1: ★ 100; ◆ 150; ▼ 200; ▲ 250; ● 300; ■ 350
3.5 Determination of the substrate deactivation rate constant kdS
With the same bioreactor, we performed the hydration reaction with fixed substrate concentration,S, at 0.8, 4, 8, 24 and 40 g·L-1separately by feeding the substrate. In Fig. 6, the product concentration is controlled at 100 g·L-1, and its effect on the deactivation constant (0.00187 min-1) has been excluded. The deactivation rate constantdSis calculated by the first-order deactivation kinetics. The relationship betweendSandScan be written as
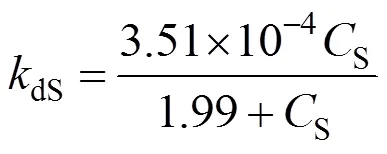
Figure 6 Experimental (●) and mathematical (—) correlation of 1/dSand 1/S
3.6 Total deactivation rate constant
Combining the effects of three factors,.., temperature, substrate concentration and product concentration, we obtained the deactivation kinetics of the NHases in free resting cells as follows.
WhenP≤250 g·L-1, the bi-steady state assumption is well applicable,
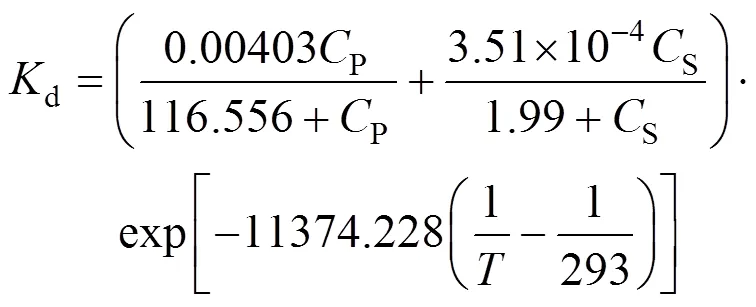
WhenP>250 g·L-1, the bi-steady state assumption is not applicable, but we can predict the value ofdin terms of the following equation

Figure 7 is a 3D-graph illustrating the correlation of deactivation rate constant with respect to product andsubstrate concentrations at 20 and 28°C, respectively, in terms of Eqs. (19) and (20). In the range of 0-60 g·L-1acrylonitrile and 0-400 g·L-1acrylamide, the effect of acrylamide on the deactivation rate constant is more significant than acrylonitrile. When the concentration of acrylamide,P, is higher than 250 g·L-1, the deactivation of NHases is more serious at higher acrylamide concentration. In the reaction with 10 g·L-1acrylonitrile and 300 g·L-1acrylamide, the contribution ofdPto the total deactivation rate constant is 3092-fold ofdS. Figs. 7 (a) and 7 (b) further reveal that the reaction temperature is another important factor governing the deactivation behavior of NHases. The total deactivation constant of NHases at 28°C [Fig. 7 (b)] is about 2.8 times of that at 20°C [Fig. 7 (a)], indicating that genetic modifications on NHases to improve their thermal stability are promising for industrial purposes.
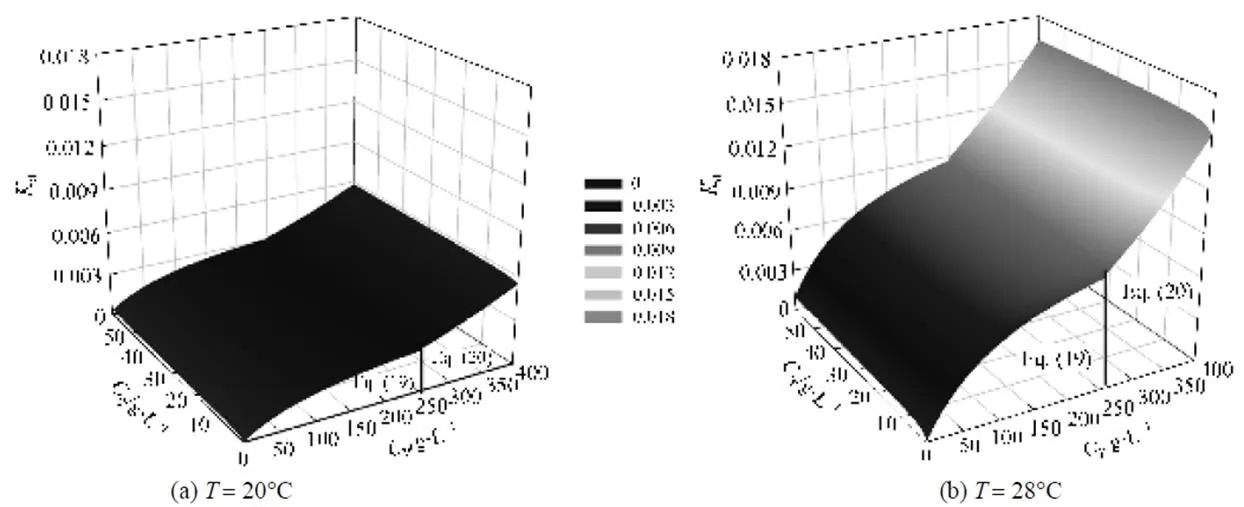
Figure 7 3D graph on the total deactivation rate constant of NHases as a function of acrylonitrile and acrylamide concentrations
4 CONCLUSIONS
We presented a bi-steady state model to simulate the deactivation behavior of NHases in the free resting cells during their hydration reaction process for transforming acrylonitrile to acrylamide. We investigated the effects of temperature, product concentration and substrate concentration on the total deactivation rate constant, and revealed that the effect of product deactivation was more serious than that of the substrate during the reaction. When acrylamide concentration was higher than 250 g·L-1, the deactivation rate increased rapidly, and the bi-steady state assumption was not applicable. The deactivation kinetics was in good agreement with the experimental deactivation behavior of NHases in free resting cells ofused for acrylamide production.
AcknowledgEments
..
NOMENCLATURE
NHactivity of nitrile hydratase, U·ml-1
NH0activity of nitrile hydratase at 293 K, U·ml-1
Econcentration of active enzymes, g·L-1
EPconcentration of enzyme-product complex, g·L-1
ESconcentration of enzyme-substrate complex, g·L-1
Pconcentration of product, g·L-1
Sconcentration of substrate, g·L-1
ttotal concentration of active NHases, g·L-1
aactivation energy, kJ·mol-1
individual deactivation rate constant, min-1
dPindividual product deactivation rate constant, min-1
dSindividual substrate deactivation rate constant, min-1
gas constant, J·mol-1·K-1
absolute temperature, K
0base-temperature of 293 K
1 Yamada, H., Kobayashi, M., “Nitrile hydratase and its application to industrial production of acrylamide”,..., 60, 1391-1400 (1996).
2 Asano, Y., Yasuda, T., Tani, Y., Yamada, H., “A new enzymatic methodof acrylamide production”,..., 46, 1183-1189 (1982).
3 Banerjee, A., Sharma, R., Banerjee, U.C., “The nitrile-degrading enzymes: current status and future prospects”,..., 60, 33-44 (2002).
4 Tsujimura, M., Dohmae, N., Odaka, M., “Structure of the photoreactive iron center of the nitrile hydratase from Rhodococcus sp. N-771: Evidence of a novel post-translational modification in the cysteine ligand”,..., 272, 29454-29459 (1997).
5 Nagasawa, T., Nanba, H., Ryuno, K., Takeuchi, K., Yamada, H., “Nitrile hydratase of Pseudomonas chlororaphis B23 purification and characterization”,..., 162, 691-698 (1987).
6 Nagasawa, T., Shimizu, H., Yamada, H., “The superiority of the third-generation catalyst,J1 nitrile hydratase, for industrial production of acrylamide”,..., 40, 189-195 (1993).
7 Sun, X.D., Shi, Y., Yu, H.M., Shen, Z.Y., “Bioconversion of acrylonitrile to acrylamide using hollow-fiber membrane bioreactor system”,..., 18, 239-243 (2004).
8 Huang, W., Jia, J., Cummings, J., Nelson, M.J., Schneider, G., Lindqvist, Y., “Crystal structure of nitrile hydratase reveals a novel iron center in a novel fold”,, 5, 691-699 (1997).
9 Miyanaga, A., Fushinobu, S., Ito, K., Wakagi, T., “Crystal Structure of cobalt-containing nitrile hydratase”,...., 288, 1169-1174 (2001).

11 Wang, C., Zhang, G.L., Xu, X.L., Li, C., “Inducing expression and reaction characteristic of nitrile hydratase from Rhodococcus sp. SHZ-1”,...., 15 (4), 573-578 (2007).
12 Kobayashi, M., Shimizu, S., “Metalloenzyme nitrile hydratase: Structure, regulation, and application to biotechnology”,.., 16, 733-736 (1998).
13 Liu, J., Yu, H.M., Shen, Z.Y., “Insights into thermal stability of thermophilic nitrile hydratases by molecular dynamics simulation”,...., 27, 529-535 (2008).
14 Yu, H.M., Shi, Y., Luo, H., Tian, Z.L., Zhu, Y.Q., Shen, Z.Y., “An over expression and high efficient mutation system of a cobalt-containing nitrile hydratase”,...:., 43, 80-85 (2006).
15 Hashimoto, Y., Sasaki, S., Herai, S., Oinuma, K.I., Shimizu, S., Kobayashi, M., “Site-directed mutagenesis for cysteine residues of cobalt- containing nitrile hydratase”,..., 91, 70-77 (2002).
16 Peplowski, L., Kubiak, K., Nowak, W., “Insights into catalytic activity of industrial enzyme Co-nitrile hydratase. Docking studies of nitriles and amides”,..., 13, 725-730 (2007).
17 Shi, Y., Yu, H.M., Sun, X.D., Tian, Z.L., Shen, Z.Y., Cloning of the nitrile hydratase gene fromsp. inandand its functional expression using site-directed mutagenesis”,..., 35, 557-562 (2004).
18 Icier, F., Yildiz, H., Baysal, T., “Polyphenoloxidase deactivation kinetics during ohmic heating of grape juice”,.., 85, 410-417 (2008).
19 Illeová, V., Polakovic, M., Štefuca, V., Acai, P., Juma, M., “Experimental modelling of thermal inactivation of urease”,.., 105, 235-243 (2003).
20 Figueroa-Soto, C.G., Valenzuela-Soto, E.M., “Kinetic study of porcine kidney betaine aldehyde dehydrogenase”,...., 269, 596-603 (2000).
21 Chen, Z., Sun, X.D., Shi, Y., Shen, Z.Y., Zhao, J.X., Sun. X.Y., “Study on production of acrylamide by microbial method (II)—Enzymatic catalytic kinetics and de-active dynamics of nitrile hydratase”,..., 18 (2), 225-230 (2002).
22 Cantarella, M., Cantarella, L., Gallifuoco, A., Frezzini, R., Spera, A., Alfani, F., “A study in UF-membrane reactor on activity and stability of nitrile hydratase fromCBS 498-74 resting cells for propionamide production”,...:. 29, 105-113 (2004).
23 Lehninger, A.L., Nelson, D.L. Cox, M.M., Principles of Biochemistry, 2nd edition, Worth Publishers Inc., New York (1992).
24 Briggs, G.E., Haldane, J.B.S., “A note on the kinetics of enzyme action”,.., 19, 338-339 (1925).
2008-12-05,
2009-04-07.
the Foundation for the Authors of National Excellent Doctoral Dissertation of China (200345), the National High Technology Research and Development Program of China (2007AA02Z201) and the National Basic Research Program of China (2007CB714304).
** To whom correspondence should be addressed. E-mail: yuhm@tsinghua.edu.cn
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of Relative Humidity on Catalytic Combustion of Toluene over Copper Based Catalysts with Different Supports*
- Prediction of Critical Endpoints Based on the PR Equation of State*
- Kinetic Studies on Wheat Straw Hydrolysis to Levulinic Acid*
- Calculation of Transport Properties of CF4+Noble Gas Mixtures
- Synthesis of 4,4′-MDC in the Presence of Sulfonic Acid-functionalized Ionic Liquids*
- A New Study on Combustion Behavior of Pine Sawdust Characterized by the Weibull Distribution*
