Volumetric and Viscometric Studies of 4-Aminobutyric Acid in Aqueous Solutions of Salbutamol Sulphate at 308.15, 313.15 and 318.15 K
K. Rajagopal and S.S. Jayabalakrishnan*
Volumetric and Viscometric Studies of 4-Aminobutyric Acid in Aqueous Solutions of Salbutamol Sulphate at 308.15, 313.15 and 318.15 K
K. Rajagopal1and S.S. Jayabalakrishnan2,*
1Department of Physics, Government College of Engg, Tirunelveli-627007 Tamil Nadu, India2Department of Physics, P.S.R. Engg. College, Sivakasi, Tamil Nadu, India
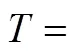
density, standard partial molar volume, hydration number, relative viscosity,-coefficient, activation parameters
1 INTRODUCTION
Amino acids are considered as model compound instead of proteins in the presence of aqueous salt solutions to obtain thermodynamic information as the structure of proteins are highly complicated [1-4]. Some amino acids in the presence of aqueous minerals, such as sodium sulphate [2], CaCl2[5], NaCl [6], potassium thiocynate [7], are available in literature. However, the study of amino acids in the presence of aqueous salbutamol sulphate has not been reported so far. We have recently investigated some homologousa-amino acids such as glycine, L-alanine, L-valine and L-leucine in the presence of aqueous salbutamol sulphate through volumetric and viscometric studies. In this paper, we report 4-aminobutyric acid in aqueous salbutamol sulphate solutions.
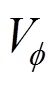
2 EXPERIMENTAL
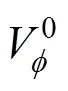
3 RESULTS AND DISCUSSION
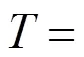
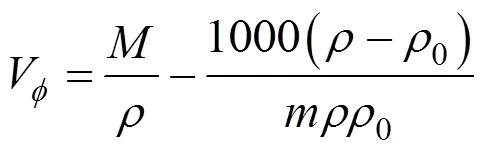
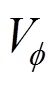
The reported values of apparent molar volume data for the amino acids were found to be adequately represented by the linear equation [13].
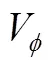
Table 1 Values of density (ρ) and apparent molar volume () of the 4-amino butyric acid in aqueous salbutamol sulphate
Table 1 (Continued)

T/KmS/mol·kg-1mA/mol·kg-1Density(r)×103/kg·m-3m3·mol-1T/KmS/mol·kg-1mA/mol·kg-1Density(r)×103/kg·m-3m3·mol-1 318.150 (in water)00.99021318.150.012500.99383 0.020.9907974.7750.020.9943776.686 0.040.9913674.8080.040.9949076.707 0.060.9919374.8410.060.9954376.729 0.080.9924974.8750.080.9959676.751 0.100.9930674.8980.100.9964976.773 0.004100.992970.020700.99547 0.020.9935276.1380.020.9959977.809 0.040.9940776.1650.040.9965077.819 0.060.9946176.2070.060.9970177.833 0.080.9951476.2410.080.9975277.845 0.100.9956876.2730.100.9980377.857
Note:S, molality of salbutamol sulphate;A, molality of amino acids.
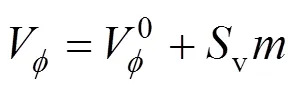
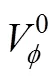
Table 2 Values of standard partial molar volume () of 4-amino butyric acid in aqueous salbutamol sulphate
① Ref. [14, 15],② Ref. [16],③ Ref. [17].
Note:S, molality of salbutamol sulphate. Parentheses indicate standard errors.
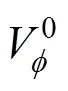
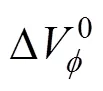
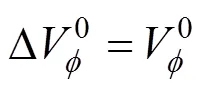
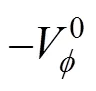
The results are given in Table 3.
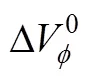
Table 3 Values of standard partial molar volumes of transfer () of 4-amino butyric acid in aqueous salbutamol sulphate solutions
Note:S, molality of salbutamol sulphate.
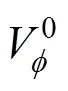





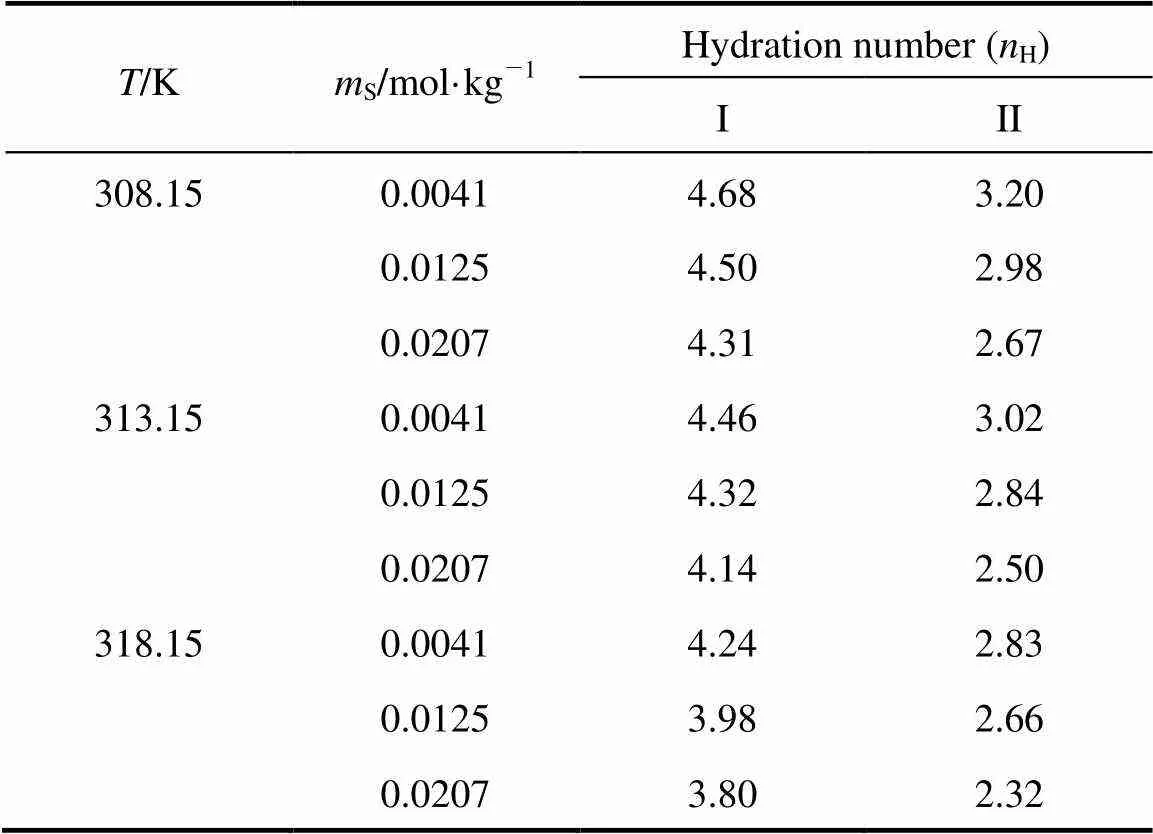
Table 4 Values of hydration number of 4-amino butyric acid in aqueous salbutamol sulphate solutions
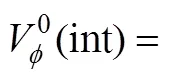
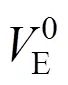
The transfer volumes of the 4-aminobutyric acid may also be expressed by the McMillan Mayer theory [30] of solutions, which permits the formal separation of the effects due to interactions between the pairs of the solute molecules and those due to interactions between three or more solute molecules by the following equation.
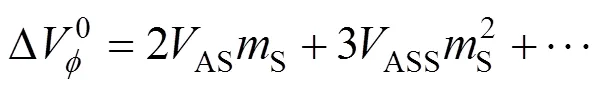
where A stands for the amino acids and S stands for salbutamol sulphate.ASandASSare the pair and triplet volumetric interaction parameters. Using the above equation volumetric interaction parameters were estimated and given in Table 5.
TheASandASSvalues are positive and negative respectively. The large positiveASvalues suggest the domination of pair interactions for the 4-aminobutyric acid over triplet volumetric interaction parameters. Similar reports are available in the literature by Banipal[31].
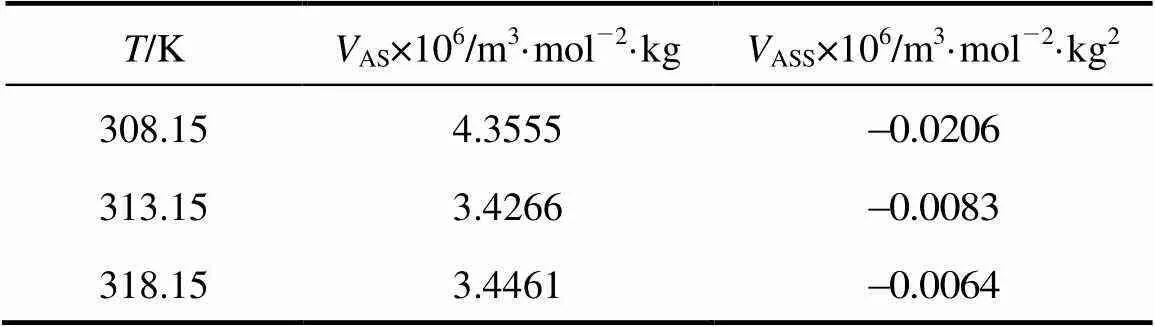
Table 5 Values of pair and triplet interaction coefficients VAS and VASS of 4-amino butyric acid in aqueous salbutamol sulphate solutions
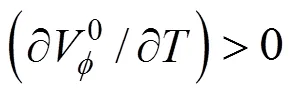
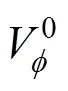
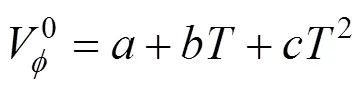
The coefficients,andhave been determined and Eq. (5) has the following forms for the amino acids reported in this work.
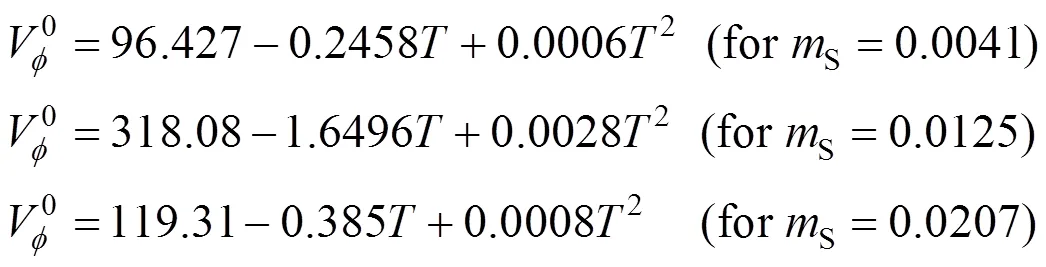
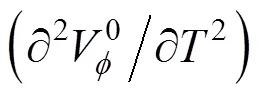
The relative viscositiesrof studied amino acid in water and in cosolute solutions were calculated using the following equation and are summarized in Table 6.
whereand0are the viscosities of the solution and solvent.
Thecoefficients were evaluated by fitting thervalues to the Jones-Dole equation by a least squares method [35] as follows
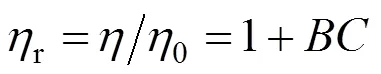
whereis the molarity (calculated from molality data). The values ofcoefficients are summarized in Table 7.
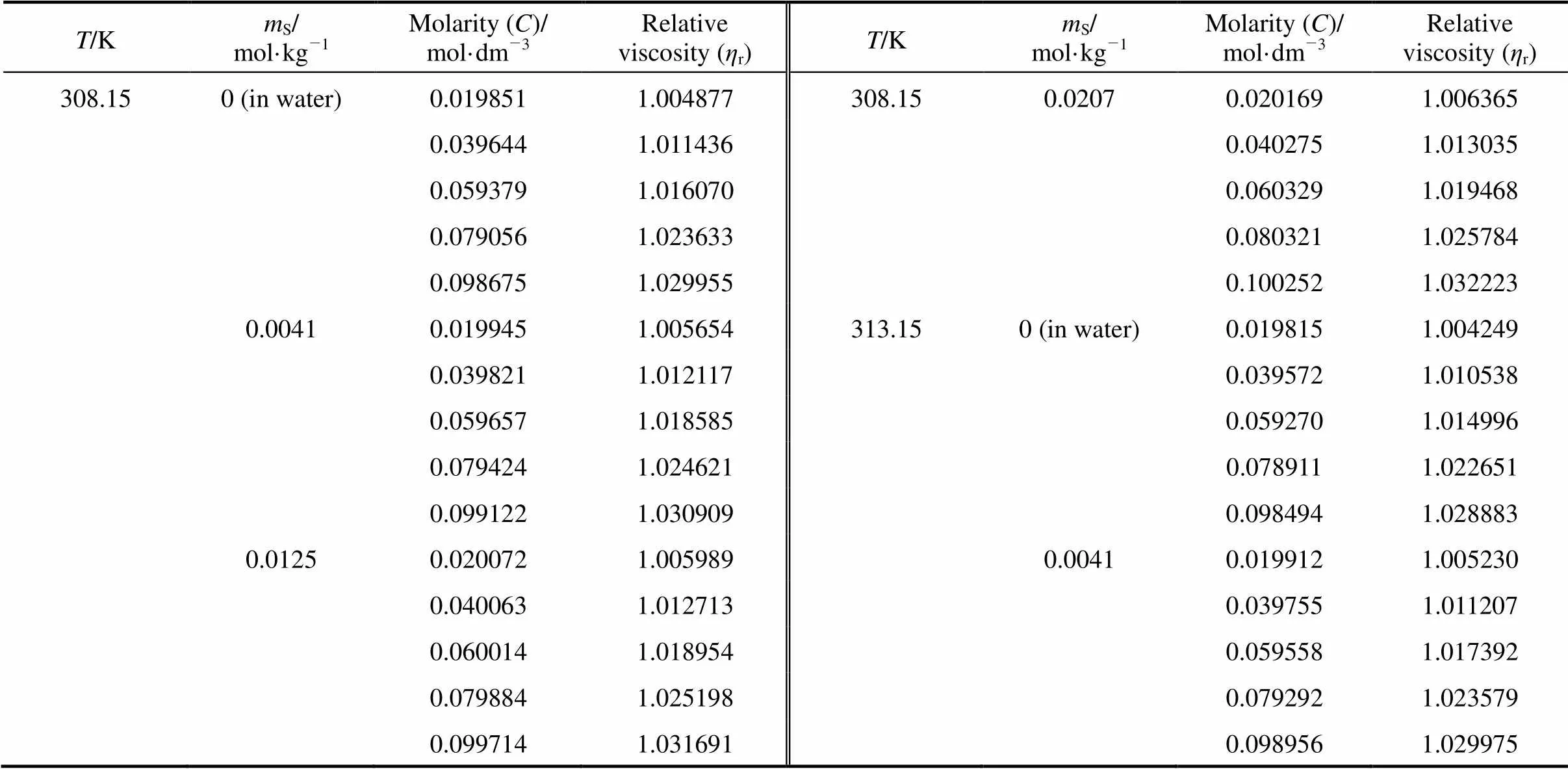
Table 6 Values of relative viscosity of the 4-amino butyric acid in aqueous salbutamol sulphate
Table 6 (Continued)
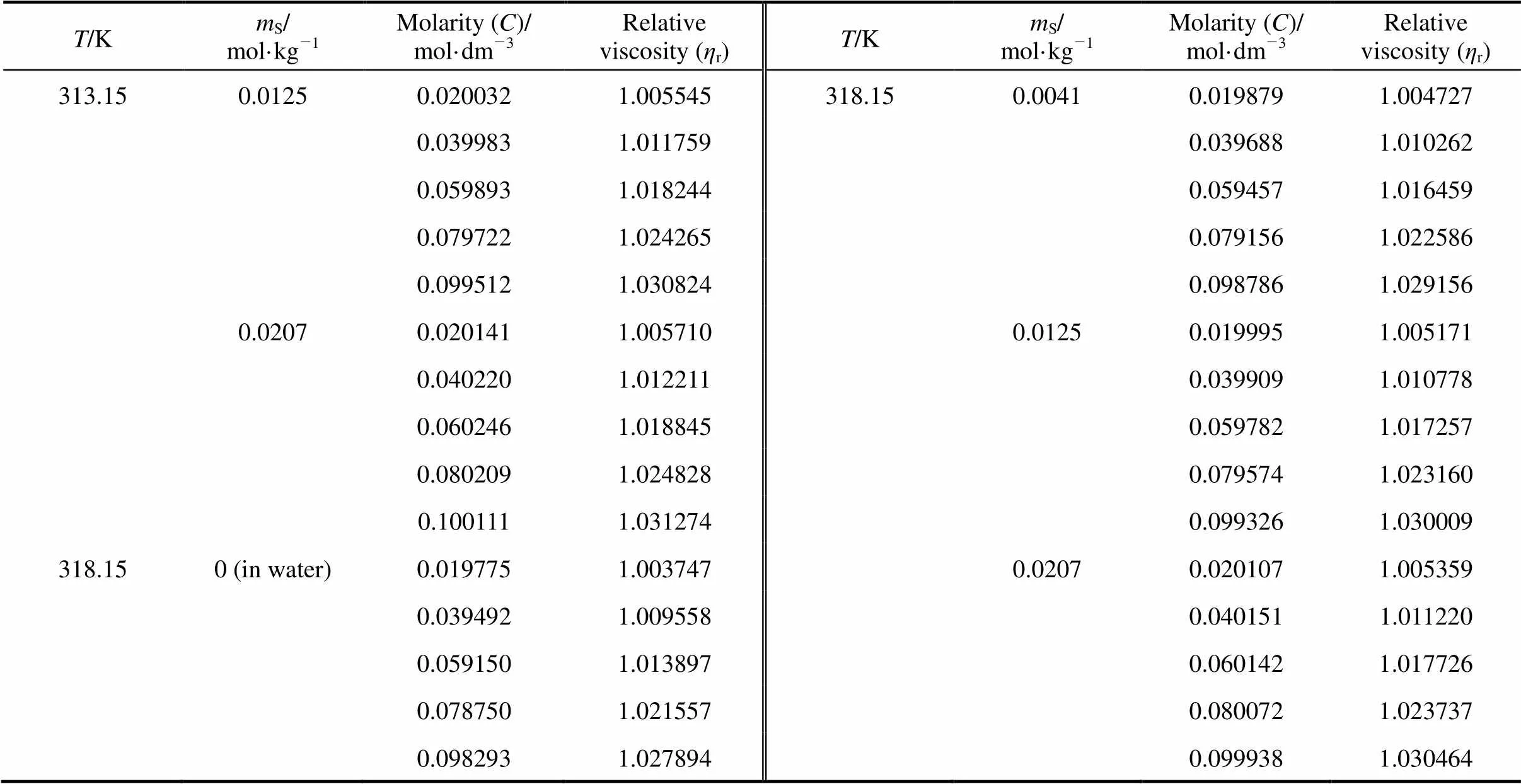
T/KmS/mol·kg-1Molarity (C)/mol·dm-3Relativeviscosity (ηr)T/KmS/mol·kg-1Molarity (C)/mol·dm-3Relativeviscosity (ηr) 313.150.01250.0200321.005545318.150.00410.0198791.004727 0.0399831.0117590.0396881.010262 0.0598931.0182440.0594571.016459 0.0797221.0242650.0791561.022586 0.0995121.0308240.0987861.029156 0.02070.0201411.0057100.01250.0199951.005171 0.0402201.0122110.0399091.010778 0.0602461.0188450.0597821.017257 0.0802091.0248280.0795741.023160 0.1001111.0312740.0993261.030009 318.150 (in water)0.0197751.0037470.02070.0201071.005359 0.0394921.0095580.0401511.011220 0.0591501.0138970.0601421.017726 0.0787501.0215570.0800721.023737 0.0982931.0278940.0999381.030464
Note:S, molality of salbutamol sulphate.
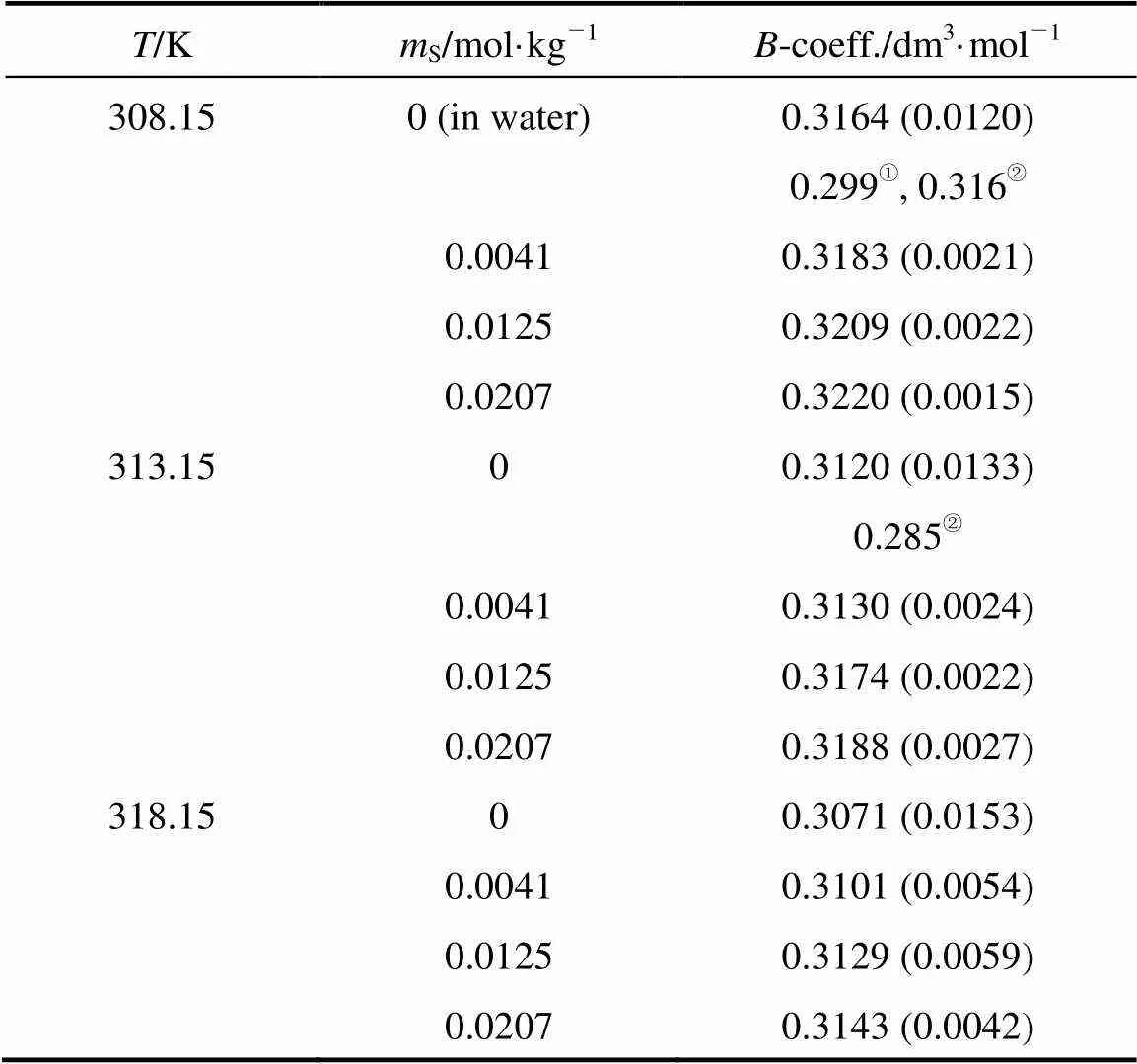
Table 7 Values of viscosity B coefficients of 4-amino butyric acid in water and in aqueous salbutamol sulphate
①Ref. [7],② Ref. [36].
Note:S, molality of salbutamol sulphate. Parentheses indicates standard errors.
The literature-values for the 4-aminobutyric acid in water are also given in Table 7 for comparison. There is a close agreement onvalues reported in this work with literature values for AA validates our viscosity data.
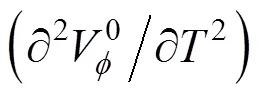
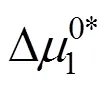
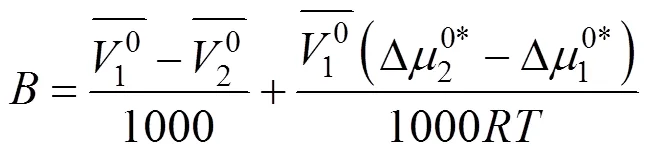
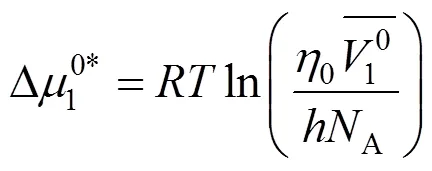
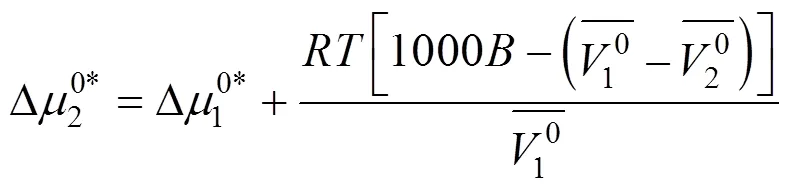
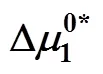
Table 8 Values of activation free energy of solvent , solute and average molar volume of solvent , solute of aqueous salbutamol sulphate solutions
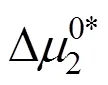
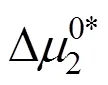
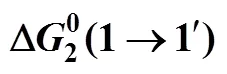
Table 9 Values of thermodynamic activation parameter transfer of 4-amino butyric acid from ground state to transition state in aqueous salbutamol sulphate solutions
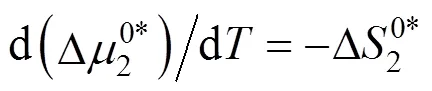
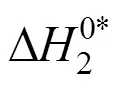
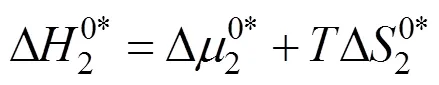
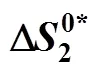
Table 10 Values of activation entropy T (kJ·mol-1) and activation enthalpy (kJ·mol-1) of 4-amino butyric acid in aqueous salbutamol sulphate solutions
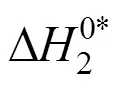
4 CONCLUSIONS
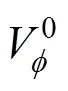
NOMENCLATURE
viscosity-coefficient, dm3·mol-1
molarity of amino acid, mol·dm-3
Planck constant
molar mass of amino acid
molality of amino acid
AAvagadro’s number
Hhydration number
universal gas constant (8.314 J·mol-1·K-1)
vcoefficient in Eq. (2)
temperature, K
flow time of solution in viscometer
rrelative viscosity
density, kg·m-3
Superscripts
0 binary solvent (in aqueous salbutamol sulphate)
Subscripts
1 binary solvent property
2 solute property
1 Ali, A., Hyder, S., Sabir, S., Chand, D., Nain, A.K., “Volumetric, viscometric and refractive index behavior ofa-amino acids and their groups contribution in aqueous D-glucose solutions at different temperatures”,.., 38, 136-143 (2006).
2 Wadi, R.K., Ramasami, P., “Partial molar volumes and adiabatic compressibilities of transfer of glycine and DL-alanine from water to aqueous sodium sulphate at 288.15, 298.15 and 308.15 K”,....., 93, 243-247 (1997).
3 Yan, Z., Wang, J., Kong, W., Lu, J., “Effects of temperature on volumetric and viscosity properties of somea-amino acids in aqueous calcium chloride solutions”,., 215, 143-150 (2004).
4 Banipal, T.S., Sehgal, G., “Partial molar adiabatic compressibility of transfer of some amino acids from water to aqueous sodium chloride and aqueous glucose solutions”,., 265, 175-183 (1995).
5 Yan, Z., Wang, J., Kong, W., Lu, J., “Effect of temperature on volumetric and viscosity properties of somea-amino acids in aqueous calcium chloride solutions”,., 215, 130-143 (2004).
6 Soto, A., Arce, A., Kheshkbarchi, M.K., “Experimental data and modeling of apparent molar volumes, isentropic compressibilities and refractive indices in aqueous solutions of glycine + NaCl”,.., 74, 165-173 (1998).
7 Wadi, R.K., Goyal, R.K., “Densities, viscosity and applications of transition-state theory for water and potassium thiocyanate + amino acid solutions at 288.15-308.15 K”,...., 37, 377-386 (1992).
8 Kharakoz, D.P., “Volumetric properties of proteins and their analogues in diluted water solutions partial molar volumes of amino acids at 15-55°C”,.., 34, 115-125 (1989).
9 Kharakoz, D.P., “Partial volumes and compressibilities of extended polypeptide chains in aqueous solutions additivity scheme and implications of protein unfolding at normal and high pressure”,-., 36, 10276-10285 (1997).
10 Sakurai, M., Nakumaura, T., Takenaka, N., “Apparent molar volumes and apparent molar adiabatic compressions of water in some alcohols”,...., 67, 352 (1994).
11 Kikuchi, M., Sakurai, M., Nitta, N., “Partial molar volumes and isentropic compressibilities of-acetyl amino acid amides in dilute aqueous solutions at (5,15,25,35 and 45)°C”,..., 41, 1439-1445 (1996).
12 Pal, A., Kumar, S., “Volumetric and viscometric studies of glycine in binary aqueous solutions of sucrose at different temperatures”,..., 44A, 469-475 (2005).
13 Ren, X., Hu, X., Lin, R., Zong, H., “Apparent molar volumes of L-glycine, L-alanine and L-serine in water + dimethyl formamide mixtures at 298.15 K”,..., 43, 700-702 (1998).
14 Islam, M.N., Wadi, R.K., “Temperature dependence of apparent molar volumes and viscosity coefficients of amino acids in aqueous sodium sulphate solutions from 15 to 35°C”,..., 41, 533-544 (2003).
15 Wadi, R.K., Goyal, R.K., “Temperature dependence of apparent molar volume and viscosity coefficients of amino acids in aqueous potassium thiocyanate solutions from 15 to 35°C”,.., 21, 163-170 (1992).
16 Bhattaacharyya, M.M., Sengupta, M., “Ion-solvent interaction of amino acids: IV. Apparent molar volumes of amino acids in natural acidic and alkaline media at different temperatures”,..., 62, 959-964 (1985).
17 Chalikian, T.V., Sarvazyan, A.V., Breslauer, K.J., “Partial molar volumes expansibilities and compressibilities ofa,w-amino carboxycylic acids in aqueous solutions between 18 and 55°C”,..., 97, 13017-13026 (1993).
18 Beli bagli, K., Ayranci, E., “Viscosities and apparent molar volumes of some amino acids in water and in 6M guanidine hydrochloride at 25°C”,.., 19, 867-882 (1990).
19 Friedman, H., Krishnan, C.V., Thermodynamics of Ion Hydration, in: Water—A comprehensive treatise, Plenum press, New York, Vol. 3 (Chapter 1), 1-118 (1973).
20 Bhat, R., Kishore, N., Ahluwalia, J.C., “Thermodynamic studies of transfer of some amino acids and peptides from water to aqueous glucose and sucrose solutions at 298.15 K”,..., 84 (8), 2651-2665 (1988).
22 Liu, Q., Hu, X., Lin, R., Sang, W., Li, S., “Limiting partial molar volumes of glycine L-alanine and L-serine in ethylene glycol + water mixtures at 298.15 K”,..., 46, 522-525 (2001).
25 Franks, F., Quickenden, M.A., Reig, D.S., Watson, B., “Calorimetric and volumetric studies of dilute aqueous of cycle ether derivatives”,...., 66, 582-589 (1970).
26 Millero, F.J., Surdo, A.L., Shin, C., “The apparent molar volumes and adiabatic compressibilities of aqueous amino acids at 25°C”,..., 82, 784-792 (1978).
27 Pal, A., Kumar, K., “Volumetric and ultrasonic studies of some amino acids in binary aqueous solutions of MgCl2·6H2O at 298.15 K”,..., 121, 148-155 (2005).
28 Millero, F.J, Leppa, G.K., Lepple, F.K., Hoff, E.V., “Isothermal compressibility of aqueous sodium chloride, magnesium chloride, sodium sulphate and magnesium sulphate solutions from 0 to 45°C at 1 atm”,..., 78, 1636-1643 (1974).
29 Romero, C.M., Negrete, F., “Effect of temperature on partial molar volumes and viscosities of aqueous solutions ofa-DL-aminobutyric and DL-norvaline and DL-norLeucine”,..., 42, 261-267 (2004).
30 McMillan, W.G., Mayer, J.E., “The statistical thermodynamics of multi component system”,..., 13, 276-305 (1945)
31 Banipal, T.S., Kaur, D., Banipal, P.K., “Effect of magnesium acetate on the volumetric and transport behaviour of some amino acids in aqueous solutions at 298.15 K”,.., 38, 1214-1226 (2006).
32 Hepler, L., “Thermal expansion and structure in water and aqueous solutions”,..., 47, 4613-4617 (1969).
33 Pal, A., Kumar, S., “Viscometric and volumetric studies of some amino acids in binary aqueous solutions of urea at various temperatures”,..., 109, 23-31 (2004).
34 Lark, B.S., Patyar, P., Banipal, T.S., Kishore, N., “Densities, parial molar volumes and heat capacities of glycine, L-alanine and L-leucine in aqueous magnesium chloride solutions at different temperatures”,...., 49, 553-565 (2004).
35 Jones, G., Dole, M., “The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride”,...., 51, 2950-2964 (1929).
36 Bhattacharyya, M.M., Sengupta, M., “Ion solvent interaction of amino acids; part II. Amino acids in aqueous solutions in the cationic, anionic and zwitterionic forms”,... (..), 133, 79-91 (1982).
37 Jenkins, H.D.B., Marcus, Y., “Viscosity-coefficients of ions in solutions”,.. ,95, 2695-2724 (1995).
38 Bai, T.C., Yan, G.B., “Viscosity-coefficients and activation parameters for viscous flow of a solution of heptanedioic acid in aqueous sucrose solutions”,, 338, 2921-2927 (2003).
39 Tsangins, J.M., Martin, R.B., “Viscosities of aqueous solutions of dipolar ions”,..., 112, 267-272 (1965).
40 Kaminsky, M., “Densities and apparent molal volumes of some aqueous rare earth solutions at 25°C”,.., 24, 171 (1957).
41 Sharma, T.S., Ahluwalia, J.C., “Experimental studies on the structure of aqueous solutions of hydrophobic solutes”,..., 2, 203-232 (1973).
42 Feakins, D., Bates, F.M., Waghorne, W.E., Lawrence, K.G., “Relative viscosities and quasi thermodynamics of solutions of-butyl alcohol in the methanol water system; a different view of the alkyl-water interaction”,...., 89, 3381-3388 (1993).
43 Glasstone, S., Laidler, K., Eyring, H., The Theory of Rate Processes, McGraw-hill, New York, 477 (1941).
44 Mishra, A.P., Gautam, S.K., “Viscometric and volumetric studies of some transition metal chlorides in glycine water solutions”,.., 40, 100-104 (2001).
45 Yan, Z., Wang, J., Lu, J., “Viscosity behaviour of somea-amino acids and their groups in water-sodium acetate mixtures”,.., 99, 199-207 (2002).
2008-11-27,
2009-02-18.
* To whom correspondence should be addressed. E-mail: krishnanpsr@yahoo.com
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Molecular Simulation of CO2/H2 Mixture Separation in Metal-organic Frameworks: Effect of Catenation and Electrostatic Interactions*
- Deactivation Kinetics of Nitrile Hydratase in Free Resting Cells*
- Corrosion Behavior of TP316L of Superheater in Biomass Boiler with Simulated Atmosphere and Deposit
- Influence of A-type Zeolite on Methane Hydrate Formation*
- Effects of Sintering Atmosphere on the Microstructure and Surface Properties of Symmetric TiO2Membranes*
- Improvement of Isomerization Process of Crude Isoamylene with Tertiary-amyl-alcohol Addition
