罗汉果甜苷Ⅲ延缓猪卵母细胞体外老化的作用机制






摘要:【目的】验证罗汉果甜苷Ⅲ是否具有延缓猪卵母细胞体外老化进程的功效,为采用植物提取物延缓卵母细胞体外老化提供参考依据。【方法】以体外培养44 h获得的猪卵母细胞为研究对象,通过孤雌激活筛选出罗汉果甜苷Ⅲ对猪卵母细胞的有效作用浓度;然后设新鲜卵母细胞(Fresh)、老化卵母细胞(Aging)及老化卵母细胞添加罗汉果甜苷Ⅲ(MogⅢ)3个处理组,采用免疫荧光染色和实时荧光定量PCR等分别检测猪卵母细胞的线粒体数量和分布、腺嘌呤核苷三磷酸(ATP)含量、线粒体膜电位、活性氧(ROS)水平、DNA损伤、抗氧化相关基因表达及早期凋亡水平等指标。【结果】老化导致猪卵母细胞孤雌激活后的胚胎分裂率及囊胚发育率极显著下降(P<0.0 下同),而添加罗汉果甜苷Ⅲ能有效提高老化卵母细胞孤雌激活后的胚胎分裂率和囊胚发育率,尤其以添加25.0µmol/L罗汉果甜苷Ⅲ的效果最优,故确定25.0µmol/L为罗汉果甜苷Ⅲ的有效作用浓度。与Fresh组猪卵母细胞相比,Aging组猪卵母细胞线粒体荧光强度显著下降(P<0.05,下同)、ATP含量极显著下降、线粒体膜电位显著下降、抗氧化相关基因相对表达量极显著下降,而ROS水平、γ-H2AX荧光强度和Annexin-V阳性率均极显著上升;但添加25.0µmol/L罗汉果甜苷Ⅲ后上述问题均得到有效改善,即罗汉果甜苷Ⅲ能有效缓解猪卵母细胞的老化进程。【结论】罗汉果甜苷Ⅲ通过改善老化猪卵母细胞线粒体数量和ATP含量的异常、恢复ROS平衡、上调抗氧化相关基因表达,以及缓解猪卵母细胞老化引起的DNA损伤和早期凋亡,而有效延缓猪卵母细胞的老化进程,进而提高猪卵母细胞的体外发育能力。
关键词:猪;卵母细胞;老化;罗汉果甜苷Ⅲ;氧化应激
中图分类号:S828.3文献标志码:A文章编号:2095-1191(2024)07-1949-12
Mechanism effects of mogrosideⅢon the alleviation of porcine oocyte aging
ZHAO Lei,ZENG Xiao-qiao,GAO Zu-wei,WU Shi-yao,PENG Ke,YAN Yu-jun,WAN Run-tian,LIANG Xing-wei*
(College of Animal Science and Technology,Guangxi University/Guangxi Key Laboratory of Animal Breeding&Disease Control and Prevention,Nanning,Guangxi 530004,China)
Abstract:【Objective】The study aimed to verify whether mogrosideⅢhad the effects to delay the aging process of porcine oocytes in vitro and provide reference for utilizing plant extracts to alleviate oocyte aging in vitro.【Method】Por-cine oocytes cultured in vitro for 44 h were used as research subjects.The effective concentration of mogrosideⅢfor por-cine oocytes was screened by parthenogenetic activation(PA).Three treatment groups were set up:fresh oocytes(Fresh),aging oocytes(Aging),and aging oocytes supplemented with mogrosideⅢ.Immunofluorescence staining andreal-time fluorescence quantitative PCR were employed to detect mitochondrial content and distribution,adenosine tri-phosphate(ATP)content,mitochondrial membrane potential,reactive oxygen species(ROS)levels,DNA damage,antioxidant-related gene expression,and early apoptosis levels in porcine oocytes.【Result】Aging extremely significantly decreased the embryonic cleavage rate and blastocyst development rate of parthenogenetic activated embryos derived from porcine oocytes(P<0.0 the same below).The addition of mogrosideⅢeffectively improved the embryonic cleavagerate and blastocyst development rate of aging oocytes,especially at 25.0µmol/L.which was therefore determined as the effective concentration for mogrosideⅢ.Compared with the Fresh group,the Aging group exhibited significant de-crease in mitochondrial fluorescence intensity(P<0.05,the same below),and extremely significant decrease in ATP con-tent,significant decrease in mitochondrial membrane potential,and extremely significant decrease in the relative expres-sion levels of antioxidant-related genes,while ROS levels,γ-H2AX fluorescence intensity,and Annexin-V positive rate were extremely significantly increased.However,the addition of 25.0µmol/L mogrosideⅢeffectively improved the above problems,indicating that mogrosideⅢcould effectively alleviate the aging process of porcine oocytes.【Conclu-sion】MogrosideⅢeffectively delays the aging process of porcine oocytes by improving mitochondrial number and ATP content,restoring ROS balance,up-regulating the expression of antioxidant-related genes,and alleviating DNA damage and early apoptosis caused by the aging of porcine oocytes,thereby enhancing the in vitro developmental competence of porcine oocytes.
Key words:pigs;oocytes;aging;mogrosideⅢ;oxidative stress
Foundation items:General Project of National Natural Science Foundation of China(32372881);Regional Project of National Natural Science Foundation of China(82160287)
0引言
【研究意义】成熟卵母细胞排出后,若在特定窗口期内没有完成受精则会迅速老化,导致受精率和发育潜能急剧下降,是卵母细胞利用率降低的重要原因之一。罗汉果(Siraitiagrosvenori)为葫芦科(Cucurbitaceae)藤本植物,原产于我国南部,能提取分离出罗汉果甜苷(Ⅱ、Ⅲ和Ⅴ)等生物活性成分(Xiao et al.,2020;Liu et al.,2021;Zhang et al.,2021;刘嘉昊等,2022)。已有研究表明,罗汉果甜苷Ⅴ能有效延缓猪卵母细胞老化及缓解生殖衰老(Nie et al.,2018;Du etal.,2022),但有关罗汉果甜苷Ⅲ在雌性生殖领域的功效尚未明确。因此,明确罗汉果甜苷Ⅲ对猪卵母细胞老化的延缓作用,有助于深入探索罗汉果生物活性成分的功效,充分挖掘其未知生理作用及拓宽罗汉果在雌性生殖领域的应用研究。【前人研究进展】卵母细胞老化会影响卵母细胞质量及其发育潜力(Czajkowska and Ajduk,2023),不利于植入前胚胎和植入后胎儿的发育(Tarínet al.,2000;Sasaki et al.,2019;Xu et al.,2024),甚至导致后代出现健康问题(Tarínet al.,2002)。在老化过程中,卵母细胞通常出现线粒体功能障碍、端粒缩短、黏连蛋白功能失调、纺锤体不稳定、线粒体膜电位下降及腺嘌呤核苷三磷酸(ATP)含量降低等异常现象(Wilding et al.,2001;Igarashi et al.,2005;Cimadomoetal.,2018)。此外,老化能诱导线粒体DNA(mtDNA)氧化损伤(Chappel,2013),还导致卵母细胞非整倍体增加(Eichenlaub-Ritter,2012;Ben-tovand Casper,2013)。卵母细胞在老化过程中会出现细胞质片段化(Chen et al.,2022)和早期细胞凋亡(Takahashi et al.,2011;Stringer et al.,2023;Wen et al.,2023),也会出现去甲基化或三甲基化水平降低等表观修饰的变化(Lianget al.,2008;Hou and Sun,2020;Petri etal.,2020)。卵母细胞老化过程导致细胞内的活性氧(ROS)过度积累,打破氧化与抗氧化间的平衡,引发氧化应激而破坏许多细胞成分,包括线粒体、脂质、蛋白、酶和DNA等(Redza-Dutordoir and Averill-Bates,2016;Sasaki et al.,2019)。氧化应激是卵巢功能随年龄增长而衰退的关键因素(Wang et al.,2020),可作用于相关信号通路而促使卵母细胞衰老(Yang et al.,2020;Xing et al.,2021)。因此,通过抗氧化措施来抑制氧化应激,能有效缓解卵母细胞的老化损伤(Cimadomo et al.,2018;Xu et al.,2024)。已有研究表明,槲皮素处理减少了衰老引起的卵母细胞形态变化和ROS积累,可缓解卵母细胞质量的下降并改善胚胎发育(Wang et al.,2017);添加抗氧化物质能促使老化卵母细胞的线粒体活性升高,并降低纺锤体和染色体的畸形率(Xian et al.,2018);褪黑激素可提高卵母细胞质量(Zhang et al.,2022),改善排卵后卵母细胞的功能障碍,延缓老化并延长其体外最佳受精的窗口期(Lord et al.,2013;Yang et al.,2018);左旋肉碱可通过减轻氧化应激来提高体外老化卵母细胞的胚胎发育能力(Jiang et al.,2020);补充阿魏酸可减少体外衰老诱导的DNA损伤,维持DNA稳定性,而有利于保持牛卵母细胞的质量(Yin et al.,2023)。可见,部分抗氧化作用物质能通过抗氧化措施减轻氧化应激,具有延缓卵母细胞老化进程的潜力,但并非所有的抗氧化物质均发挥类似作用(Suttirojpattana et al.,2017)。源自药用植物的抗氧化剂具有出色的抗氧化应激能力,可作用于多种疾病,包括卵巢衰老(Yang et al.,2020)。罗汉果提取物具有强抗氧化性,尤其是罗汉果甜苷Ⅴ能降低老化卵母细胞内的ROS水平,减轻早期凋亡和DNA损伤,有效保护猪卵母细胞免受热应激的损伤(Nie et al.,2024;Peng et al.,2024);或通过促进SIRT1基因上调表达而延缓猪卵母细胞的体外老化进程(Nie et al.,2019)。除了罗汉果甜苷Ⅴ外,罗汉果提取物中还含有其他成分具有抗氧化作用。其中,罗汉果甜苷Ⅲ是罗汉果中的主要甜味成分,分子式为C48H82O19,属于蒽醌甙类化合物,具有高甜度、低热量的特点,其甜度相当于蔗糖的200倍左右。罗汉果甜苷Ⅲ可通过激活AMPK-SIRT1信号通路而缓解高糖诱导的塞托利细胞炎症和氧化应激反应(Xue et al.,2020),还能通过抑制TLR4/MyD88信号和炎症细胞因子释放以抵抗心肌纤维化(Shi et al.,2023)。【本研究切入点】罗汉果甜苷作为一种绿色无毒害的人工非糖类甜味剂,具有保护肝脏(王勤和肖刚,2007;陈薇等,2010)、抗肿瘤抗病毒(Chen et al.,2019;Luo et al.,2022)及抗炎症(Xiao et al.,2020;Liu et al.,2021;Zhang et al.,2021)等生物学功效,其中,罗汉果甜苷Ⅴ在改善雌性生殖机能及提高家畜繁殖力方面具有明显优势(Nie et al.,2020;Du etal.,2022),而罗汉果甜苷Ⅲ是否具有类似功效还需进一步探究。【拟解决的关键问题】在猪卵母细胞体外老化过程中添加不同浓度的罗汉果甜苷Ⅲ,分别检测卵母细胞线粒体数量与分布、ATP含量、膜电位、ROS水平、抗氧化相关基因表达变化、DNA损伤及早期凋亡水平等指标,验证罗汉果甜苷Ⅲ是否具有延缓猪卵母细胞体外老化进程的功效,为采用植物提取物延缓卵母细胞体外老化提供参考依据。
1材料与方法
1.1试验材料
从广西南宁市当地屠宰场获取新鲜猪卵巢,装入盛有32~34℃无菌生理盐水的保温瓶,2 h内送回实验室。采用注射器进行抽卵,并挑选健康的卵丘卵母细胞复合体(COCs)置于CO2培养箱(38.5℃,5%CO 最大湿度)中培养44h,以获得成熟后的猪卵母细胞。透明质酸酶、聚乙烯醇(PVA)、牛血清白蛋白(BSA)、DNA染料(Hoechst 33342)及羊毛脂购自美国Sigma公司;罗汉果甜苷Ⅲ(纯度99.11%)购自成都埃法生物科技有限公司;线粒体检测荧光探针(Mito Tracker)购自美国Invitrogen公司;TCM-199培养基购自美国Gibco公司;二甲基亚砜(DMSO)、4%多聚甲醛(4%PFA)、曲拉通X-100(Triton X-100)、吐温-20(Tween-20)及抗荧光衰减封片剂购自北京索莱宝生物科技有限公司;FITC标记山羊抗小鼠IgG(H+L)购自北京中杉金桥生物技术有限公司;RNA提取试剂盒购自天根生化科技(北京)有限公司;Premix型反转录试剂(PrimeScriptTM RT Master Mix)、高特异性qPCR试剂(TB Green Premix Ex TaqTMⅡ)及实时荧光定量PCR预混液(SYBRⅡMix)购自日本TaKaRa公司;cDNA第一链合成试剂盒购自美国Bio-Rad公司;DNA损伤抗体(Anti-γ-H2AX)购自英国Abcam公司;ROS检测试剂盒、增强型ATP检测试剂盒、线粒体活性检测试剂盒、膜电位检测试剂盒及细胞凋亡检测试剂盒购自上海碧云天生物技术股份有限公司。
1.2试验设计
COCs成熟培养44 h后,将其转移至1.8 mL洗卵液中,加入200µL 0.1%透明质酸酶,用200µL移液枪去除卵母细胞周围的卵丘细胞,此时猪卵母细胞开始老化。以DMSO溶解罗汉果甜苷Ⅲ制备100 mmol/L的原液,-20℃保存备用。每次老化处理前,将罗汉果甜苷Ⅲ原液添加至TCM-199培养基中使其试验浓度分别为12.5、25.0和50.0µmol/L。将成熟后的猪卵母细胞分别转移到含罗汉果甜苷Ⅲ(MogⅢ组)或不含罗汉果甜苷Ⅲ(Aging组)的培养基中继续24h,将新鲜猪卵母细胞设为Fresh组。各处理组培养基的DMSO终浓度均低于0.1%。试验经广西大学实验动物伦理与使用委员会(IACUC)批准,批准号GXU-2023-0049。
1.3测定指标及方法
1.3.1胚胎分裂率、囊胚发育率及有效作用浓度在各处理组中分别挑选出形态良好的猪卵母细胞进行孤雌激活(电压1.2 kV/cm,脉冲时间30µs,2次连续直流电脉冲),电激活后将胚胎清洗并置于38.5℃、5%CO2的培养箱中继续培养,培养48和144h后通过体视显微镜观察胚胎发育情况,分别统计胚胎分裂率和囊胚发育率,同时使用倒置显微镜拍摄明场典型图像。选择作用效果最显著的浓度作为有效作用浓度,后续试验均按有效作用浓度进行处理。
1.3.2线粒体数量及分布使用荧光探针(Mito Tracker)对猪卵母细胞的线粒体进行标记及观察统计。将各处理组猪卵母细胞置于预热的0.1%PVA-DBPS中清洗3次,向培养皿中添加200 nmol/L荧光探针并转移至培养箱(38.5℃、5%CO2)中染色30 min,再次将猪卵母细胞置于0.1%PVA-DBPS中清洗3次。于培养皿中滴加35µL缓冲液制成微滴,每组取10枚猪卵母细胞置于同一微滴中,通过荧光显微镜进行观察并拍摄。
1.3.3 ATP含量利用增强型ATP检测试剂盒检测猪卵母细胞中的ATP含量,以检验线粒体功能。挑选出处于MII期且卵丘细胞完全脱离的猪卵母细胞,置于预热的0.1%PVA-DBPS中清洗3次。按100枚猪卵母细胞为一组装入1.5 mL EP管中,液氮速冻后-80℃保存。使用裂解液对ATP标准液进行梯度稀释以确定标准曲线,按1∶4的比例将稀释后的溶液制成混合工作液;向各处理组EP管中添加160µL裂解液,离心制备样品;然后按使用说明,采用发光检测仪检测相对光单位(RLU)并计算各处理组猪卵母细胞中的ATP含量。
1.3.4线粒体膜电位使用膜电位检测试剂盒检测猪卵母细胞线粒体内膜的膜电位,以评估线粒体功能。将各处理组猪卵母细胞置于预热的0.1%PVA-DBPS中清洗3次,分组后转移至浓度为10µmol/L的JC-1染液(以成熟液稀释JC-1探针)中,置于培养箱(38.5℃、5%CO2)中染色30 min,再次将猪卵母细胞清洗3次。在共聚焦培养皿中滴加35µL缓冲液制成微滴,将清洗好的猪卵母细胞转移至微滴中,使用激光共聚焦显微镜进行观察。
1.3.5 ROS水平使用ROS检测试剂盒检测猪卵母细胞内的ROS水平。猪卵母细胞染色前以预热的0.1%PVA-DBPS清洗3次,按1∶1000的比例用0.1%PVA-DBPS将10 mmol/L二氯荧光素二乙酸(DCFH-DA)稀释至10µmol/L,各处理组取60~70枚猪卵母细胞放入稀释后的DCFH-DA溶液中,置于培养箱(38.5℃、5%CO2)中染色30 min,再次将猪卵母细胞清洗3次。在培养皿中滴加35µL缓冲液制成微滴,每处理组取10枚猪卵母细胞置于同一微滴中,通过荧光显微镜进行观察拍摄,并统计荧光强度。
1.3.6抗氧化相关基因表达情况按照RNA提取试剂盒使用说明,从100个猪卵母细胞样品(每处理组3个重复)中提取总RNA;使用PrimeScriptTM RT Master Mix将提取的10µL RNA(相当于100个猪卵母细胞)反转录合成cDNA第一链,然后进行实时荧光定量PCR检测。反转录合成体系20µL:5×PrimeScriptTM RT Master Mix 4µL,RNA模板10µL,无RNase蒸馏水6µL。反转录程序:37℃15 min,85℃5 s,4℃冷却。实时荧光定量PCR反应体系20µL:TB Green Premix Ex TaqTM II 10µL,cDNA模板2µL(相当于10个猪卵母细胞),上、下游引物(浓度2µmol/L)各2µL,ddH2O 4µL。在Bio-Rad CFX96仪器中进行扩增,扩增程序:95℃预变性30 s;95℃5 s,60℃30 s,进行44个循环。以GAPDH为内参基因,采用2-ΔΔCt法计算目的基因相对表达量。实时荧光定量PCR扩增引物序列信息如表1所示。
1.3.7 DNA损伤使用DNA损伤抗体和FITC标记山羊抗小鼠IgG(H+L)进行免疫荧光染色,以评估猪卵母细胞中DNA双链断裂水平。将固定液(4%PFA)制成100µL的微滴,各处理组猪卵母细胞经预热的0.1%PVA-DBPS清洗3次后,转移至固定液微滴中室温固定30min,再次将猪卵母细胞清洗3次。配制400µL透膜液(1%Triton X-100+PBS)并注入4孔细胞板,将清洗后的猪卵母细胞转移至4孔细胞板,置于湿盒(4℃)中透膜10 h,再次清洗。配制400µL封闭液(含1%BSA的DPBS)并注入4孔细胞板,将透膜后的猪卵母细胞放入4孔细胞板中并湿盒室温封闭1h。将DNA损伤抗体(一抗)和FITC标记山羊抗小鼠IgG(H+L)(二抗)与封闭液按1∶300稀释比例分别制成100µL的一抗微滴和二抗微滴,猪卵母细胞移至一抗微滴并湿盒(4℃)孵育10h,然后转入抗体清洗液(0.01%Tween-20+0.01%Tri-ton X-100+PBS)中清洗3次;再移至二抗微滴并湿盒室温孵育30 min,孵育后的猪卵母细胞以抗体清洗液清洗3次。DNA染料(Hoechst 33342)以缓冲液按1∶1000稀释比例制成10µg/mL的工作染液,抗体孵育后的猪卵母细胞以工作染液核染15 min,核染后重复清洗。将核染后的猪卵母细胞转移至均匀涂抹有2µL抗荧光衰减封片剂的载玻片上,利用羊毛脂进行压片操作,并以透明指甲油封片,通过激光共聚焦显微镜进行观察拍摄,并统计荧光强度。
1.3.8早期凋亡水平采用细胞凋亡检测试剂盒对猪卵母细胞的早期凋亡水平进行评估。各处理组猪卵母细胞用预热的0.1%PVA-DBPS清洗3次,随机分组后转移至Annexin-V-FITC探针按1∶9稀释比例制成的100µL微滴中,置于湿润培养箱(38.5℃、5%CO2)中染色30 min,再次将猪卵母细胞清洗3次。在共聚焦培养皿中滴加35μL缓冲液制成微滴,将染色后的猪卵母细胞移至微滴中,通过激光共聚焦显微镜进行观察拍摄。
1.4统计分析
使用Image J采集荧光强度,并以GraphPad Prism 9.0对所有试验数据进行单因素方差分析(One-way ANOVA)及制图。
2结果与分析
2.1罗汉果甜苷Ⅲ对老化猪卵母细胞后续胚胎发育能力的影响
胚胎发育能力是评价卵母细胞质量的关键指标,而胚胎分裂和囊胚发育是胚胎发育能力的主要体现(Nie et al.,2019;Zilberberg et al.,2021)。各处理组猪卵母细胞孤雌激活后的胚胎分裂率和囊胚发育率如图1所示。与Fresh组的胚胎分裂率[(94.29±2.77)%]相比,Aging组的胚胎分裂率[(13.13±2.67)%]极显著下降(r<0.0 下同),但添加不同浓度的罗汉果甜苷Ⅲ能有效提高胚胎分裂率,以添加25.0µmol/L罗汉果甜苷Ⅲ的效果最优,胚胎分裂率极显著回升到(55.00±3.27)%。此外,与Fresh组的囊胚发育率[(31.67±3.07)%]相比,Aging组的囊胚发育率[(0.80±0.80)%]也极显著下降,添加25.0µmol/L罗汉果甜苷Ⅲ能极显著提高囊胚发育率[(10.00±1.58)%]。综上所述,老化导致猪卵母细胞孤雌激活后的胚胎分裂率及囊胚发育率极显著下降,而添加罗汉果甜苷Ⅲ能有效提高老化卵母细胞孤雌激活后的胚胎分裂率和囊胚发育率,尤其以添加25.0µmol/L罗汉果甜苷Ⅲ的效果最优,故确定25.0µmol/L为罗汉果甜苷Ⅲ的有效作用浓度。
2.2罗汉果甜苷Ⅲ对老化猪卵母细胞线粒体及ATP含量的影响
线粒体是细胞产生ATP的场所,与细胞能量供应密切相关,是评价卵母细胞质量的重要指标之一(van der Reest et al.,2021;Yu et al.,2022)。由图2可看出,与Fresh组猪卵母细胞相比,Aging组猪卵母细胞的线粒体荧光强度(18.84±0.97)显著下降(P<0.05,下同),但添加25.0µmol/L罗汉果甜苷Ⅲ能显著提高猪卵母细胞线粒体荧光强度(22.99±1.12)。此外,与Fresh组猪卵母细胞相比,Aging组猪卵母细胞的ATP含量极显著下降,而添加25.0µmol/L罗汉果甜苷Ⅲ能显著增加猪卵母细胞的ATP含量。说明罗汉果甜苷Ⅲ可改善卵母细胞老化导致的线粒体数量异常和ATP含量异常,即罗汉果甜苷Ⅲ可延缓猪卵母细胞因老化引起的线粒体数量减少和功能衰退。
2.3罗汉果甜苷Ⅲ对老化猪卵母细胞线粒体膜电位的影响
膜电位直接影响卵母细胞线粒体的产ATP能力,也是评估卵母细胞质量的重要指标之一(Yuet al.,2022;Zhou et al.,2022)。由图3可看出,与Fresh组猪卵母细胞相比,Aging组猪卵母细胞线粒体膜电位(1.33±0.03)显著下降,而添加25.0µmol/L罗汉果甜苷Ⅲ后,猪卵母细胞线粒体膜电位极显著回升至1.58±0.03。说明罗汉果甜苷Ⅲ可改善老化所致的线粒体膜电位异常,即罗汉果甜苷Ⅲ可延缓猪卵母细胞老化所引起的线粒体功能衰退。
2.4罗汉果甜苷Ⅲ对老化猪卵母细胞ROS水平及抗氧化相关基因表达的影响
在卵母细胞中,ROS为细胞代谢的正常产物,其含量保持相对稳态;但在卵母细胞老化期间,ROS不断积累而造成氧化应激,因此ROS水平也是评估卵母细胞质量的重要指标之一(Prasad et al.,2016)。由图4-A和图4-B可看出,与Fresh组猪卵母细胞相比,Aging组猪卵母细胞的ROS水平(35.50±2.58)极显著升高,但添加25.0µmol/L罗汉果甜苷Ⅲ能极显著降低老化猪卵母细胞的ROS水平(22.52±1.42)。进一步检测与氧化应激相关的抗氧化基因(SOD、CAT和GPX)在老化猪卵母细胞中的表达情况,结果(图4-C)显示,与Fresh组猪卵母细胞相比,Aging组猪卵母细胞的抗氧化相关基因相对表达量均极显著下降,而添加25.0µmol/L罗汉果甜苷Ⅲ能极显著提升抗氧化相关基因的相对表达量。综上所述,罗汉果甜苷Ⅲ能有效延缓卵母细胞老化后ROS积累导致的氧化应激,并上调抗氧化相关基因的表达。
2.5罗汉果甜苷Ⅲ对老化猪卵母细胞DNA损伤的影响
卵母细胞老化引起的氧化应激可导致细胞DNA损伤,进而影响卵母细胞发育(Turan and Oktay,2020)。由图5可看出,与Fresh组猪卵母细胞相比,Aging组猪卵母细胞的γ-H2AX荧光强度(174.10±8.23)极显著增强,添加25.0µmol/L罗汉果甜苷Ⅲ后能极显著降低老化猪卵母细胞的γ-H2AX荧光强度(121.6±11.57)。说明罗汉果甜苷Ⅲ可有效缓解猪卵母细胞老化引起的DNA损伤。
2.6罗汉果甜苷Ⅲ对老化猪卵母细胞早期凋亡水平的影响
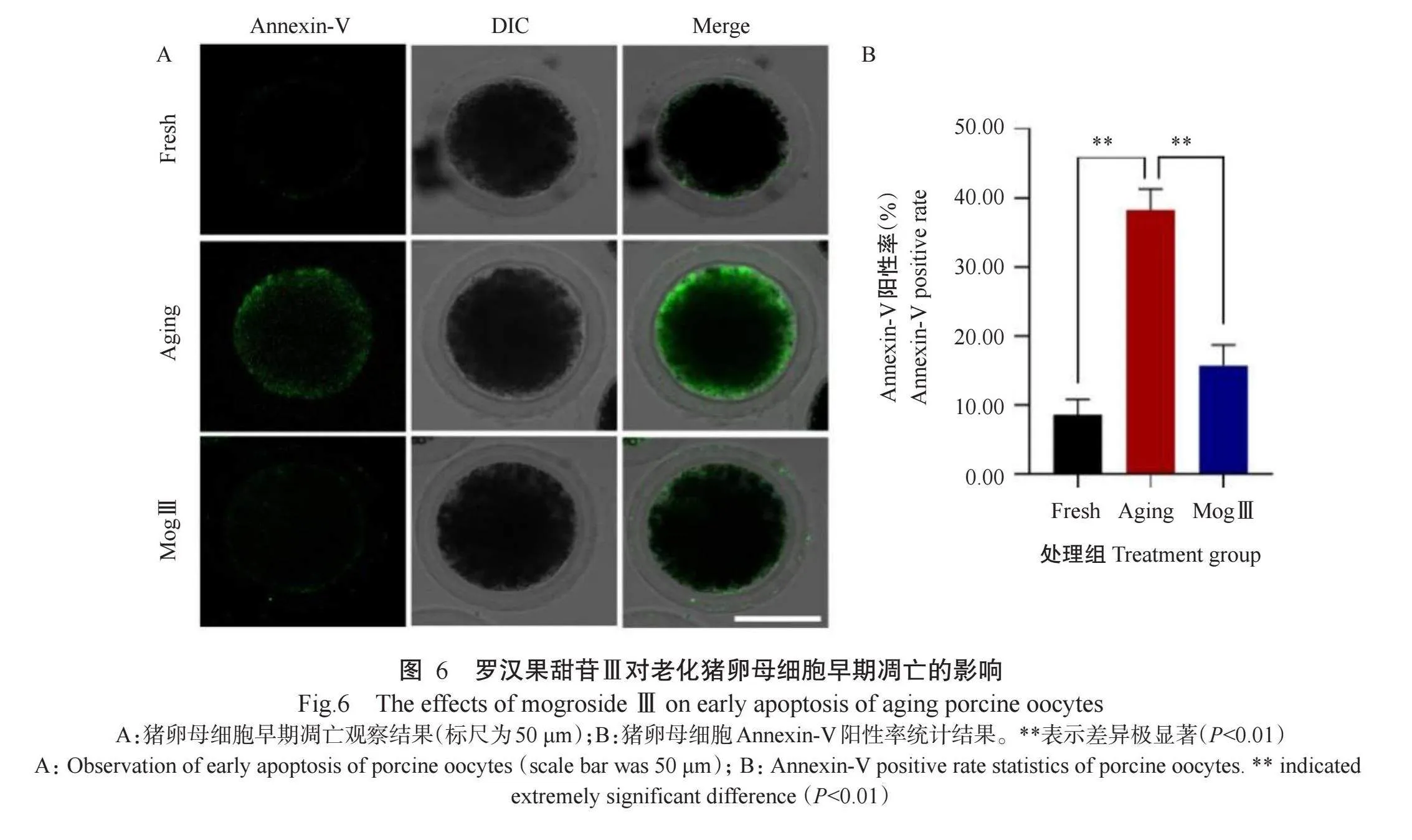
卵母细胞老化引起的氧化应激可诱导细胞早期凋亡(Prasad et al.,2016;Nie et al.,2019;Wang et al.,2021)。由图6可看出,与Fresh组猪卵母细胞相比,Aging组猪卵母细胞的Annexin-V阳性率[(38.27±3.03)%]极显著升高,但添加25.0µmol/L罗汉果甜苷Ⅲ能极显著降低老化猪卵母细胞的Annexin-V阳性率[(15.72±2.99)%]。可见,罗汉果甜苷Ⅲ能有效缓解猪卵母细胞老化导致的早期凋亡。
3讨论
本研究通过孤雌激活评估老化猪卵母细胞的胚胎分裂率和囊胚发育率,最终确定罗汉果甜苷Ⅲ的有效作用浓度为25.0µmol/L,在后续研究中均采用该浓度探究罗汉果甜苷Ⅲ是否具有延缓猪卵母细胞老化的功效。结果表明,老化猪卵母细胞出现的氧化应激、线粒体数量与功能及DNA损伤等均不利于后续胚胎发育,但添加罗汉果甜苷Ⅲ后上述问题得到有效改善,说明罗汉果甜苷Ⅲ可延缓猪卵母细胞老化,进而提高其胚胎发育能力。该结论为延缓猪卵母细胞体外老化提供了新思路,同时为采用植物提取物延缓卵母细胞体外老化提供了参考依据。
线粒体是卵母细胞内进行有氧呼吸和产生能量的主要场所,其数量和质量与卵母细胞的质量密切相关。线粒体参与卵母细胞纺锤体的形成,以及后续卵母细胞的发育、受精和胚胎发育过程(van der Reestetal.,2021)。线粒体的质量还与氧化应激有关(Sena and Chandel,2012),氧化应激可导致线粒体功能障碍,进而引起ROS积累与氧化应激(Wang et al.,2013)。线粒体通过呼吸链产生ATP,为卵母细胞的各项生理活动提供能量,因此ATP含量是评判线粒体功能的常用指标之一。ATP含量直接影响猪卵母细胞核与细胞质的成熟,ATP为运动蛋白供能,促使纺锤体形成及染色体移动(张碧菡等,2023)。为此,本研究通过荧光探针(Mito Tracker)和ATP检测试剂盒分别验证罗汉果甜苷Ⅲ对猪卵母细胞线粒体数量及ATP含量的影响,结果表明,细胞老化导致猪卵母细胞线粒体数量显著减少和ATP含量极显著降低,而添加罗汉果甜苷Ⅲ可有效延缓细胞老化引起的线粒体数量减少和功能衰退。膜电位在维持线粒体结构和功能中发挥着至关重要的作用,是评判线粒体功能的重要指标之一。卵母细胞凋亡的局部表现就是线粒体膜电位下降,且线粒体膜电位与后续胚胎发育能力相关(李厚儒等,2024),还与ATP的生成及卵母细胞中纺锤体的形成存在关联。本研究通过JC-1荧光探针检测罗汉果甜苷Ⅲ对线粒体膜电位的影响,结果显示,细胞老化会导致猪卵母细胞线粒体膜电位显著下降,而添加罗汉果甜苷Ⅲ可有效改善细胞老化导致的线粒体膜电位异常。
ROS是卵母细胞的正常代谢产物,正常的ROS水平对维持卵母细胞的结构与功能至关重要(Tra-choothametal.,2008;Shao et al.,2016)。ROS水平过高,会诱导细胞凋亡,严重影响卵母细胞的质量(Wildinget al.,23jQRtCHJ72jZd+/wewkxdg==003;Valko et al.,2007)。氧化应激是卵母细胞体外培养面临的严峻挑战之一(Pizzino et al.,2017),而缓解氧化应激能有效改善卵母细胞及胚胎的后续发育质量(Li etal.,2015;Pang et al.,2018;An et al.,2019;Zhang et al.,2021)。ROS积累会诱导氧化应激的发生,降低卵母细胞内的ATP含量及影响第一极体的排出,进而影响卵母细胞的进一步成熟及胚胎发育(Schneider et al.,2000)。本研究结果表明,Aging组猪卵母细胞的ROS水平极显著高于Fresh组猪卵母细胞,而MogⅢ组猪卵母细胞的ROS水平极显著低于Aging组猪卵母细胞,说明罗汉果甜苷Ⅲ能有效恢复老化猪卵母细胞中的ROS平衡,从而抑制氧化应激的发生。抗氧化相关基因(SOD、CAT和GPX)的表达与卵母细胞中的ROS水平有关(Guérin et al.,2001;Baird et al.,2005;Landis and Tower,2005;Combelles et al.,2009;Heck et al.,2010)。在本研究中,SOD、CAT和GPX基因的表达均被罗汉果甜苷Ⅲ激活,其相对表达量较Aging组猪卵母细胞极显著上升。可见,罗汉果甜苷Ⅲ是通过上调抗氧化相关基因表达和刺激抗氧化酶活性,促使猪卵母细胞的抗氧化能力增强,从而改善卵母细胞的减数分裂与成熟。
在老化的卵母细胞中,氧化应激导致DNA损伤。DNA损伤传感器复合物Mre11-Rad50-NBS1(MRN),尤其是减数分裂重组Ⅱ在双绞线断裂(DSBs)检测和γ-H2AX积累中发挥重要作用(Mayer et al.,2016)。在此基础上,本研究利用DNA损伤抗体(Anti-γ-H2AX)通过免疫荧光染色检测猪卵母细胞中DNA双链断裂程度,结果表明,老化的猪卵母细胞会造成DNA损伤,但经罗汉果甜苷Ⅲ处理后能极显著减轻老化引起的DNA损伤。氧化应激可通过多种途径作用于卵母细胞而引起细胞凋亡。已有研究表明,卵母细胞老化时,细胞的凋亡程度加剧(Lord et al.,2013)。细胞膜上的磷脂酰丝氨酸(Phosphatidylserine,PS)表达可作为卵母细胞凋亡的标志。由于Annexin-V与PS具有较高的亲和力,通过Annexin-V-FITC探针能检测PS表达情况而评估老化卵母细胞的早期凋亡程度。本研究结果表明,罗汉果甜苷Ⅲ能有效缓解猪卵母细胞老化导致的早期凋亡。
4结论
罗汉果甜苷Ⅲ通过改善老化猪卵母细胞线粒体数量和ATP含量的异常、恢复ROS平衡、上调抗氧化相关基因表达,以及缓解猪卵母细胞老化引起的DNA损伤和早期凋亡,而有效延缓猪卵母细胞的老化进程,进而提高猪卵母细胞的体外发育能力。
参考文献(References):
陈薇,段小群,刘永明,卢曦.2010.罗汉果甜苷对体外诱导肝细胞脂肪变性的影响[J].时珍国医国药,21(12):3092-3094.[Chen W,Duan X Q,Liu Y M,Lu X.2010.Effects of mogrosides(Mog)on hepatocyte steatosis induced by tetracycline in vitro[J].Lishizhen Medicine and Materia Medica Research,21(12):3092-3094.]doi:10.3969/j.issn.1008-0805.2010.12.024.
李厚儒,白佳琛,王晶晶,海桂萍,郝哨鹏,刘倩,刘昱成,万鹏程,傅祥伟.2024.原花青素对青春期前羔羊卵母细胞体外成熟的影响[J].中国畜牧杂志,60(3):172-176.[Li H R,Bai J C,Wang J J,Hai G P,Hao S P,Liu Q,Liu Y C,Wan P C,Fu X W.2024.Effect of proanthocyanidins on the in vitro maturation of oocytes from prepubertal goat lambs[J].Chinese Journal of Animal Science,60(3):172-176.]doi:10.19556/j.0258-7033.20230510-06.
刘嘉昊,王金兴,胡俊杰,冯明,蒋慧敏,李孟琪,卢克焕,杨小淦,梁兴伟.2022.罗汉果甜苷V对持续光照小鼠脂肪积累的缓解作用[J].南方农业学报,53(9):2624-2633.[Liu J H,Wang J X,Hu J J,Feng M,Jiang H M,Li M Q,Lu K H,Yang X G,Liang X W.2022.Mogroside V attenuated constant light exposure-induced accumulation of body fat mass in mice[J].Journal of Southern Agriculture,53(9):2624-2633.]doi:10.3969/j.issn.2095-1191.2022.09.025.
王勤,肖刚.2007.罗汉果甜苷对大鼠慢性肝损伤保护作用的实验研究[J].广西中医药,30(5):54-56.[Wang Q,Xiao G.2007.Experimental study of protective effect of Mog on chronic liver injury of rats[J].Guangxi Journal of Tra-ditional Chinese Medicine,30(5):54-56.]doi:10.3969/j.issn.1003-0719.2007.05.030.
张碧菡,赵宝宝,高静,沈成龙,王勇胜,马会明,卿素珠.2023.甘草酸单铵盐对猪卵母细胞体外成熟及胚胎发育能力的影响[J].中国兽医学报,43(11):2361-2367.[Zhang B H,Zhao B B,Gao J,Shen C L,Wang Y S,Ma H M,Qing S Z.2023.Effects of monoammonium glycyrrhi-zinate on oocyte maturation and embryonic developmentin porcine[J].Chinese Journal of Veterinary Science,43(11):2361-2367.]doi:10.16303/j.cnki.1005-4545.2023.11.23.
An Q L,Peng W,Cheng Y Y,Lu Z Z,Zhou C,Zhang Y,Su J M.2019.Melatonin supplementation during in vitro matu-ration of oocyte enhances subsequent development of bovine cloned embryos[J].Journal of Cellular Physiology,234(10):17370-17381.doi:10.1002/jcp.28357.
Baird D T,Collins J,Egozcue J,Evers L H,Gianaroli L,Leri-don H,Sunde A,Templeton A,van Steirteghem A,Cohen J,Crosignani P G,Devroey P,Diedrich K,Fauser B C J M,Fraser L,Glasier A,Liebaers I,Mautone G,Penney G,Tarlatzis B,Collins J,Crosignani P G.2005.Fertility and ageing[J].Human Reproduction Update,11(3):261-276.doi:10.1093/humupd/dmi006.
Bentov Y,Casper R F.2013.The aging oocyte—Can mitochon-drial function be improved?[J].Fertility and Sterility,99(1):18-22.doi:10.1016/j.fertnstert.2012.11.031.
Chappel S.2013.The role of mitochondria from mature oocyte to viable blastocyst[J].Obstetrics and Gynecology Interna-tional,2013:183024.doi:10.1155/2013/183024.
Chen J,Jiao D M,Li Y,Jiang C Y,Tang X L,Song J,Chen Q Y.2019.Mogroside V inhibits hyperglycemia-induced lung cancer cells metastasis through reversing EMT and dama-ging cytoskeleton[J].Current Cancer Drug Targets,19(11):885-895.doi:10.2174/1568009619666190619154240.Chen R R,Wang J,Zhang M,Kong Q Q,Sun G Y,Jin C H,Luo M J,Tan J H.2022.Restraint stress of female miceduring oocyte development facilitates oocyte postovula-tory aging[J].Aging,14(22):9186-9199.doi:10.18632/aging.204400.
Cimadomo D,Fabozzi G,Vaiarelli A,Ubaldi N,Ubaldi F M,Rienzi L.2018.Impact of maternal age on oocyte and embryo competence[J].Frontiers in Endocrinology,9:327.doi:10.3389/fendo.2018.00327.
Combelles C M,Gupta S,Agarwal A.2009.Could oxidative stress influence the in-vitro maturation of oocytes?[J].Re-productive BioMedicine,18(6):864-880.doi:10.1016/s1472-6483(10)60038-7.
Czajkowska K,Ajduk A.2023.Mitochondrial activity and redox status in oocytes from old mice:The interplay be-tween maternal and postovulatory aging[J].Theriogeno-logy,204:18-30.doi:10.1016/j.theriogenology.2023.03.022.
Du Y,Liu J H,Liu S Y,Hu J H,Wang S Y,Cui K X,Yan K,Liu X X,Wu N R,Yang X G,Liang X W.2022.Mogroside-rich extract from Siraitiagrosvenorii fruits pro-tects against the depletion of ovarian reserves in aging mice by ameliorating inflammatory stress[J].Food&Func-tion,13:121-130.doi:10.1039/d 1fo03194e.
Eichenlaub-Ritter U.2012.Oocyte ageing and its cellular basis[J].The International Journal of Developmental Biology,56:841-852.doi:10.1387/ijdb.120141ue.
Guérin P,El Mouatassim S,Ménézo Y.2001.Oxidative stress and protection against reactive oxygen species in the preim-plantation embryo and its surroundings[J].Human Re-production Update,7(2):175-189.doi:10.1093/humupd/7.2.175.
Heck D E,Shakarjian M,Kim H D,Laskin J D,Vetrano A M.2010.Mechanisms of oxidant generation by catalase[J].Annals of the New York Academy of Sciences,1203(1):120-125.doi:10.1111/j.1749-6632.2010.05603.x.
Hou G M,Sun Q Y.2020.Maternal ageing causes changes in DNA methylation and gene expression profiles in mouse oocytes[J].Zygote,28(5):360-366.doi:10.1017/s09671 99420000143.
Igarashi H,Takahashi T,Takahashi E,Tezuka N,Nakahara K,Takahashi K,Kurachi H.2005.Aged mouse oocytes fail to readjust intracellular adenosine triphosphates at fertiliza-tion[J].Biology of Reproduction,72(5):1256-1261.doi:10.1095/biolreprod.104.034926.
Jiang W J,Li Y H,Zhao Y H,Gao Q S,Jin Q G,Yan C G,Xu YN.2020.L-carnitinesupplementation during in vitro cul-ture regulates oxidative stress in embryos from bovine agedoocytes[J].Theriogenology,143:64-73.doi:10.1016/j.theriogenology.2019.11.036.
Landis G N,Tower J.2005.Superoxide dismutase evolution and life span regulation[J].Mechanisms of Ageing and Development,126(3):365-379.doi:10.1016/j.mad.2004.08.012.
Li Y,Zhang Z Z,He C L,Zhu K F,Xu Z Y,Ma T,Tao J L,Liu G S.2015.Melatonin protects porcine oocyte in vitro matu-ration from heat stress[J].Journal of Pineal Research,59(3):365-375.doi:10.1111/jpi.12268.
Liang X W,Zhu J Q,Miao Y L,Liu J H,Wei L,Lu S S,HouY,Schatten H,Lu K H,Sun Q Y.2008.Loss of methyla-tion imprint of Snrpn in postovulatory aging mouse oocyte[J].Biochemical and Biophysical Research Communica-tions,371(1):16-21.doi:10.1016/j.bbrc.2008.03.105.
Liu YY,Zhang B X,Liu J H,Qiao C Y,Xue N Y,Lv H M,Li S Z.2021.Mogroside V alleviates lipopolysaccharide-induced neuroinflammation via inhibition of TLR4-MyD88 and activation of AKT/AMPK-Nrf2 signaling pathway[J].Evidence-Based Complementary and Alterna-tive Medicine,2021:5521519.doi:10.1155/2021/5521519.
Lord T,Nixon B,Jones K T,Aitken R J.2013.Melatonin pre-vents postovulatoryoocyte aging in the mouse and extends the window for optimal fertilization in vitro[J].Biology of Reproduction,88(3):1-9.doi:10.1095/biolreprod.112.10 6450.
Luo H J,Peng C X,Xu X F,Peng Y T,Shi F,Li Q H,Dong J H,Chen M.2022.The protective effects of mogroside V against neuronal damages by attenuating mitochondrial dysfunction via upregulating Sirtuin3[J].Molecular Neu-robiology,59:2068-2084.doi:10.1007/s 12035-021-02689-z.
Mayer A,Baran V,Sakakibara Y,Brzakova A,Ferencova I,Motlik J,Kitajima T S,Schultz R M,Solc P.2016.DNA damage response during mouse oocyte maturation[J].Cell Cycle,15(4):546-558.doi:10.1080/15384101.2015.1128 592.
Nie J Y,Sui L M,Zhang H T,Zhang H Y,Yan K,Yang X G,Lu S S,Lu K H,Liang X W.2019.Mogroside V protects porcine oocytes from in vitro ageing by reducing oxidative stress through SIRT1 upregulation[J].Aging,11(19):8362-8373.doi:10.18632/aging.102324.
Nie J Y,Xiao P,Wang X F,Yang X G,Xu H Y,Lu K H,Lu S S,Liang X W.2018.Melatonin prevents deterioration in quality by preserving epigenetic modifications of porcine oocytes after prolonged culture[J].Aging,10(12):3897-3909.doi:10.18632/aging.101680.
Nie J Y,Xiao P,Xiong Q Q,Liang X W,Zhao X L.2024.Smart seq2 revealed distinct molecular responses during in vitro porcine oocyte maturation before or after the addition of mogroside V[J].Reproduction in Domestic Animals,59(5):e14595.doi:10.1111/rda.14595.
Nie J Y,Yan K,Sui L M,Zhang H T,Zhang H,Yang X Y,Lu S S,Lu K H,Liang X W.2020.Mogroside V improves porcine oocyte in vitro maturation and subsequent embryo-nic development[J].Theriogenology,141:35-40.doi:10.1016/j.theriogenology.2019.09.010.
Pang Y W,Zhao S J,Sun Y Q,Jiang X L,Hao H S,Du W H,Zhu H B.2018.Protective effects of melatonin on the in vitro developmental competence of bovine oocytes[J].Ani-mal Science Journal,89(4):648-660.doi:10.1111/asj.12970.
Peng K,Cui K X,Li P,Liu X X,Du Y,Xu H Y,Yang X G,Lu S S,Liang X W.2024.Mogroside V alleviates the heat stress-induced disruption of the porcine oocyte in vitro maturation[J].Theriogenology,217:37-50.doi:10.1016/j.theriogenology.2024.01.008.
Petri T,Dankert D,Demond H,Wennemuth G,Horsthemke B,Grümmer R.2020.In vitro postovulatory oocyte aging affects H3K9 trimethylation in two-cell embryos after IVF[J].Annals of Anatomy-Anatomischer Anzeiger,227:151424.doi:10.1016/j.aanat.2019.151424.
Pizzino G,Irrera N,Cucinotta M,Pallio G,Mannino F,Arco-raci V,Squadrito F,Altavilla D,Bitto A.2017.Oxidative Stress:Harms and benefits for human health[J].Oxidative Medicine and Cellular Longevity,2017:8416763.doi:10.1155/2017/8416763.
Prasad S,Tiwari M,Pandey A N,Shrivastav T G,Chaube S K.2016.Impact of stress on oocyte quality and reproductive outcome[J].Journal of Biomedical Science,23:36.doi:10.1186/s 12929-016-0253-4.
Redza-Dutordoir M,Averill-Bates D A.2016.Activation of apoptosis signalling pathways by reactive oxygen species[J].Biochimica et Biophysica Acta,1863(12):2977-2992.doi:10.1016/j.bbamcr.2016.09.012.
Sasaki H,Hamatani T,Kamijo S,Iwai M,Kobanawa M,Ogawa S,Miyado K,Tanaka M.2019.Impact of oxidative stress on age-associated decline in oocyte developmental competence[J].Frontiers in Endocrinology,10:811.doi:10.3389/fendo.2019.00811.
Schneider Y,Vincent F,Duranton B,Badolo L,GosséF,Berg-mann C,Seiler N,Raul F.2000.Anti-proliferative effect of resveratrol,a natural component of grapes and wine,on human colonic cancer cells[J].Cancer Letters,158(1):85-91.doi:10.1016/s0304-3835(00)00511-5.
Sena LA,Chandel N S.2012.Physiological roles of mitochon-drial reactive oxygen species[J].Molecular Cell,48(2):158-167.doi:10.1016/j.molcel.2012.09.025.
Shao Y Y,Ni Y B,Yang J,Lin X T,Li J,Zhang L X.2016.Astaxanthin inhibits proliferation and induces apoptosis and cell cycle arrest of mice H22 hepatoma cells[J].Medi-cal Science Monitor,22:2152-2160.doi:10.12659/msm.899419.
Shi Y N,Li B H,Sun S F,Tian W D,Ma Z Z,Liu W.2023.Inhibition of mogroside IIIE on isoproterenol-induced myocardial fibrosis through the TLR4/MyD88/NF-κB sig-naling pathway[J].Iranian Journal of Basic Medical Scien-ces,26(1):114-120.doi:10.22038/ijbms.2022.67908.148 48.
Stringer J M,Alesi L R,Winship A L,Hutt K J.2023.Beyond apoptosis:Evidence of other regulated cell death pathways in the ovary throughout development and life[J].Human Reproduction Update,29(4):434-456.doi:10.1093/humu-pd/dmad005.
Suttirojpattana T,Somfai T,Matoba S,Parnpai R,Nagai T,Geshi M.2017.Effect of medium additives during liquid storage on developmental competence of in vitro matured bovine oocytes[J].Animal Science Journal,88(2):231-240.doi:10.1111/asj.12623.
Takahashi T,Igarashi H,Amita M,Hara S,Kurachi H.2011.Cellular and molecular mechanisms of various types of oocyte aging[J].Reproductive Medicine and Biology,10(4):239-249.doi:10.1007/s 12522-011-0099-0.
Tarín J J,Pérez-AlbaláS,Cano A.2000.Consequences on off-spring of abnormal function in ageing gametes[J].Human Reproduction Update,6(6):532-549.doi:10.1093/humupd/6.6.532.
Tarín J J,Pérez-AlbaláS,Pérez-Hoyos S,Cano A.2002.Posto-vulatory aging of oocytes decreases reproductive fitness and longevity of offspring[J].Biology Reproduction,66(2):495-499.doi:10.1095/biolreprod66.2.495.
Trachootham D,Lu W Q,Ogasawara M A,del Valle N3HqHVsrR3TDjuWHQsQaWy8mat61Q0AZ1FJW1QSsG7Uw= R,Huang P.2008.Redox regulation of cell survival[J].Antio-xidants&Redox Signaling,10(8):1343-1374.doi:10.1089/ars.2007.1957.
Turan V,Oktay K.2020.BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging[J].Human Reproduction Update,26(1):43-57.doi:10.1093/humupd/dmz043.
Valko M,Leibfritz D,Moncol J,Cronin M T D,Mazur M,Telser J.2007.Free radicals and antioxidants in normal physiological functions and human disease[J].The Interna-tional Journal of Biochemistry&Cell Biology,39(1):44-84.doi:10.1016/j.biocel.2006.07.001.
van der Reest J,Cecchino G N,Haigis M C,Kordowitzki P.2021.Mitochondria:Their relevance during oocyte ageing[J].Ageing Research Reviews,70:101378.doi:10.1016/j.arr.2021.101378.
Wang C H,Wu S B,Wu Y T,Wei Y H.2013.Oxidative stress response elicited by mitochondrial dysfunction:Implica-tion in the pathophysiology of aging[J].Experimental Biology and Medicine,238(5):450-460.doi:10.1177/1535370213493069.
Wang H Y,Jo Y J,Oh J S,Kim N H.2017.Quercetin delays postovulatory aging of mouse oocytes by regulating SIRT expression and MPF activity[J].Oncotarget,8:38631-38641.doi:10.18632/oncotarget.16219.
Wang L,Tang J H,Wang L,Tan F,Song H B,Zhou J W,Li F G.2021.Oxidative stress in oocyte aging and female repro-duction[J].Journal of Cellular Physiology,236(12):7966-7983.doi:10.1002/jcp.30468.
Wang S,Zheng Y X,Li J Y,Yu Y,Zhang W Q,Song M S,Liu Z P,Min Z Y,Hu H F,Jing Y,He X J,Sun L,Ma L F,Esteban C R,Chan P,Qiao J,Zhou Q,Izpisua Belmonte J C,Qu J,Tang F C,Liu G H.2020.Single-cell transcrip-tomic atlas of primate ovarian aging[J].Cell,180(3):585-600.doi:10.1016/j.cell.2020.01.009.
Wen X,Yang Q,Sun D,Jiang Z Y,Wang T,Liu H R,Han Z,Wang L,Liang C G.2023.Cumulus cells accelerate posto-vulatory oocyte aging through IL1-IL1R1 interaction in mice[J].International Journal of Molecular Sciences,24(4):3530.doi:10.3390/ijms24043530.
Wilding M,Dale B,Marino M,di Matteo L,Alviggi C,Pisa-turo M L,Lombardi L,de Placido G.2001.Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos[J].Human Reproduction,16(5):909-917.doi:10.1093/humrep/16.5.909.
Wilding M,de Placido G,de Matteo L,Marino M,Alviggi C,Dale B.2003.Chaotic mosaicism in human preimplanta-tion embryos is correlated with a low mitochondrial mem-brane potential[J].Fertility and Sterility,79(2):340-346.doi:10.1016/s0015-0282(02)04678-2.
Xian Y X,Liang L F,Qi S T,Xie Y J,Song B,Ouyang S M,Xie Y H,Sun X F,Wang W H.2018.Antioxidants retard the ageing of mouse oocytes[J].Molecular Medicine Re-ports,18(2):1981-1986.doi:10.3892/mmr.2018.9167.
Xiao J,Huang K,Lin H M,Xia Z J,Zhang J,Li D P,Jin J F.2020.Mogroside II(E)inhibits digestive enzymes via sup-pression of interleukin 9/interleukin 9 receptor signalling in acute pancreatitis[J].Frontiers in Pharmacology,11:859.doi:10.3389/fphar.2020.00859.
Xing X P,Zhang J J,Wu T,Zhang J C,Wang Y S,Su J M,Zhang Y.2021.SIRT1 reduces epigenetic and non-epigenetic changes to maintain the quality of postovulatory aged oocytes in mice[J].Experimental Cell Research,399(2):112421.doi:10.1016/j.yexcr.2020.112421.
Xu M T,Zhang M,Wang G L,Gong S,Luo M J,Zhang J,Yuan H J,Tan J H.2024.Postovulatory aging of mouse oocytes impairs offspring behavior by causing oxidative stress and damaging mitochondria[J].Cells,13(9):758.doi:10.3390/cells 13090758.
Xue W,Mao J,Chen Q,Ling W,Sun Y.2020.Mogroside IIIE alleviates high glucose-induced inflammation,oxidative stress and apoptosis of podocytes by the activation of AMPK/SIRT1 signaling pathway[J].Diabetes,Metablic Syndrome and Obesity,13:3821-3830.doi:10.2147/dmso.S276184.
Yang L Q,Chen Y,Liu Y,Xing Y,Miao C Y,Zhao Y,Chang X W,Zhang Q.2020.The role of oxidative stress and natu-ral antioxidants in ovarian aging[J].Frontiers in Pharma-cology,11:617843.doi:10.3389/fphar.2020.617843.
Yang Q L,Dai S J,Luo X Y,Zhu J,Li F Y,Liu J H,Yao G F,Sun Y P.2018.Melatonin attenuates postovulatory oocyte dysfunction by regulating SIRT1 expression[J].Reproduc-tion,156(1):81-92.doi:10.1530/rep-18-0211.
Yin Y J,Zhang Y H,Wang Y,Jiang H,Zhang J B,Liang S,Yuan B.2023.Ferulic acid ameliorates the quality of in vitro-aged bovine oocytes by suppressing oxidative stress and apoptosis[J].Aging,15(21):12497-12512.doi:10.18632/aging.205193.
Yu X X,Meng F,Huang J,Li W D,Zhang J M,Yin S,Zhang L R,Wang S X.2022.1-Nitropyrene exposure induces mito-chondria dysfunction and impairs oocyte maturation in mice[J].Ecotoxicology and Environmental Safety,242:113921.doi:10.1016/j.ecoenv.2022.113921.
Zhang H,Li C,Wen D X,Li R Y,Lu S H,Xu R,Tang Y J,Sun Y D,Zhao X E,Pan M H,Ma B H.2022.Melatonin improves the quality of maternally aged oocytes by main-taining intercellular communication and antioxidant metabolite supply[J].Redox Biology,49:102215.doi:10.1016/j.redox.2021.102215.
Zhang Z G,Mu Y Q,Ding D,Zou W W,Li X Y,Chen B L,Leung P C K,Chang H M,Zhu Q,Wang K J,Xue R F,Xu Y Q,Zou H J,Zhou P,Wei Z L,Cao Y X.2021.Melatoninimproves the effect of cryopreservation on human oocytes by suppressing oxidative stress and maintaining the permea-bility of the oolemma[J].Journal of Pineal Research,70(2):e12707.doi:10.1111/jpi.12707.
Zhou Y T,Li R,Li S H,Ma X,Liu L,Niu D,Duan X.2022.Perfluorooctanoic acid(PFOA)exposure affects early embryonic development and offspring oocyte quality via inducing mitochondrial dysfunction[J].Environment Inter-national,167:107413.doi:10.1016/j.envint.2022.107413.
Zilberberg E,Casper R,Meriano J,Barzilay E,Aizer A,Kirsh-enbaum M,Orvieto R,Haas J.2021.Cleavage vs blasto-cyst stage embryos:How are they interrelating?[J].Arc-hives of Gynecology and Obstetrics,304(4):1083-1088.doi:10.1007/s00404-021-06003-z.
(责任编辑兰宗宝)

