Improvement of Hydrophilicity and Blood Compatibility on Polyethersulfone Membrane by Blending Sulfonated Polyethersulfone*
WANG Haitao (王海涛), YANG Liu (杨刘), ZHAO Xuehui (赵学辉), YU Tian (于湉)and DU Qiyun (杜启云)
Improvement of Hydrophilicity and Blood Compatibility on Polyethersulfone Membrane by Blending Sulfonated Polyethersulfone*
WANG Haitao (王海涛)1,**, YANG Liu (杨刘)2, ZHAO Xuehui (赵学辉)1, YU Tian (于湉)1and DU Qiyun (杜启云)1
1The Key Lab of Hollow Fiber Membrane Materials and Membrane Process of Ministry of Education, Tianjin Polytechnic University, Tianjin 300160, China2Department of Bio-medical Engineering, Tianjin Polytechnic University, Tianjin 300160, China
Polyethersulfone (PES) is widely used as biomaterials due to its thermal stability, mechanical strength, and chemical inertness. Nevertheless, their blood compatibility is still not adequate for hemodialysis and blood purification. In this study, the sulfonated polyethersulfone (SPES) was synthesized through an electrophilic substitution reaction, and PES/SPES blending membranes were prepared. The characterization of the SPES was studied by FTIR. The water adsorption and water contact angle experiments show that the hydrophilicity of PES/SPES blend membrane was improved as for the sulfonate group existing in the SPES. Moreover, PES/SPES blend membrane could effectively reduce bovine serum albumin adsorption and prolong the blood coagulation time compared with the PES membrane, thereby improving blood compatibility.
sulfonated polyethersulfone, polyethersulfone, hydrophilicity, blood compatibility, blending
1 INTRODUCTION
However, the hydrophobic nature of PES is disadvantageous in these blood-contacting applications [9]. The adsorption of serum proteins onto PES hemodialysis membranes can cause serious or life-threatening complications due to activation of the complement alternativepathway [10]. Anticoagulants are often injected during the process of dialysis to avoid clot formation.
To resolve the problem, many studies have aimed to improve the blood compatibility of biomaterials by surface modification. The most well-known method is to modify the materials themselves into antithrombogenic materials [11, 12]. Being the most widely used blood anticoagulant, heparin can catalytically increase the formation rate of antithrombin III and inhibit thrombin and other coagulation proteases. Another method is grafting hydrophilic polymer onto membrane material surface using chemical modification [13-16]. The common modified membrane materials may be polysulfone, cellulose, polyurethane, and polyacrylonitrile. However, both grafting antithrombogenic materials and grafting hydrophilic polymer onto membrane material surface required a long reaction time and reaction process.
Sulfonation is a simple chemical modification method to enhance the hydrophilicity of PES materials. Hydrophilic groups can be chemically introduced onto PES backbones using chlorosulfonic acid as sulfonating agent. Guan. [17] found that the hydrophilicity of sulfonated polyethersulfone (SPES) membrane was increased compared with PES membrane. Cheng. [18] used five purification materials to examine the degree of contact activation and found that the SPES has the best blood compatibility.
Blending modification is an important method to improve the membrane hydrophilicity [19, 20]. In this study, we succeeded in synthesizing the sulfonated PES through an electrophilic substitution reaction and preparing the membrane with PES blending sulfonated PES (PES/SPES). Surface properties of PES/SPES were investigated by Fourier Transform Infrared Spectroscopy. The hydrophilicity of PES/SPES blend membranes were investigated by the experiment of water adsorption and water contact angle. The blood compatibility of PES/SPES membranes were evaluated by protein adsorption assay and blood coagulation time.
2 EXPERIMENTAL
2.1 Materials
2.2 Synthesis of SPES
The PES flake (20 g) was dissolved in concentrated sulfuric acid (98%, 160 ml) at 25°C for 4 h with stirring in a three-neck reaction flask, and the stirring speed was 500 r·min-1. When PES was dissolved completely, about 40 ml chlorosulfonic acid was dropped in the solution and reacted for 2 h. Then, the solution was placed in the deionized water and washed several times until the pH is about 7. Finally, the SPES was dried for 4 h at 90°C in vacuum oven. The degree of sulfonation for the SPES was measured by titration and the result is 8%.
2.3 Preparation of PES membrane blended with the SPES polymer
Five mass percent polymers of both PES and SPES were prepared separately, thereinto the SPES mass percent were 0, 5%, 15%, 30%, and 50%, respectively. The mixed polymers were dissolved in DMAC at 70°C for 24 h with vigorous stirring, which was allowed to stand overnight to remove air bubbles. The pore forming agent is PEG400. After the air bubbles were removed, the casting solution was cast onto a clean glass plate using a casting knife with 100 μm gate opening. The nascent membrane was placed in the air for 10 min and then immersed in ultrafiltrated water for 24 h before use.
2.4 Hydrophilicity measurements
The hydrophilicity of the PES and PES/SPES membranes surface were characterized on the basis of water contact angle (CA) and water absorption measurements. Using a sessile drop method [21], water CA was measured at room temperature by a CA goniometer (JY-82). A total of 15 μl of deionized water was dropped onto a dry membrane using a microsyringe in an atmosphere of saturated water vapor, and then CA was measured after 10 s. At least 5 CAs were averaged to get a reliable value. Water absorption was defined as1and2, where1and2represent the mass of the dry membrane (1 cm×1 cm), and the membrane was soaked in deionized water for 12 h at (25±0.5)°C, respectively. The reported value was the average of at least five experiments.
2.5 Characterization
The cross-section morphologies of membranes were observed by scanning electron microscopy (SEM) using an XL 30ESME scanning microscope. The membranes frozen in liquid nitrogen were broken and sputtered with gold before SEM analysis.
ATR-FTIR study was performed using a VECTOR22 (BROKER) Fourier-Transform Infrared Spectrometer equipped with an OMNI sampler over 32 scans. The spectra were recorded at a 45º incident angle using a Ge crystal.
2.6 Protein adsorption assay on the membrane
2.7 Blood coagulation time
Thecoagulation times, including activated partial thrombin time (APTT) and prothrombin time (PT), were determined using an automated blood coagulation analyzer. Blood coagulation times were measured as follows [22]: a sample membrane (1 cm× 1 cm) was placed carefully in tubes and incubated in 0.5 ml of platelet-poor plasma (PPP) at 37°C for 30 min. Afterward, 0.1 ml residual PPP solution was mixed well with 0.1 ml of Dade Actin®FS (Date Behring Marburg GmbH) agent at 37°C for 3 min. Simultaneously, with addition of CaCl2, a stopwatch was started and the solution was mixed well. After 20 s, the time of clot formation was recorded using a chronometer. The tests were repeated three times for each sample.
3 RESULTS AND DISCUSSION
3.1 Synthesis of SPES

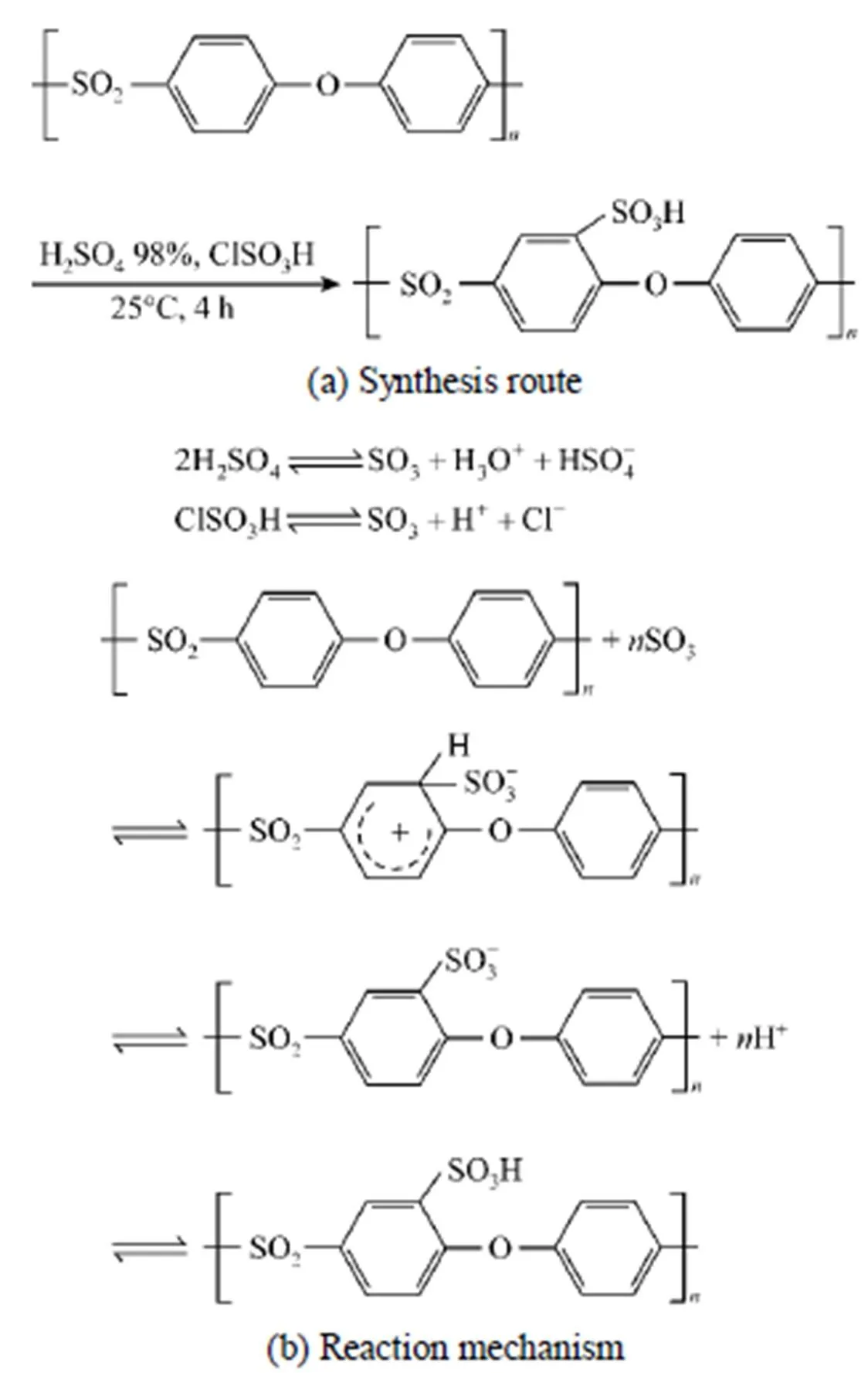
Figure 1 Synthesis route and reaction mechanism for grafting sulfonic onto PES


Figure 2 Infrared spectra (ATR) of PES material and SPES material
3.2 Hydrophilicity of the membranes

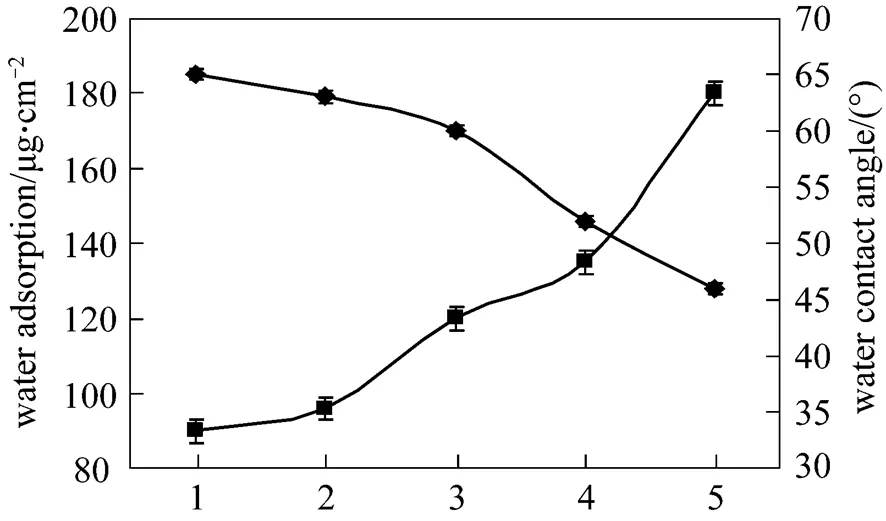
Figure 3 Water CA and water absorption for PES and PES/SPES membranes 1—PES; 2—PES/SPES 5%; 3—PES/SPES 15%; 4—PES/SPES 30%; 5—PES/SPES 50%■ water adsorption;◆ water contact angle
3.3 Filtration properties and BSA adsorption
Table 1 shows the results of water and BSA filtration to investigate whether blending SPES affects the filtration performance of the membranes or not because the pore shape and size may be changed due to the existing of SPES. It was found that the BSA rejection of PES membrane was 99.8%, whereas the PES/SPES membranes containing 5%, 15%, 30%, and 50% SPES showed 99.6%, 99.9%, 99.7%, and 99.2%, respectively. This result shows that the molecular weight cut off (MWCO) of the PES membrane was not affected seriously by blending SPES. Moreover, Table 1 also shows that with the increase of SPES content, the flux of water and BSA solution increase. All the membranes blending with SPES have higher water flux and BSA filtration than the PES membrane. These results indicate that blending SPES can improve the filtration performance of the membrane due to the enhancement of hydrophilicity.
Figure 4 shows the cross-sectional SEM micrographs of the PES membrane and PES/SPES membranes. A characteristic morphology of asymmetric membrane consisting of a dense top-layer and porous sublayer with a finger-like structure was observed for all the membranes. However, the porous sublayer of PES/SPES membranes was more inerratic and obvious than that of the PES membrane. This result also explained why all the membranes blending with SPES have higher water flux than the PES membrane.

Table 1 Filtration and rejection performances of the PES and PES/SPES membranes
Note: The filtration test was conducted at a constant pressure of 0.1 MPa and a system temperature of (37±0.5)°C.w: water flux;b: BSA solution flux.

Figure 4 SEM images of the cross-section of PES membrane and PES/SPES membranes
When SPES content is 5% and 15%, the mechanical strength of the PES/SPES membranes was found to be similar to the pure PES membrane through the stretching examination by hand and eye. However, when SPES content is 30% and 50%, the mechanical strength of the PES/SPES membranes were found to be lower than the pure PES membrane obviously. About this problem, we will discuss in the future research.
Protein adsorption on the materials is a common phenomenon during thrombogenic formation. The amount of proteins adsorbed on the membrane materials is reported to be one of the important factors in evaluating the blood compatibility of materials [12]. Fig. 5 shows the adsorption of BSA on PES membrane and PES/SPES membranes with different content. It was found that all the PES/SPES membranes have lower BSA adsorption than the PES membrane. It has been well known that the hydrophobic interaction between the material surface and proteins plays a very important role in the nonselective adsorption of protein. Materials possessing hydrophilic surface normally show relatively low nonselective adsorption for proteins or cells [21]. So the hydrophilicity of the PES/SPES membranes was improved and accordingly the BSA adsorption was decreased.

Figure 5 Adsorption of BSA on PES membrane and PES/SPES membrane with different content
1—PES; 2—PES-5%SPES; 3—PES-15%SPES; 4—PES-30%SPES; 5—PES-50%SPES
3.4 Anticoagulant properties


Figure 6 Comparison of anticoagulant properties of PES and PES/SPES membranes
1—PES; 2—PES-5%SPES; 3—PES-15%SPES; 4—PES-30%SPES; 5—PES-50%SPES
4 CONCLUSIONS

AcknowledgEments
..
1 Moachon, N., Boullanger, C., Fraud, S., Vial, E., Thomas, M., Quash, G., “Influence of the charge of low molecular weight proteins on their efficacy of filtration and/or adsorption on dialysis membranes with different intrinsic properties”,, 23, 651-658 (2002).
2 Sun, H.X., Zhang, L., Chai, H., Chen, H.L., “Removing endotoxin from protein solution by chitosan modified affinity membrane”,... Eng., 13 (4), 457-463 (2005).
3 Barzin, J., Feng, C., Khulbe, K.C., Matsuura, T., Madaeni, S.S., Mirzadeh, H., “Characterization of polyethersulfone hemodialysis membrane by ultrafiltration and atomic force microscopy”,..., 237, 77-85 (2004).
4 Wang, T., Wang, Y.Q., Su, Y.L., Jiang, Z.Y., “Antifouling ultrafiltration membrane composed of polyethersulfone and sulfobetaine copolymer”,..., 280, 343-350 (2006).
5 Shi, Q., Su, Y.L., Zhu, S.P., Li, C., Zhao, Y.Y., Jiang, Z.Y., “A facile method for synthesis of pegylated polyethersulfone and its application in fabrication of antifouling ultrafiltration membrane”,..., 303, 204-212 (2007).
6 Zhu, L.P., Zhu, B.K., Xu, L., Feng, Y.X., Liu, F., Xu, Y.Y., “Corona-induced graft polymerization for surface modification of porous polyethersulfone membranes”,..., 253, 6052-6059 (2007).
7 Zhang, H.F., Zhang, Q.B., Sun, C.Y., “Nonisothermal crystallization kinetics of polypropylene/polyethersulfone blend”,.., 60, 291-300 (2008).
8 Su, B.H., Fu, P., Li, Q., Tao, Y., Li, Z., Zao, H.S., Zhao, C.S., “Evaluation of polyethersulfone high flux hemodialysis membraneand”,....., 19, 745-751 (2008).
9 Lu, D.P., Zou, H., Guan, R., Dai, H., Lu, L., “Sulfonation of polyethersulfone by chlorosulfonic acid”,.., 54, 21-28 (2005).
10 Singh, N.P., Thakur, A., Kohli, R., “Effect of membrane composition on cytokine production and clinical symptoms during hemodialysis: A crossover study”,, 25, 419-430 (2003).
11 Kazuhiko, I., Kikuko, F., Yasuhiko, I., Nakabayashi, N., “Modification of polysulfone with phospholipid polymer for improvement of the blood compatibility. Part 1. Surface characterization”,, 20, 1545-1551 (1999).
12 Kazuhiko, I., Kikuko, F., Yasuhiko, I., Nakabayashi, N., “Modification of polysulfone with phospholipid polymer for improvement of the blood compatibility. Part 2. Protein adsorption and platelet adhesion”,, 20, 1553-1559 (1999).
13 Bernacca, G.M., Gulbransen, M.J., Wilkinson, R., Wheatley, D.J., “blood compatibility of surface-modified polyurethanes”,, 19, 1151-1165 (1998).
14 Yuan, J., Zhang, J., Zang, X.P., Shen, J., Lin, S., “Improvement of blood compatibility on cellulose membrane surface by grafting betaines”,., 30, 147-155 (2003).
15 Sang, H.Y., Junji, W., Yasuhiko, I., Ishihara, K., “Novel cellulose acetate membrane blended with phospholipid polymer for hemocompatible filtration system”,..., 210, 411-421 (2002).
16 Jane, Y.P., Metin, H.A., Ariya, A., Kuhlman, W., Mayes, A.M., “Polysulfone-graft-poly(ethylene glycol) graft copolymers for surface modification of polysulfone membranes”,, 27, 856-865 (2006).
17 Guan, R., Zou, H., Lu, D.P., Gong, C.L., Liu, Y.F., “Polyethersulfone sulfonated by chlorosulfonic acid and its membrane characteristics”,..., 41, 1554-1560 (2005).
18 Cheng, L.P., Sun, S.D., Yue, Y.L., Huang, J., “Effect of blood purification materials and anticoagulants on the contact activity of factor XI”,.. (), 36, 411-421 (2005). (in Chinese)
19 Mathias, U., Oliver, S., Wolfgang, A., Martin, R., Peter, S., “Improvement of hydrophilicity and blood compatibility on polyethersulfone membrane by blending sulfonated polyethersulfone”,..., 57, 63-73 (2007).
20 Chen, H.L., Cheng, L.H., “Study on PVA membranes blended with acrylic ester-acrylic acid copolymer for dehydration of ethanol”,...., 8 (2), 184-188 (2000).
21 Xu, Z.K., Nie, F.Q., Qu, C., Wan, L.S., Wu, J., Yao, K., “Tethering poly(ethylene glycol)s to improve the surface biocompatibility of poly (acrylonitrile-co-maleic acid) asymmetric membranes”,, 26, 589-598 (2005).
22 Lin, W.C., Yu, D.G., Yang, M.C., “Blood compatibility of thermoplastic polyurethane membrane immobilized with water-soluble chitosan/dextran sulfate”,., 44, 82-92 (2005).
23 Nolte, R., Ledjeff, K., Bauer, M., Mülhaupt, R., “Partially sulfonated poly(arylene ether sulfone)—A versatile proton conducting membrane material for moden energy conversion technologies”,..., 83, 211-220 (1993).
24 Kim, I.C., Choi, J.G., Tak, T.M., “Sulfonated polyethersulfone by heterogeneous method and its membrane performances”,..., 74, 2046-2055 (1999).
2008-08-28,
2008-12-19.
the Special Fund for International Cooperation Projects of China (2005DFA50160).
** To whom correspondence should be addressed. E-mail: whaitao134@163.com
 Chinese Journal of Chemical Engineering2009年2期
Chinese Journal of Chemical Engineering2009年2期
- Chinese Journal of Chemical Engineering的其它文章
- Design and Performance Analysis of Micro Proton Exchange Membrane Fuel Cells*
- Isolation of Cordyceps ophioglossoides L2 from Fruit Body and Optimization of Fermentation Conditions for Its Mycelial Growth*
- Efficient and Comprehensive Utilization of Hemicellulose in the Corn Stover*
- Simulating Surface Aeration Systems at Different Scale of Mixing Time*
- Kinetics of Reaction-Crystallization of Struvite in the Continuous Draft Tube Magma Type Crystallizers—Influence of Different Internal Hydrodynamics
- Preparation and Characterization of Tungsten-substituted Molybdophosphoric Acids and Catalytic Cyclodehydration of 1,4-Butanediol to Tetrahydrofuran*
