放疗抵抗宫颈鳞状细胞癌circRNAs表达谱的初步研究

[摘" "要]" "目的:分析放疗敏感及放疗抵抗宫颈鳞状细胞癌(cervical squamous cell carcinoma, CSCC)患者肿瘤组织中差异表达环状RNA(circular RNAs, circRNAs)。方法:采用Illumina PE150高通量测序技术法检测3例放疗抵抗及3例放疗敏感CSCC患者肿瘤组织中差异表达circRNAs,对测序获得的序列进行信息挖掘及分析。利用逆转录定量聚合酶链式反应(quantitative real time-polymerase chain reaction, qRT-PCR)法对测序获得的60个差异显著circRNAs进行验证。结果:放疗敏感与放疗抵抗CSCC组织间差异表达在2倍及以上共有1 316个circRNAs。与放疗敏感CSCC相比,放疗抵抗CSCC组织中显著上调的circRNAs共594个,显著下调的circRNAs共722个。对差异circRNAs来源基因进行基因本体(Gene Ontology, GO)分析发现:就细胞生物学过程而言,差异circRNAs主要富集于细胞代谢过程、细胞器组织、初级代谢过程、杂环代谢过程等;就分子功能而言,差异circRNAs主要富集于蛋白质结合、激酶结合、染色质结合、杂环化合物结合等。京都基因和基因组大百科全书数据库(Kyoto encyclopedia of genes and genomes, KEGG)信号通路富集分析发现,差异circRNAs富集于甲状腺激素信号通路、Hippo信号通路、FoxO信号通路等通路。qRT-PCR分析证实,与放疗敏感CSCC相比,hsa_circ_0003373、hsa_circ_0004337、hsa_circ_0005114、hsa_circ_0031431、novel_circ_0006491、novel_
circ_0070688、novel_circ_0070695在放疗抵抗CSCC组织中显著下调(均Plt;0.05)。结论:与放疗敏感CSCC相比,hsa_circ_
0003373、hsa_circ_0004337、hsa_circ_0005114、hsa_circ_0031431、novel_circ_0006491、novel_circ_0070688、novel_circ_
0070695在放疗抵抗CSCC组织中显著下调,为进一步研究circRNAs在CSCC放疗抵抗中的作用提供实验数据。
[关键词]" "宫颈鳞状细胞癌;放疗抵抗;环状核糖核酸
[中图分类号]" "R737.33" " " " " " " "[文献标志码]" "A" " " " " " " "[文章编号]" "1674-7887(2024)02-0145-05
Preliminary study on circRNAs expression profile of radioresistant cervical squamous cell carcinoma*
ZHANG Xingsong1**, JIA Meiqun2***" " " " (1Department of Pathology, 2Department of Gynecologic Oncology, Tumor Hospital Affiliated Nantong University, Nantong Tumor Hospital, Jiangsu 226361)
[Abstract]" "Objective: To analyze the differential expression of circular RNAs(circRNAs) in the tumor tissues of patients with cervical squamous cell carcinoma(CSCC) that are sensitive to and resistant to radiotherapy. Methods: The Illumina PE150 high-throughput sequencing technology was used to detect the differentially expressed circRNAs in tumor tissues of 3 radioresistant and 3 radiosensitive CSCC patients, and information mining and analysis of the sequence were carried out. The 60 differential expression of circRNAs were verified by quantitative real time-polymerase chain reaction(qRT-PCR). Results: There were 1 316 circRNAs that were twice or more differentially expressed between radiosensitive and radioresistant CSCC tissues. Compared with radiosensitive CSCC tissues, there were 594 differential circRNAs that were significantly up-regulated in radioresistant CSCC tissues, and 722 differential circRNAs were significantly down-regulated. Gene Ontology(GO) analysis showed that in terms of cell biological processes, differential circRNAs are mainly enriched in cellular metabolic processes, organelles, primary metabolic processes, heterocyclic metabolic processes, etc. In terms of molecular functions, differential circRNAs are mainly enriched in protein binding, kinase binding, chromatin binding, heterocyclic compound binding, etc. Enrichment analysis of Kyoto encyclopedia of genes and genomes(KEGG) signaling pathway revealed that differential circRNAs were enriched in thyroid hormone signaling pathway, Hippo signaling pathway, FoxO signaling pathway and other pathways. qRT-PCR analysis showed that hsa_circ_0003373,
hsa_circ_0004337, hsa_circ_0005114, hsa_circ_0031431, novel_circ_0006491, novel_circ_0070688 and novel_circ_0070695 were significantly down-regulated in radioresistant CSCC tissues compared with radiosensitive CSCC tissues(all Plt;0.05). Conclusions: Hsa_circ_0003373, hsa_circ_0004337, hsa_circ_0005114, hsa_circ_0031431, novel_circ_0006491,novel_circ_
0070688 and novel_circ_0070695 were significantly down-regulated in radioresistant CSCC tissues compared with radiosensitive CSCC tissues, which provides an experimental basis for further study on the role of circRNAs in CSCC radiotherapy resistance.
[Key words]" "cervical squamous cell carcinoma; radio-resistance; circular ribonucleic acid
宫颈鳞状细胞癌(cervical squamous cell carci-noma, CSCC)是女性常见的恶性肿瘤,严重威胁女性的健康。放疗是治疗中晚期CSCC的重要方法之一,但仍有约50%的患者对放疗不敏感,导致治疗失败。目前,放疗抵抗产生的确切机制尚不明确[1-2]。
环状RNA(circular RNAs, circRNAs)是一类特殊的内源性非编码RNA,不具有5′帽和3′poly(A)尾结构,主要由前体mRNA(pre-mRNA)通过可变剪切加工产生,其5′端和3′端通过共价结合形成闭合环状结构。这种特殊的环状结构使其不易被RNA酶降解,从而比线性RNA具有更强的稳定性[3-4]。现已证实,circRNAs可参与基因的转录、转录后及翻译调控,在结直肠癌、肝细胞肝癌、乳腺癌、胰腺导管腺癌等恶性肿瘤中均存在circRNA的表达异常[5]。然而,circ-RNAs在CSCC放疗抵抗中的作用还不十分清楚。本文通过Illumina PE150(Pair end 150 bp)高通量测序技术对3例放疗抵抗及3例放疗敏感CSCC患者肿瘤组织中circRNAs进行检测,筛选差异表达circR-NAs,为CSCC放疗抵抗分子机制的研究奠定基础。
1" "材料与方法
1.1" "材料来源" "放疗抵抗及放疗敏感CSCC组织来自于南通市肿瘤医院就诊患者,组织离体后迅速置于液氮冷冻,24 h后转移至-80 ℃冰箱冻存。所有样本均经病理诊断确认为CSCC。入院前所有患者均未接受放、化疗等治疗,入院后均给予根治性放射治疗。根据实体肿瘤疗效评定标准,将患者分为完全缓解(complete response, CR):病灶消失,无新病灶出现;部分缓解(partial response, PR):病灶最大径之和减少≥30%;进展(progressive disease, PD):病灶最大径之和至少增加≥20%,或出现新病灶;稳定(stable disease, SD):病灶最大径之和缩小未达PR,或增大未达PD。CR+PR为放疗敏感组,SD+PD为放疗抵抗组。6例患者中CR共2例,PR共1例,SD共3例。本研究由南通市肿瘤医院伦理委员会批准,样本的采集经患者知情同意。
1.2" "建库测序" "建库测序委托上海吉凯基因科技有限公司完成。主要流程:(1)RNA提取及完整性检测;(2)采用去除核糖体RNA的方法构建链特异性文库;(3)库检以保证文库质量;(4)Illumina PE150高通量双端测序。
1.3" "CircRNAs差异分析及其来源基因富集分析" "差异circRNAs筛选条件为Plt;0.05、log2(差异倍数)lt;-1或gt;1(组间差异倍数超过2倍)。根据circRNAs与其来源基因的对应关系,对差异表达circRNAs的来源基因的集合分别进行基因本体(Gene Ontology, GO)和京都基因和基因组大百科全书数据库(Kyoto ency-clopedia of genes and genomes, KEGG)富集分析。
1.4" "逆转录定量聚合酶链式反应(quantitative real time-polymerase chain reaction, qRT-PCR)验证" "总RNA提取、逆转录反应及real-time PCR反应试剂盒均购自北京天根公司,按试剂盒说明书操作,PCR仪为ABI 7500。CircRNAs引物购自上海吉凯基因科技有限公司。
2" "结" " " 果
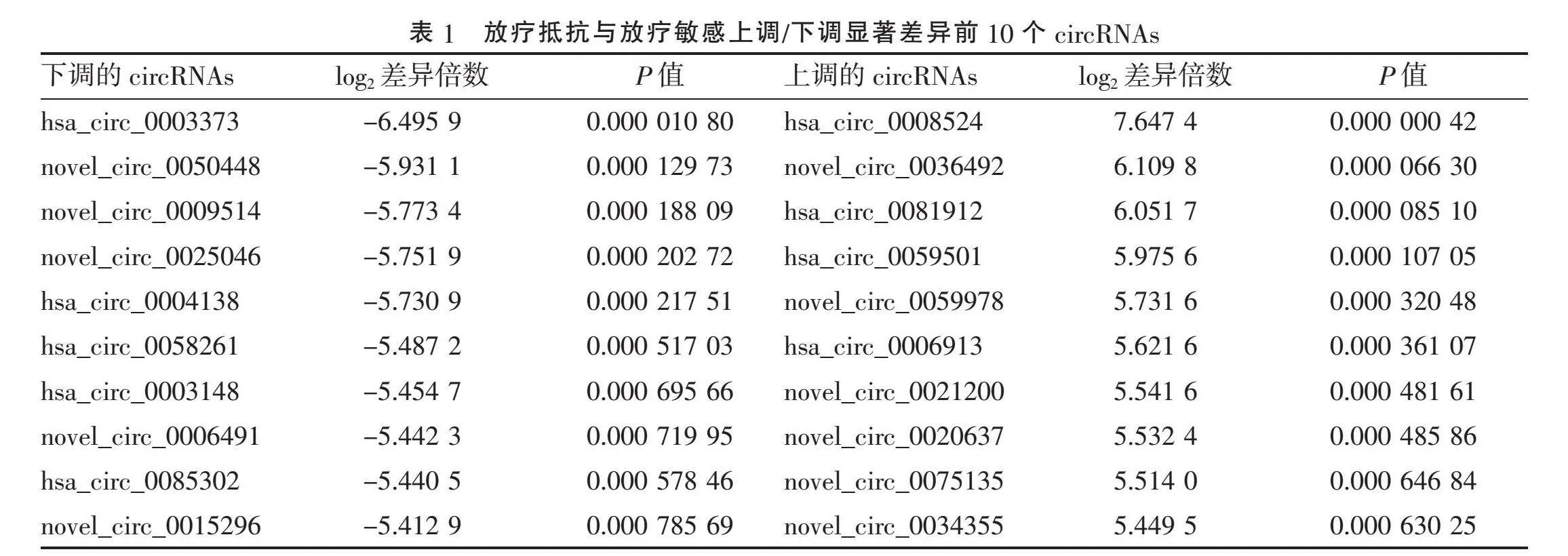
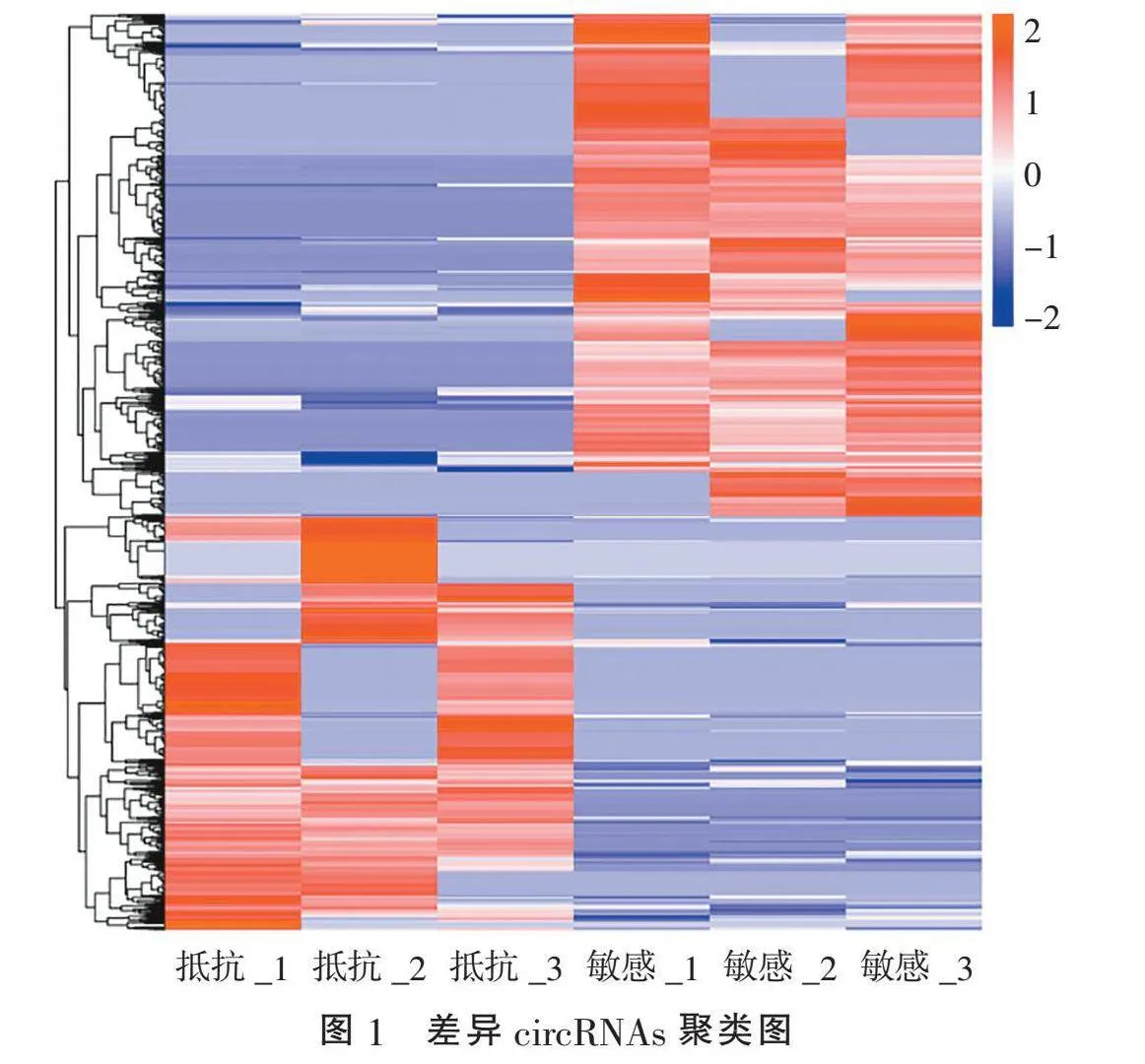
2.1" "CircRNAs差异分析" "通过比较放疗敏感与放疗抵抗CSCC组织circRNAs的表达得到差异circRNAs聚类图(图1)。火山图显示:放疗敏感与放疗抵抗CSCC组织间差异表达在2 倍及以上共有1 316个circRNAs。与放疗敏感CSCC组织相比,放疗抵抗CSCC组织中显著上调的circRNAs共594个,显著下调的circRNAs共722个(图2)。表1列出了上调和下调最明显的10个circRNAs。其中,下调最显著的是hsa_circ_0003373;上调倍数最大的是hsa_circ_0008524,上调倍数为7.647 4。
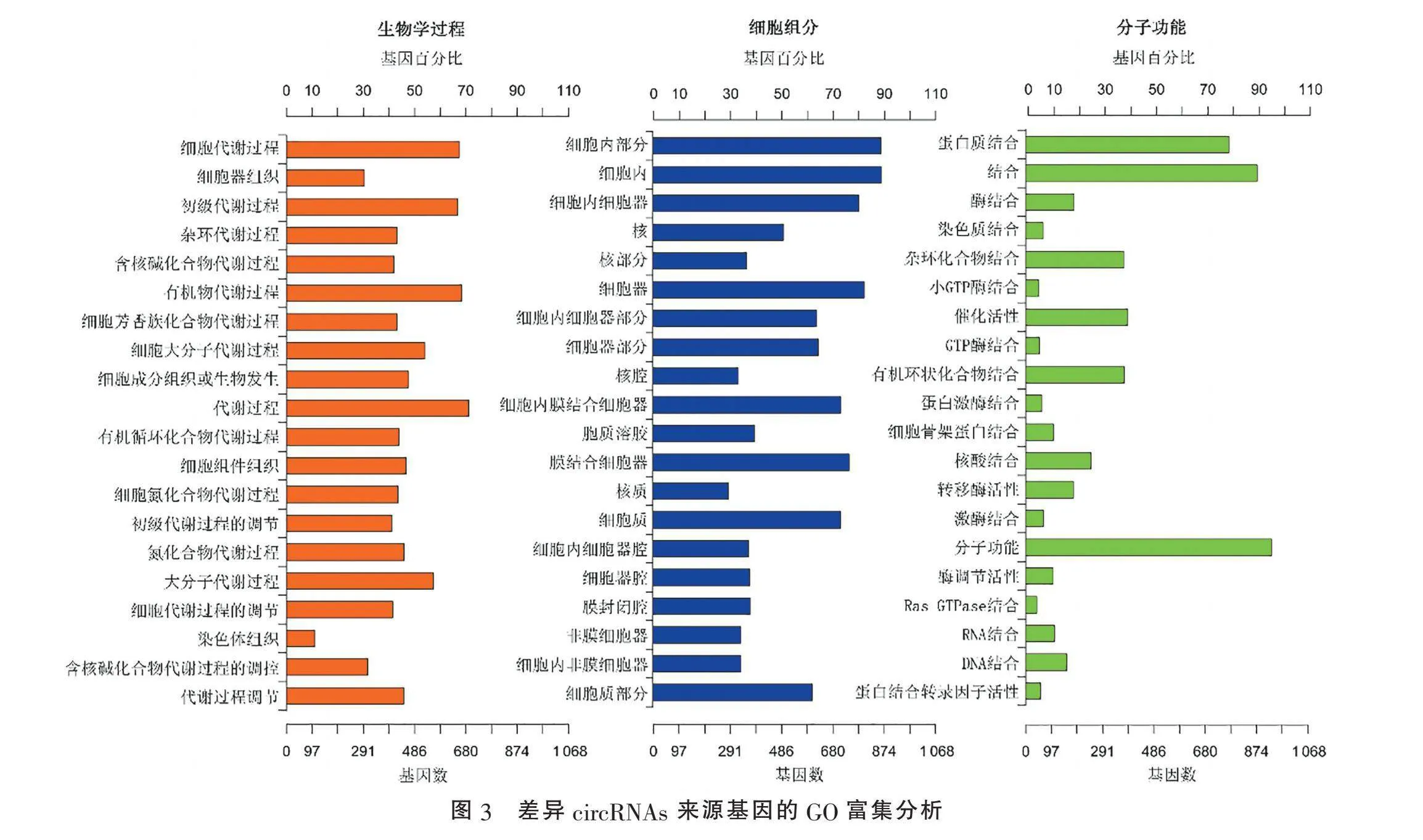
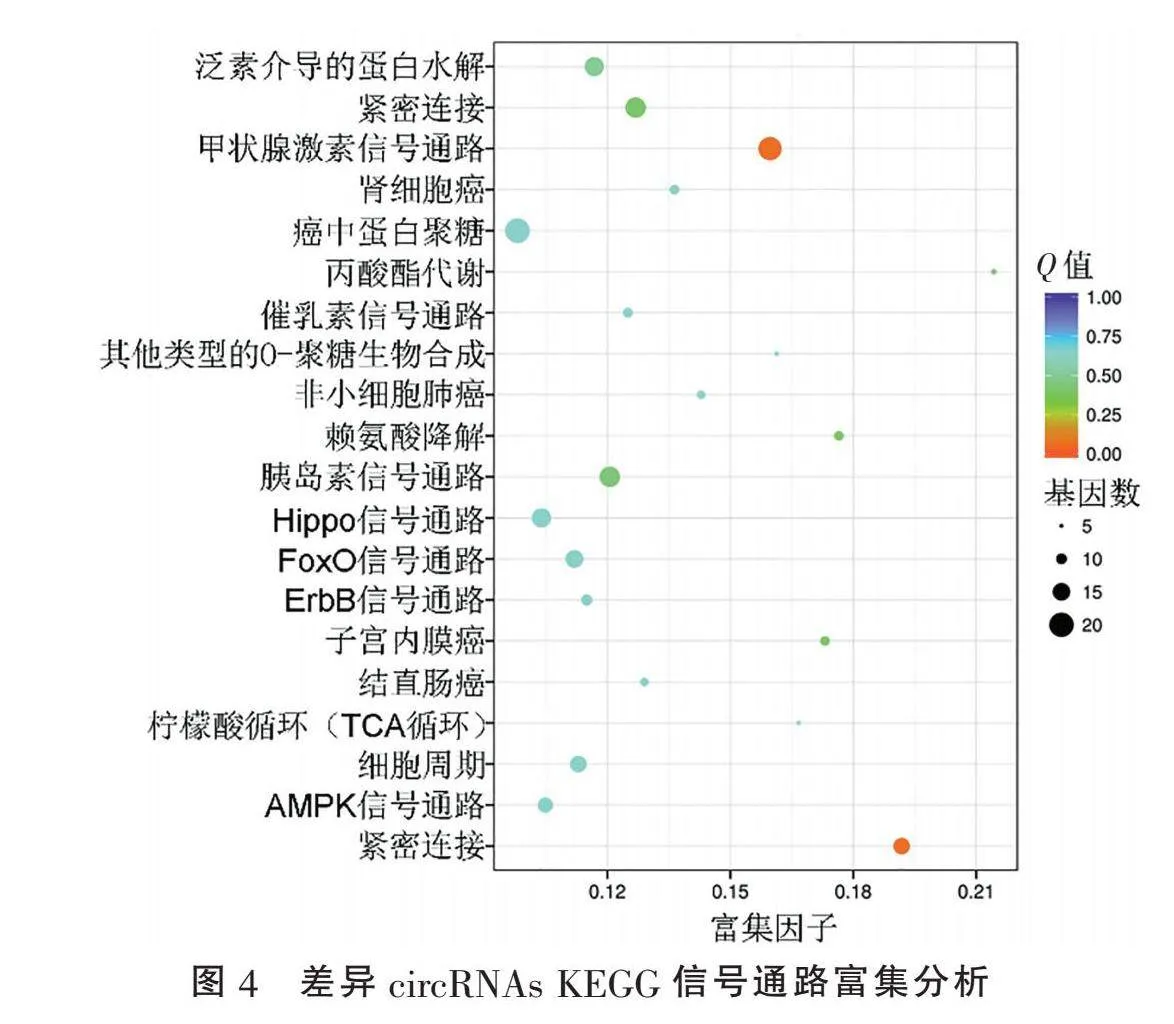
2.2" "差异circRNAs来源基因的GO和KEGG富集分析" "对差异circRNAs来源基因进行GO分析发现:就细胞生物学过程(biological process)而言,差异circRNAs主要富集于细胞代谢过程(cellular metabolic process, GO:0044237)、细胞器组织(organelle organization, GO:0006996)、初级代谢过程(primary metabolic process, GO:0044238)、杂环代谢过程(heterocycle metabolic process, GO:0046483)等;就分子功能(molecular function)而言,差异circRNA主要富集于蛋白质结合(protein binding, GO:0005515)、激酶结合(enzyme binding, GO:0019899)、染色质结合(chromatin binding, GO:0003682)、杂环化合物结合(heterocyclic compound binding, GO:1901363)等(图3)。KEGG信号通路富集分析发现,差异circRNAs富集于甲状腺激素信号通路、Hippo信号通路、FoxO信号通路等通路,但富集不显著(Qgt;0.05)(图4)。
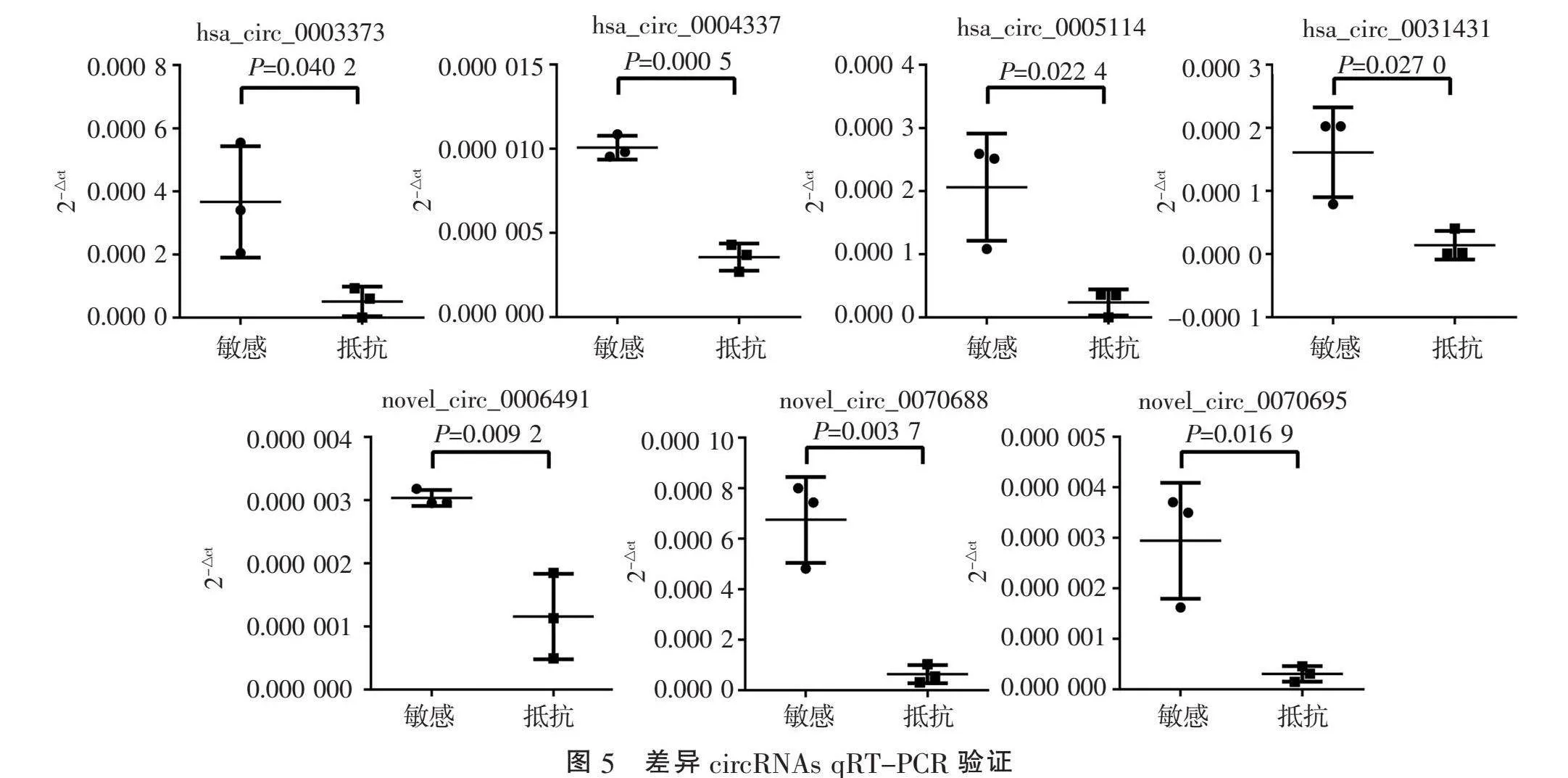
2.3" "差异circRNAs qRT-PCR验证" "对筛选获得的60个差异circRNAs进行qRT-PCR验证发现,与放疗敏感CSCC相比,hsa_circ_0003373、hsa_circ_0004337、hsa_circ_0005114、hsa_circ_0031431、novel_circ_0006491、
novel_circ_0070688、novel_circ_0070695在放疗抵抗CSCC组织中显著下调(均Plt;0.05)(图5)。
3" "讨" " " 论
CircRNAs是一类表达广泛的非编码RNA,其末端共价封闭,长度在100至数千个核苷酸之间。与其他非编码RNA不同,circRNAs在不同物种之间都很保守。CircRNAs主要通过反向剪接而产生,在该过程中,前体RNA的3′和5′末端通过剪接机制切割并连接。由于缺乏5′和3′末端,circRNAs不会被RNA核酸外切酶降解,因此circRNAs非常稳定。CircRNAs可定位在细胞质和细胞核中,也可定位在细胞外的囊泡中[6-8]。许多circRNAs可通过充当microRNAs或蛋白质抑制剂(“海绵”)调节蛋白质功能或翻译,从而发挥重要的生物学功能[9]。
随着高通量测序和分析技术的发展,circRNAs已被发现在卵巢癌、子宫内膜癌、非小细胞肺癌、结肠直肠癌、食管鳞状细胞癌等肿瘤的发展中起重要的调节作用[7, 10]。有研究[11]利用circRNAs芯片对宫颈癌组织中circRNAs的表达进行筛选发现:有45个circRNAs在癌和癌旁组织中的表达差异在4倍以上;其中hsa_circ_0018289在35对宫颈癌组织中的表达显著高于癌旁组织;功能分析显示:敲低hsa_circ_
0018289可显著抑制宫颈癌细胞的增殖、迁移和侵袭。进一步研究证实hsa_circ_0018289通过直接与miR-497结合发挥作用。
D.YU等[12]对经/未经放射处理的Hela细胞高通量测序和生物信息学分析发现:与未经放射处理组相比,放射处理组有153个差异表达的circRNAs,其中上调76个,下调77个。本研究显示放疗敏感与放疗抵抗CSCC组织间差异表达在2倍及以上共有1 316个circRNAs。与放疗敏感CSCC组织相比,放疗抵抗CSCC组织中显著上调的circRNAs共594个,显著下调的circRNAs共722个。其中,下调最显著的是hsa_circ_0003373,上调最显著的是hsa_circ_0008524。D.YU等[12]通过GO分析发现:就生物学过程而言,主要涉及肽基酪氨酸去磷酸化和细胞迁移的调控;就细胞组分而言,主要涉及细胞间黏附连接,核浆和胞浆;就分子功能而言,主要涉及蛋白质结合和蛋白酪氨酸磷酸酶活性。本研究通过对差异circRNAs来源基因进行GO分析发现:就细胞生物学过程而言,差异circRNAs主要富集于细胞代谢过程、细胞器组织、初级代谢过程、杂环代谢过程等;就分子功能而言,差异circRNAs主要富集于蛋白质结合、激酶结合、染色质结合、杂环化合物结合等。现已证实,糖酵解、氧化磷酸化、脂肪酸氧化等代谢途径在肿瘤放疗敏感性的调控中发挥重要作用,如靶向代谢重编程可为鼻咽癌放疗增敏提供新的策略[13]。因此,靶向代谢相关circRNAs可为CSCC放疗抵抗提供新的思路。D.YU等[12]通过KEGG富集分析发现Hela细胞放疗耐受主要涉及MAPK信号通路、内吞作用、轴突引导、神经营养因子信号通路等。本研究通过KEGG信号通路富集分析发现,差异circRNAs主要富集于甲状腺激素信号通路、Hippo信号通路、FoxO信号通路等通路。近期,Z.L.LI等[14]报道,USP21可通过去泛素化FOXM1调控Hippo信号通路,进而促进宫颈癌放疗抵抗,与本研究KEGG信号通路富集分析结果相吻合。提示Hippo信号通路可能是CSCC放疗抵抗的重要靶点。值得注意的是,尽管KEGG富集分析显示Qgt;0.05,但上述通路是否具有生物学意义还需后续功能实验确认。
本研究对筛选获得的60个差异circRNAs进行qRT-PCR验证发现,与放疗敏感CSCC相比,hsa_
circ_0003373、hsa_circ_0004337、hsa_circ_0005114、hsa_circ_0031431、novel_circ_0006491、novel_circ_
0070688、novel_circ_0070695在放疗抵抗CSCC组织中显著下调。目前,上述circRNAs在肿瘤中的作用报道较少。研究[15]发现,hsa_cir_0005114可能通过影响胰岛素分泌途径参与胶质瘤的发生、发展。除了胰岛素分泌机制外,hsa_cir_0005114还可能通过上调miR-142-3p和miR-590-5p发挥肿瘤抑制功能,从而抑制抗原呈递细胞的表达。上述circRNAs在宫颈癌放疗抵抗中的具体作用未见报道,还需后续细胞生物学实验进一步分析。
[参考文献]
[1]" "刘红, 房朝晖, 张倩影, 等. CD44+/CD24+宫颈癌细胞和耐辐射宫颈癌细胞特性研究[J]. 中华放射医学与防护杂志, 2018, 38(2):87-92.
[2]" "施璠, 汪涛, 王娟, 等. 宫颈癌组织中Survivin、UHRF1 mRNA表达及其与宫颈癌放疗敏感性的相关性[J]. 西安交通大学学报(医学版), 2020, 41(1):23-26.
[3]" "张春梅, 杨清. 环状RNA在妇科恶性肿瘤中的研究进展[J]. 现代肿瘤医学, 2019, 27(7):1234-1237.
[4]" "王思远, 张国英. 环状RNA在子痫前期发病机制和预测中的研究进展[J]. 现代妇产科进展, 2019, 28(8):634-636.
[5]" "ZHANG H D, JIANG L H, SUN D W, et al. CircRNA: a novel type of biomarker for cancer[J]. Breast Cancer, 2018, 25(1):1-7.
[6]" "PEREZ DE ACHA O, ROSSI M, GOROSPE M. Circular RNAs in blood malignancies[J]. Front Mol Biosci, 2020, 7:109.
[7]" "朱亭佳, 王新宇. 环状RNA在妇科恶性肿瘤中的研究进展[J]. 现代妇产科进展, 2020, 29(6):463-466.
[8]" "刘欢妹, 靖长友, 张曙光, 等. 环状RNA在肿瘤领域中的研究进展[J]. 癌症进展, 2019, 17(22):2612-2616.
[9]" "KRISTENSEN L S, ANDERSEN M S, STAGSTED L V W, et al. The biogenesis, biology and characterization of circular RNAs[J]. Nat Rev Genet, 2019, 20(11):675-691.
[10]" "GONG J, JIANG H, SHU C, et al. Integrated analysis of circular RNA-associated ceRNA network in cervical cancer: Observational Study[J]. Medicine, 2019, 98(34):e16922.
[11]" "GAO Y L, ZHANG M Y, XU B, et al. Circular RNA exp-ression profiles reveal that hsa_circ_0018289 is up-regulated in cervical cancer and promotes the tumorigenesis[J]. Oncotarget, 2017, 8(49):86625-86633.
[12]" "YU D, LI Y F, MING Z H, et al. Comprehensive circular RNA expression profile in radiation-treated HeLa cells and analysis of radioresistance-related circRNAs[J]. PeerJ, 2018, 6:e5011.
[13]" "陈小慧, 黄栗盛, 刘林, 等. 鼻咽癌代谢重编程与放疗抵抗[J]. 肿瘤药学, 2022, 12(3):287-294.
[14]" "LI Z L, LIU X J, YU H Z, et al. USP21 regulates Hippo signaling to promote radioresistance by deubiquitinating FOXM1 in cervical cancer[J]. Hum Cell, 2022, 35(1):333-347.
[15]" "WEI B, WANG L, ZHAO J W. Circular RNA hsa_circ_
0005114-miR-142-3p/miR-590-5p-adenomatous polyposis coli protein axis as a potential target for treatment of glioma[J]. Oncol Lett, 2021, 21(1):58.
[收稿日期] 2022-11-15

