Research progress of intestinal microbiota in targeted therapy and immunotherapy of colorectal cancer
Xinying Zhou, Yu Zhao, Rongchuan Zhao, Shaheryar Shafi, Yue Yang, Guangxing Liu, Song-Bai Liu
1Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, Suzhou 215163, Jiangsu, China.
2Jiangsu Province Engineering Research Center of Molecular Target Therapy and Companion Diagnostics in Oncology, Suzhou Vocational Health College, Suzhou 215009, Jiangsu, China.
Abstract Colorectal cancer (CRC) is one of the most common malignancies of the digestive tract, with increasing morbidity and mortality worldwide, and is the third most common malignancy in the world.At present, the main treatment methods for CRC include surgery, chemotherapy, targeted therapy, and immunotherapy.Regulation of the gut microbiota is one of the most promising new strategies for CRC treatment.Gut microbiota interacts with host cells to regulate many physiological processes, such as energy acquisition, metabolism, and immune responses.Recent studies have found that a combination of gut microbiota with targeted therapy and immunotherapy could improve the therapeutic effect of colon cancer compared with treatment alone.This article reviews the mechanism of microbiota regulation in CRC and the latest progress of intestinal microbiota in targeted therapy and immunotherapy of CRC, which is helpful in developing potential prevention or treatment strategies for colorectal cancer.
Keywords: CRC, gut microbiota, targeted therapy, immunotherapy
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer-related death worldwide, with a 5-year survival rate of 15%[1].With the deepening of research, more and more factors increasing the incidence of colorectal cancer have been discovered, such as cancers of unknown primary (CUP), metastatic cancer with no identifiable primary site following adequate evaluation[2].Early screening has been shown to improve CRC survival in developed countries.While surgery alone or combined with radiotherapy or chemotherapy has been the mainstay of CRC treatment, targeted therapies such as cetuximab (Cet) and bevacizumab (Bev)have led to significantly improved overall survival since their development in the 21st century[3].Even though older patients with pT4 disease are more prone to severe postoperative complications, there is no consensus that age affects survival outcomes.The prognosis of older patients may be confounded by differences in stage at presentation, tumor site, preexisting comorbidities, and type of treatment received[4].There are two main chemotherapy regimens for the treatment of colorectal cancer: FOLFOX [5-fluorouracil(5-FU), leucovorin, and oxaliplatin] and FOLFIRI (5-FU, leucovorin, and irinotecan).Cet, panitumumab(Pan), and Bev are often added to these two chemotherapy regimens for synergistic therapeutic effects[5].In recent years, immunotherapy has achieved great therapeutic effects in solid tumors such as melanoma and lung cancer; this success has led to its gradual application in treating CRC[6].Microsatellite instability (MSI)is a change in the number of short repetitive sequences (1-6 bases) in DNA and is a form of genetic instability in tumors.dMMR is a system for correcting errors during DNA replication, and if MMR is defective, it can lead to MSI.In general, CRC patients with high MSI (MSI-H) or MMR deficiency (dMMR)have better outcomes for ICI, while CRC patients with low MSI (MSI-L) or microsatellite stabilization(MSS) have ineffective or limited treatment for ICI.
The recommended screening for defective DNA mismatch repair includes immunohistochemistry (IHC)and/or MSI test.However, there are challenges in distilling the biological and technical heterogeneity of MSI testing down to usable data.It has been reported in the literature that IHC testing of the mismatch repair machinery may give different results for a given germline mutation, and it has been suggested that this may be due to somatic mutations[7].However, prognosis remains poor for many CRC patients[8],highlighting the need for more effective treatments.
The intestinal microbiota represents an integral part of the gastrointestinal microenvironment and has been implicated in CRC development and progression, as well as response to therapy.Under homeostatic conditions, commensal microbes confer resistance against pathogens in part by regulating epithelial cell proliferation and DNA damage[9].Thus, maintaining the gut microbial ecosystem appears critical for CRC prevention and treatment.There is mounting evidence that the intestinal microbiota contributes to CRC via various mechanisms.Consequently, preservation of gut microbial balance could mitigate CRC advancement[10].Furthermore, modulation of the microbiota may improve outcomes for immunotherapy and targeted therapy.
MICROBIOTA IN TARGETED THERAPY Fusobacterium nucleatum
Fusobacterium nucleatum(F.nucleatum) is a gram-negative, spore-free anaerobic bacterium.It mainly colonizes the mouth and is an opportunistic pathogen that may cause oropharyngeal and external oral diseases[11].In recent years, a large number of tissue samples and stool samples of clinical patients with colorectal cancer have been detected with an increased abundance ofF.nucleatum[12].Studies have shown thatF.nucleatumcan cooperate with other intestinal microorganisms to regulate the tumor immune microenvironment and promote the progression of colorectal cancer, and its high abundance is often associated with poor prognosis and drug resistance in the late stage of chemotherapy[13].Therefore, the exploration of molecular targets targetingF.nucleatumto inhibit the progression of colorectal cancer is reliable.This part summarizes the existing potential therapeutic targets and molecular mechanisms related toF.nucleatum.
Pathogenic mechanism
FadA is aF.nucleatumadhesin that can bind to E-cadherin on human colon epithelial cells, activating the Wnt/β-catenin signaling pathway.This promotes CRC cell proliferation, migration, and invasion[14].Fninfection can also prompt CRC development by stimulating toll-like receptor 4 (TLR4) signaling, resulting in NF-κB activation and upregulation of the pro-carcinogenic microRNA-21 (miR-21)[15].Lipopolysaccharide (LPS), a cell wall component ofF.nucleatum, can bind to TLR4 on the surface of tumor cells, activating MyD88 and other signaling pathways.This will further promote the transformation of LC3-I to LC3-II and the formation of autophagosomes, contributing to the degradation of chemotherapy drugs and resistance.Activation of the TLR4-MyD88 signaling pathway can also inhibit the expression of two microRNAs involved in autophagy signaling molecules, thereby further enhancing the activation of autophagy[16].
Therapeutic targets
Current approaches to impedeF.nucleatum-mediated CRC progression primarily fall into three categories:
(1) Small molecule compound inhibitors.Previous studies have shown that CEACAM1 and TIGIT inhibitors can be used as potential adjuvant drugs for CRC[17].At present, some small molecule drugs under clinical trial are screened, such as azelnidipine and liothyronine[18].A recent study has shown that Sodium New Houttuyfonate (SNH) significantly inhibits the progression of CRC by targetingF.nucleatum's membrane-associated protein FadA, leading to FadA polymerization, membrane rupture, and penetration,which blocks the tumor-promoting activity ofF.nucleatumandF.nucleatum-associated cancer-driven inflammation, protecting the intestinal barrier[19].Studies have also shown that antimicrobial peptides(AMPs) are a new class of antimicrobial drugs.Br-J-I, a derivative of antimicrobial peptides Jelleine-I,inhibitsF.nucleatumcolonization, colon inflammation, andF.nucleatum-induced CRC growth by targeting FadA.Br-J-I can also enhance the antitumor effect of 5-fluorouracil[20].
(2) Phage-guided intestinal microbiome regulation therapy.Irinotecan-loaded dextran nanoparticles covalently linked to azide-modified phages were demonstrated to selectively inhibit the proliferation ofF.nucleatumand autophagy of colon tumor, thus restoring the sensitivity of tumor cells to chemotherapy drugs[21].
Bacteroides fragilis
Bacteroides fragilisis divided intonontoxigenic Bacteroides fragilis(NTBF) andenterotoxigenic Bacteroides fragilis(ETBF) according to the ability to produce Bacteroides fragilis toxin (BFT).ETBF is an obligate anaerobic gram-negative bacterium that can secrete BFT to destroy the intestinal epithelial barrier, induce inflammation and precancerous lesions, and ultimately promote the tumorigenesis and progression of colorectal cancer[22].
Pathogenic mechanism
In a steady state, mucosal barrier can separate gut microbiota from the immune system.Destruction of mucosal barrier will cause chronic inflammation, which will further inhibit apoptosis of abnormal cells and eventually lead to cancer.Wuet al.found that Bacteroides fragilis toxin (BFT) produced by ETBF destroys colon mucosal barrier by degrading E-cadherin of colon epithelial cells[23].BFT could also induce Wnt/βcatenin signaling[24].In addition, the transcription factor STAT3 in colon epithelial cells and immune cells activated by ETBF could induce the production of growth factors, promote survival and proliferation of tumor cells, and inhibit antitumor immune response[25].ETBF was also involved in the cytokine secretion of T cells, which would further increase the risk of intestinal inflammation and cancer[26].ETBF can also affect the inflammatory response of cells, the degradation of extracellular matrix, and the regulation of gene expression through various signaling pathways, such as NF-κB and MAPK signaling pathways, which will further promote tumorigenesis and cancer progression[27,28].Biofilms are structures composed of bacteria and extracellular polymers that protect bacteria from being attacked by the host immune system and antimicrobial agents[29].ETBF could participate in biofilm formation and alter the tumor microenvironment, thereby promoting carcinogenesis[30].
Therapeutic targets
Currently, the potentialanti-enterotoxigenic Bacteroides fragilistherapy to inhibit CRC progression can be divided into three categories:
(1) Natural product screening.Kimet al.found that zerumbone could effectively inhibit biofilm formation inBacteroides fragiliscontaining toxic BFT-2 by downregulating the effector pump gene (bmeB12)associated with biofilm formation[29].α-humulene is also effective in killing enterotoxigenicBacteroides fragilisand destroying biofilms, which is expected to treat intestinal infections[31].In addition, a recent study showed that natural polysaccharides extracted from agricultural wastes exhibited antibacterial effects through involvement in DNA damage and plasma membrane permeability alteration in carcinogenic bacteria[32].
(2) Anti-inflammatory drugs.Administration of the anti-inflammatory drug auranofin can inhibit bacterial survival and eradicate bacterial biofilms, alleviating intestinal inflammatory response caused by ETBF, and may reduce the occurrence of inflammation-related cancers by reducing the expression of outer membrane protein (OmpA) gene and effector pump-related gene bmeB3[33].
(3) Probiotic therapy.The use of probiotic blends such asClostridium butyricumandBifidobacteriumcan regulate the intestinal microecological balance by inhibiting the production of intestinal pathogenic biofilms, thereby inhibiting the colonization and proliferation of ETBF, and reducing the possibility of intestinal inflammation and cancer[34].It was also shown in the article that the synthetic recombinant BFT-2 can inhibit the incidence of colon cancer, by decreasing the expression of Ki-67 and increasing the expression of Caspase-3, leading to apoptosis of colon cancer cells[35].
Clostridium butyricum
Clostridium butyricum(CB) is a spore-bearing, gram-positive anaerobic bacterium of the genusClostridium.It can inhibit the growth of intestinal pathogenic bacteria and promote the amplification of intestinal probiotics such as Bifidobacterium.In addition,Clostridium butyricumferments undigested dietary fiber to produce short-chain fatty acids (SCFA), by which butyric acid is one of the main products.These SCFAs affect host intestinal homeostasis and barrier function.
Therapeutic targets
Clostridium butyricumcan enhance the mucosal barrier by increasing MUC gene expression in goblet cells and thereby increasing the content of mucin[36].Zhouet al.found thatCBinhibited the occurrence and progression of colon cancer by regulating the levels of inflammatory factors such as IL-6 and IL-10 through the MyD88-NF-κB signaling pathway[37].Another study showed thatCBcan reduce the proliferation and promote apoptosis of intestinal tumor cells by inhibiting the Wnt/β-catenin signaling pathway.It can also reduce the secondary bile acid content in the stool, and increase the SCFA content in the cecum, which could further activate G protein-coupled receptors (GPRs) and activate the antiproliferative effect of probiotics[38].Xuet al.found thatClostridium butyricumcan also reduce the stability of MYC protein by enhancing ubiquitin-mediated degradation, which contributes to the inhibition of the MYC-TYMS signaling pathway involved in CRC[39].In addition,Clostridium butyricumcan also inhibit the progression of colorectal cancer by regulating the expression levels of TGF-β, miR-200c, and Bcl-2[40,41].
Previous studies have reported Prebiotics-Encapsulated Probiotic Spores, spores-dex, which could be specifically enriched in colon cancer lesions after oral administration.Oligosaccharides are fermented byClostridium butyricumto produce anticancer short-chain fatty acids (SCFAs).Meanwhile, spores-dex could also regulate the gut microbiota, increase the abundance of SCFA-producing bacteria, and significantly increase the overall diversity of the microbiota[42].
Streptococcus gallolyticus
Streptococcus gallolyticus, formerly known asStreptococcus bovis, is a Gram-positive pathogenic bacteria that causes endocarditis in humans.It is also the first bacterium identified to be associated with the development of CRC[43,44].Studies have shown that patients withStreptococcus gallolyticusinfection have an elevated risk of developing CRC[44].In 2021, Rahwa Taddeseet al.found thatS.gallolyticusinterrelates with AhRmediated pathways for cellular biotransformation (CYP1), which could potentially contribute to combating DNA damage and/or play a role in removing intestinal pathogens and protecting the epithelial barrier.S.gallolyticusincreased the expression and activity of CYP1 biotransformation capacity in colorectal epithelial cells[45].
Peptostreptococcus anaerobius
Peptostreptococcus anaerobiusis an anaerobic bacterium selectively enriched in the fecal and mucosal microbiota of CRC patients[46].In 2019, Longet al.found that,in vivo, anaerobic bacteria attach to the mucosa of colorectal cancer, accelerating the development of colorectal cancer; anaerobes selectively adhere to colorectal cancer cell lines in vitro.Mechanistically, anaerobic bacteria drive colorectal cancer through the PCWBR2-integrin α2/β1-PI3K-AKT-Nf-κb signaling axis, and the PCWBR2-integrin α2/β1 axis is identified as a potential therapeutic target for CRC.α2/β1 is a receptor frequently overexpressed in human CRC tumors and cell lines.PCWBR2 can interact with α2/β1, which can induce the activation of the PI3K–Akt pathway in CRC cells.This leads to increased cell proliferation and NF-κB activation[47].
The pathogenic microbiota and their therapeutic targets are summarized in Table 1.
MICROBIOTA IN IMMUNOTHERAPY Gram negative bacillus
Gram-negative bacillus generally refers to bacteria with red Gram staining reaction.It mainly includes plateau Escherichia coli and Pseudomonas aeruginosa.LPS is one of the important products of Gramnegative bacteria.As an immunostimulatory ligand, LPS can activate TLR4 and nuclear factor NF-κB pathway.As early as 2011, Hsuet al.found that LPS could promote CRC liver metastasis by stimulating the TLR4 signaling pathway and increasing cell adhesion mediated by β1 integration[48].In 2018, Songet al.found that LPS content was significantly increased in the blood and tissues of patients with early adenoma and CRC.High levels of LPS in orthotopic colon cancer tissues were associated with low response to PD-L1 monoclonal antibody treatment in immunotherapy.Clearance of Gram-negative bacteria in gut with polymyxin B (PmB) or blockade of TLR4 with TAK-242 alleviated the immunosuppressive microenvironment and promoted T-cell infiltration into CRC tumors.Based on this finding, Songet al.considered neutralizing or modulating LPS produced by gut microbes as a potential treatment for colorectal cancer.Therefore, they suggest that genetically engineered bacteria that secrete LPS traps or PmB produced byBacillus polymyxaare promising therapeutic approaches to kill Gram-negative bacillus instead ofantibiotics.Transplanting these bacteria into the host gut in an appropriate manner could be an effective trial to clear LPS and regulate the CRC microenvironment[49].
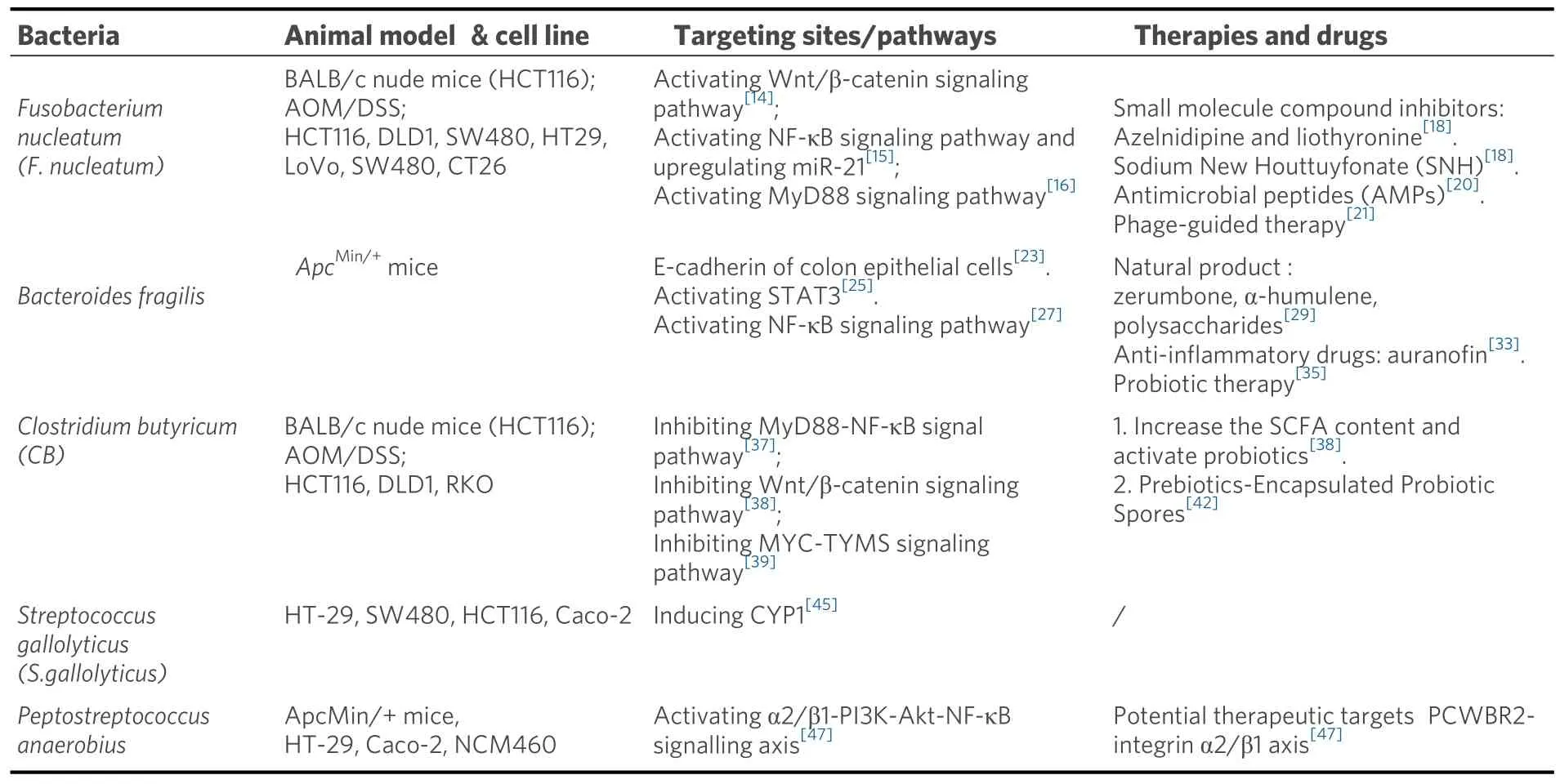
Table 1.Gut microbiota in CRC-targeted therapy
Lachnospiraceae
Lachnospiraceaebelongs to Firmicutes, an obligate anaerobes found in most healthy people.Lachnospiraceaeis involved in the production of SCFAs, an important energy source for intestinal epithelial cells[50].Spirochetesparticipate in host immune system by producing short-chain fatty acids, converting primary to secondary bile acids, and promoting colonization resistance to enteric pathogens[51].In 2023,Zhanget al.found that commensal bacteriaRuminococcus(Rg),Bacillus(Bp), andProteus(Df) of the Spirillaceae family were enriched in normal tissues, while Fusobacterium nucleatum (Fn) and anaerobicStreptococcus gasteri(Pa) were enriched in tumor tissues.Depletion ofRgandBppromoted the antitumor effect of CD8+T cells, and thus inhibited the progression of CRC, which could be a potential combination approach with immunotherapy[52].
Lactobacillus gallinarum
In 2022, Sugimuraet al.identifiedLactobacillus gallinarumas a potential adjuvant that could enhance the effect of anti-PD1 immunotherapy on CRC.Indole-3-lactic acid (ILA), a tryptophan metabolite produced byLactobacillus gallinarum, can be further derived to indole-3-carboxylic acid (ICA), a functional metabolite that could inhibit the production of kynurenine (Kyn) in tumors by inhibiting the expression of indoleamine 2,3-dioxygenase (IDO1).In addition,Lactobacillus gallinarum-derived ICA competed with Kyn for the binding site of AHR and antagonized the binding of Kyn to CD4+T cells.In conclusion,Lactobacillus gallinarumenhanced the efficiency of anti-PD1 therapy in CRC by inhibiting differentiation of Treg, and enhancing CD8+T cell function through IDO1/Kyn/AHR axis[53].
Streptococcus thermophilus
Streptococcus thermophilusis a Gram-positive anaerobes that protects the intestinal epithelium by preventing invasive Escherichia coli[54]; it has also been shown to increase ceramide levels both in vitro andin vivo[55].Liet al.found in 2021 thatS.thermophilusinhibited colorectal tumorigenesis by secreting βgalactosidase.β-galactosidase secreted byS.thermophilusinhibits cell proliferation, reduces colony formation, induces cell cycle arrest, promotes CRC cell apoptosis, and retards the growth of CRC xenografts.MutantS.thermophiluswithout functional β-galactosidase loses its tumor suppressive effect.As a novel drug for CRC prevention, S.thermophilushas a promising application prospect[56].
Lactobacillus paracasei
Lactobacillus paracaseiis a Gram-positive and facultative anaerobic fermentativeLactobacillusthat is widely distributed in fermented dairy products, vegetables, cereal products, and the gastrointestinal tract of humans and animals[57].In recent years,L.paracaseihas been shown to have a variety of health effects, such as antitumor immunity, improvement of functional dyspepsia, and improvement of lipid metabolism[58,59].In 2018, Changet al.found that the combination of NTU101-FM extract with 5-fluorouracil (5-FU), a chemotherapeutic drug, could induce CRC cell viability without toxicity to colonic epithelial cells[60].In 2022, Zhanget al.demonstrated thatL.paracasei sh2020could induce antitumor immunity in mice and synergize with anti-PD-1 to delay tumor progression.The team found that the microbiome from healthy individuals had considerable sensitivity to anti-PD-1, which was absent from the gut microbiome of CRC patients.L.paracasei sh2020was identified to exhibit significant antitumor immunity in mice with gut dysbiosis.Mechanistically,L.paracasei sh2020stimulation led to the upregulation of CXCL10 expression in tumors and enhanced CD8+T cell recruitment[57].In the same year, Shiet al.found thatL.paracasei PC-H1could use extracellular vesicles to induce apoptosis of colorectal cancer cells and delay the progression of CRC by using the PDK1/AKT/Bcl-2 signaling pathway[61].Taken together, the regulation of intestinal microbiota byL.paracaseimay play a synergistic role in enhancing immunotherapy and provide a new target for the treatment of CRC, which may have a broad prospect in the clinical treatment of CRC.
Clostridium
Clostridiumis a general term for gram-positive, anaerobic, microaerobic and coarse bacillus species.Montalbanet al.found that the abundance ofClostridiumwas decreased in CRC patients.Mice administrated orally with four Clostridium species (CC4) exhibited the activation of IFN-γ+, granzyme B+,CD8+T cells and downregulation of immune checkpoint molecules, which resulted in the activation of antitumor immune response and further CRC inhibition[62].
Fusobacterium nucleatum
In addition to targeted treatment of CRC,Fusobacterium nucleatumcan also work with immune pathways to inhibit CRC progression.TIGIT (T cell Ig and ITIM domain) is an inhibitory receptor expressed on regulatory T cells (Treg), activated T cells, and natural killer cells (NK cells).CEACAM1 is a cell adhesion molecule involved in tumor metastasis by binding to TIGIT.Fap2 is an outer membrane protein capable of binding to TIGIT/CEACAM1, thereby inhibiting the activity of natural killer cells and promoting the development and progression of CRC[63].Specific antibodies targeting anti-FAP2 protein antibodies/anti-CEACAM1 and TIGIT can be used to block the interaction betweenFnand immune cells, thereby enhancing the function of natural killer cells and inhibiting the progression of colorectal cancer.
We summarized the mechanism of action and target cells of gut microbiota in CRC- immunotherapy in Table 2.
MICROBIOTA IN RELATION TO DIETS AND CRC Dietary impacts and drug resistance
There are many other factors that influence the gut microbiota in CRC, and the current approach to analyzing these factors involves the concept of Molecular pathological epidemiology.Molecular pathological epidemiology (MPE) is based on molecular pathology and disease heterogeneity, using epidemiological study design methods to comprehensively analyze the effects of exposure factors, lifestyle habits, andchanges at the molecular level on disease development, prognosis, and outcomes[64,65].Therefore, analyzing the impact of factors such as diet on disease progression in a clinical sample of colorectal cancer patients is a novel and interesting aspect.
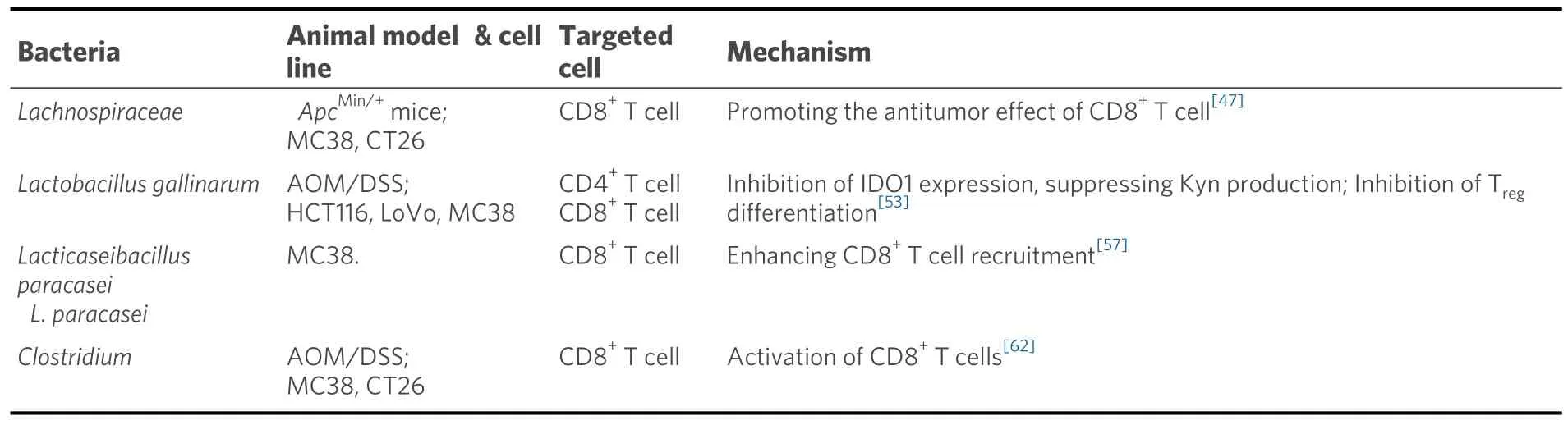
Table 2.Gut microbiota in CRC- immunotherapy
Yanget al.found that a high-fat diet can lead to an increase in some bacteria associated with colorectal cancer in the intestinal tract of mice, and a high-fat diet can alter the levels of intestinal metabolites, for example, an increase in the expression of lysophosphatidic acid (LPA) can stimulate proliferation and inflammation of intestinal epithelial cells[66].
In addition, a high-fat diet can impair the function of the intestinal epithelial barrier, making the gut more vulnerable to bacteria and metabolites.It has also been shown that high-fat diets and APC mutations can alter bile acid (BA) levels in the body, leading to proliferation and DNA damage in intestinal stem cells,which promotes the formation and progression of CRC[67].
In addition, calorie restriction, fasting food during fasting phase, Vitamin D and ketone bodies can decrease CRC risk in humans/animal models while fructose in diets can increase it in animal models and some epidemiological associations[68].All of the above evidence suggests a correlation between diet and the development of colorectal cancer, and diet control may be able to serve as a new target and strategy for the prevention and treatment of colorectal cancer.
The emergence of drug-resistant pathogens can also have an impact on the efficacy of clinical treatment.The presence of antibiotic resistance genes (ARGs) in the microbiota is characterized by quantity, identity,and function, which are collectively referred to as resistome[69].There are already a number of strategies to combat drug-resistant microorganisms, such as those that utilize the CRISPR Cas9 system to eliminate selected bacterial targets[70].Furthermore, combining molecular pathological epidemiology with microbial resistance and other factors, the strategy of rationally creating treatment methods to achieve better clinical treatment outcomes may have broad prospects.
Probiotic interventions
Studies have shown that the composition of gut microbiota changes significantly with the progression of CRC[71].Driver bacteria increase in the early stages of CRC, providing conditions for tumor formation.Passenger bacteria are able to maintain CRC through metabolites.Furthermore, some probiotics can inhibit the progression of colorectal cancer.In conclusion, alterations in the ratio of gut microbiota may lead to dysregulation of homeostasis in the intestinal environment.Therefore, effective utilization of gut microbiota that play different functions in CRC may provide new ideas and methods for its prevention and treatment.
Notable among these is probiotic supplementation.Probiotics are active bacteria isolated from the gut or fermented foods that can be used as food or food supplements for improving gut microbiota homeostasis or for preventive treatment of specific diseases[72].Some studies have shown that patients who underwent surgical resection of CRC displayed a decrease in CRC-associated bacteria (Fusobacterium,Porphyromonas,andAlloprevotella) after being given probiotic (Clostridium butyricum,Lactobacillus plantarum, andBifidobacterium) treatments and a reduction in postoperative complications[73,74].
CONCLUSION
Although our current understanding of microorganisms is limited, it is undeniable that microorganisms, as an important part of the animal body, have broad therapeutic prospects.In recent years, more and more attention has been paid to the study of gut microbes.Existing studies confirmed that the efficiency of gut microbiota has been extensively studied in CRC treatment, and certain results have been achieved, such as the enhancement of activation of CD8+T cells by microorganisms and the enhancement of anti-PD-1 immunotherapy.This signals that gut microbes may be able to act as an adjuvant to enhance immunotherapy.In addition, intestinal microbes can also use some targeted pathways, such as the MyD88-NF-κB signaling pathway, to regulate the levels of inflammatory factors such as IL-6 and IL-10, thereby inhibiting CRC progression.However, most of the results have only been verifiedin vivoin mice and have not been applied in clinical practice.Due to the similarities in the immune environment between humans and mice, there are still great limitations in clinical application.
Despite many difficulties, current research still shows that therapeutic strategies targeting microbiota will become a key area of personalized cancer treatment, and the strategies of microbes as adjuvants in various therapies also have broad prospects.
DECLARATIONS
Authors’ contributions
Made substantial contributions to the conception and design of the study and performed data analysis and interpretation: Zhou X, Zhao Y, Zhao R, Shafi S, Yang Y
Performed data acquisition, as well as providing administrative, technical, and material support: Liu SB, Liu G
Availability of data and materials
Not applicable.
Financial support and sponsorship
This study was supported by the Project of Jiangsu Province Engineering Research Center of Molecular Target Therapy and Companion Diagnostics in Oncology, Jiangsu higher education institution innovative research team for science and technology (2021), Program of Jiangsu vocational college engineering technology research center (2023), Key technology program of Suzhou people's livelihood technology projects (Grant No.SKY2021029), Key Programs of the Suzhou Vocational Health College(SZWZYTD202201), Qing-Lan Project of Jiangsu Province in China (2021, 2022).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
 Journal of Cancer Metastasis and Treatment2024年3期
Journal of Cancer Metastasis and Treatment2024年3期
- Journal of Cancer Metastasis and Treatment的其它文章
- Neutrophils in the tumor microenvironment: role in tumor progression and potential targeted therapeutic strategy
- Research advances in immunotherapy combined with DNA damage response inhibitors for liver cancer therapy
- ImmuneScore as a novel RNA-based prognostic signature superior to PD-L1 in advanced nonsquamous NSCLC patients receiving chemotherapy combined with immune checkpoint inhibitor therapy
