Consideration of liquid biomarkers for surveillance of HPV-related oropharyngeal cancer in veteran populations
Joshua D.Smith, Matthew E.Spector, J.Chad Brenner,3,#, Jessica H.Maxwell
1Department of Otolaryngology - Head & Neck Surgery, University of Michigan, Ann Arbor, MI 48109, USA.
2Department of Otolaryngology - Head & Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA.
3Rogel Cancer Center, University of Michigan, Ann Arbor, MI 48109, USA.
4Section of Otolaryngology, Pittsburgh Veterans Affairs Medical Center, Pittsburgh, PA 15240, USA.
#Authors contributed equally.
Abstract The incidence of human papillomavirus (HPV)-related oropharyngeal squamous cell carcinoma (OPSCC) will continue to rise in the United States over the next several decades.Thus, efforts to reduce treatment intensity,mitigate long-term physical and psychological sequelae of treatment, and simplify surveillance regimens for patients with HPV-related OPSCC are critical.Liquid biomarkers, namely plasma circulating tumor HPV DNA(ctDNA), have shown considerable promise for improvements in these domains by guiding personalized and adaptive treatment de-escalation paradigms and predicting disease recurrence in the survivorship period.Preliminary reports suggest an even broader impact of plasma HPV ctDNA assays for HPV-related OPSCC surveillance beyond the mere detection of cancer recurrence and metastasis.For instance, such assays may reduce the need for costly imaging studies, alleviate the financial toxicities of survivorship care, and improve care access and patient satisfaction.Currently, veterans and underserved populations are disproportionately affected by the financial burden of cancer surveillance and survivorship care.These disparities negatively impact oncologic outcomes, healthcare access, and utilization, specifically among veterans with HPV-related OPSCC.As such, we posit that HPV ctDNA monitoring may be of unique benefit and impact in the surveillance period for these patients specifically.Herein, we provide a narrative review of the current literature supporting the formal clinical evaluation of HPV ctDNA monitoring in veterans with HPV-related OPSCC.
Keywords: HPV, ctDNA, liquid biomarker, oropharynx, squamous cell carcinoma, surveillance, veterans
INTRODUCTION
The incidence of human papillomavirus (HPV)-related oropharyngeal squamous cell carcinoma (OPSCC)has reached epidemic proportions in the United States[1].Initiatives to promote widespread HPV vaccination for primary prevention are critical and ongoing, though they will not appreciably impact rising OPSCC rates for several decades[2].Certain vulnerable patient populations, including our United States(U.S.) veterans, are and will continue to be afflicted disproportionately by this disease and the sequelae of its treatment[3].Generally, HPV-related OPSCC is associated with excellent five-year survival after standard-ofcare treatment[4].Thus, efforts are underway to de-escalate definitive treatment and reduce long-term toxicities in select patients with HPV-related OPSCC[5,6].
The cancer survivorship period begins at the time of diagnosis and continues post-treatment, representing a dynamic and complex time for patients with HPV-related OPSCC.For patients and providers alike,surveillance for cancer recurrence is arguably the greatest priority, as a small but sizable subset of patients(15%-20%) with HPV-related OPSCC will develop locoregional recurrence or distant metastasis[4,7].Importantly, in patients with HPV-related OPSCC, distant metastases are proportionally more common and develop later than in HPV-negative OPSCC[8,9].Furthermore, HPV-related recurrences and metastases are often asymptomatic and undetectable on physical exam[10].As such, HPV-related OPSCC demands unique surveillance strategies to identify these recurrences at an early, and potentially treatable, stage.
Beyond cancer surveillance, important considerations for the survivorship care of patients with HPVrelated OPSCC include rapid identification and amelioration of physical toxicities (e.g., cervical fibrosis,osteoradionecrosis, dysphagia)[11]and psychosocial distress (e.g., depression, anxiety)[12].Dedicated efforts to address comorbid substance use (e.g., tobacco, alcohol) and promote overall physical health (e.g., oral/dental hygiene) are essential[13].Ideal survivorship care for these patients should also maximize costeffectiveness and reduce the burden to minimize socioeconomic stressors that disproportionately affect vulnerable populations with HPV-related OPSCC[14].In the following sections, we review current recommendations for optimal surveillance for HPV-related OPSCC after definitive treatment and how the emergence of HPV ctDNA as a liquid biomarker has the potential to drastically alter such paradigms.In recognition of a paucity of published literature on the topic, we specifically discuss how HPV ctDNA monitoring may be of unique benefit and impact in the survivorship period for veteran populations.
GUIDELINES FOR SURVEILLANCE OF HPV-RELATED OPSCC
Current National Comprehensive Cancer Network (NCCN) guidelines for post-treatment surveillance of patients with head and neck cancers do not accurately reflect the unique prognosis, recurrence patterns, and toxicity considerations for those with HPV-related OPSCC[15].For all head and neck squamous cell carcinoma (HNSCC), regardless of subsite or HPV status, NCCN recommends frequent (year one: every 1-3 months; year two: every 2-6 months; year three through five: every 4-8 months and annual visits after year five) in-person clinic visits for physical and flexible endoscopic exam.Beyond the three-month response assessment with CT or FDG PET/CT, current NCCN guidelines note a lack of consensus recommendations for the frequency and modality of post-treatment imaging studies in asymptomatic patients[15].
Importantly, the feasibility and efficacy of contemporary surveillance protocols for HPV-related OPSCC are largely unproven.In a retrospective cohort study of 233 patients with HPV-related OPSCC, 23 patients experienced recurrences[16].All but one of these recurrences were detected after patients reported related symptoms.Only one was asymptomatic and detected on a routine clinical exam[16].They further demonstrated that adherence to NCCN guidelines for follow-up did not portend improved disease-specific survival.As such, the authors of this study ultimately advocated for de-escalation of contemporary NCCN follow-up recommendations for HPV-related OPSCC[16].Notably, current surveillance practices exhibit insensitivity in detecting distant metastases, which are disproportionately more common than locoregional recurrences in this population[17].Furthermore, the economic costs of frequent in-person clinic visits to patients are often substantial, influencing their preference for altered surveillance paradigms[18].Thus, novel patient-centered approaches to surveillance of HPV-related OPSCC with enhanced practicality and efficacy are desperately needed.Of course, these approaches must be evidence-based, and their efficacy and safety compared against the current NCCN standards prior to implementation.
LIQUID BIOMARKERS FOR SURVEILLANCE OF HPV-RELATED OPSCC
As a minimally invasive approach for dynamic assessment of tumor burden, plasma HPV ctDNA monitoring is actively transforming traditional surveillance paradigms for HPV-related OPSCC[19].These assays leverage ultrasensitive sequencing methods to detect cell-free HPV ctDNA shed from the primary tumor or metastatic deposits into circulation[19-21].Numerous robust studies have shown promising sensitivity, specificity, and positive and negative predictive values of these assays for detecting locoregional and distant recurrences of HPV-related OPSCC[22-25].We direct the reader to Kuhset al.for a contemporary review of published plasma HPV ctDNA test parameters[19].
The primary value of these assays for cancer surveillance lies in their ability to detect recurrences before they manifest symptomatically or can be detected on routine physical exams or imaging studies[26].Although large prospective, controlled trials are needed, several retrospective observational studies have shown promising lead times of between 19 days to 18 months for a positive plasma HPV ctDNA test[21,23,24].While currently unproven, this lead time may permit earlier surgical salvage or initiation of systemic therapy with tangible survival benefit[27].However, whether such treatment should be routinely initiated prior to confirmation of recurrence with anatomic or functional imaging remains an area where clinical trials are still needed to formally assess clinical utility.A commercially available assay (NavDx®, Naveris, Waltham,MA) has now permitted widespread, though heterogeneous, incorporation of plasma HPV ctDNA monitoring into routine surveillance paradigms for HPV-related OPSCC[28], though this is not yet recommended for surveillance by the NCCN guidelines.
Several other advantages of plasma HPV ctDNA monitoring for survivorship care of patients with HPVrelated OPSCC have been proposed, albeit with less empirical support.First is cost-effectiveness compared to standard NCCN follow-up guidelines, as described above.In a sophisticated cost modeling study,Wardet al.showed plasma HPV ctDNA monitoring to be cost-saving for surveillance when its use reduced the frequency of imaging studies[29].The possibility of reducing the frequency of surveillance imaging with subsequent cost reduction is promising, though it certainly demands further prospective study to confirm the safety of this approach.Second is the potential for broadening survivorship care access and convenience for patients with specific sociodemographic or geographic barriers.Our group is currently developing a robust, “second generation”, urine-based HPV ctDNA assay that would permit at-home collection at regular intervals with specimens mailed to a central laboratory for analysis[30].Saliva-based assays may hold similar promise, though presently lack similar empirical support to plasma- and urine-based assays[31].Third is the harmonization of surveillance protocols with patients’ priorities and preferences.Surveys of patients with early-stage HPV-related OPSCC show strong interest in blood- or urine-based HPV ctDNA monitoring, reflective of a desire for altered surveillance paradigms that attenuate surveillance-related burdens[18].
A notable limitation of the published literature on plasma HPV ctDNA monitoring is a lack of assessment of its efficacy, feasibility, and applicability for surveillance of specific patient subgroups.Whether plasma HPV ctDNA monitoring has equivalent power to detect recurrence after definitive (chemo)radiationvs.transoral robotic surgery (TORS) for upfront treatment of HPV-related OPSCC remains to be seen.Moving forward, prospective studies should consider stratification by age, sex, race and ethnicity, smoking status,and HPV genotype[19].For instance, as HPV-related OPSCC disproportionately affects males, sex-specific differences in the clinical utility of plasma HPV ctDNA require further study.The literature is currently lacking in this regard.Further, despite the promise and proliferation of plasma HPV ctDNA assays as clinically useful biomarkers, pre-clinical investigations into other possible biomarkers with translational relevance (e.g., oral microbiome composition, tumor-derived exosomes) are needed.
BURDEN OF HPV-RELATED OPSCC IN VETERANS
Epidemiological trends in HPV-related OPSCC incidence within the U.S.Veteran population largely mirror that of the civilian population.From 2006-2012, Zevalloset al.reported an annual percent change of +7.19%in incident cases of HPV-related OPSCC within the Veterans Affairs Healthcare System (VHA)[3].This statistically significant rise was noted across all age and ethnicity groups.Presently, the incidence of HPVrelated OPSCC within the U.S.Veteran population is estimated to be between 2-3-fold higher than the estimated rate of 45,000 incident cases/yearly in the U.S.general population[32-34].Unfortunately, the prevalence of HPV vaccination among eligible U.S.veterans is roughly one-half that of their civilian counterparts.For example, among Veterans aged 18 to 26, only 30.2% of females and 18.7% of males have received HPV vaccination compared to 62.4% of females and 37.0% of males in the U.S.general population[34].Thus, it will be several more decades before the incidence of HPV-related OPSCC in this population peaks and begins to decline[34].
In comparison to the civilian population, veterans with HPV-related OPSCC are distinguished by higher rates of significant tobacco use and “intermediate-risk” disease (i.e., HPV-related OPSCC with > 10 packyear tobacco use and advanced-stage disease)[4,35].This, coupled with the disproportionate burden of comorbidities and poor social support characteristics of Veteran populations, poses unique challenges for treatment and surveillance[34].Additionally, individual VHA centers vary widely in their infrastructure to support research and clinical trials, thus limiting access to potentially beneficial deintensification paradigms for veterans[35].Clearly, the landscape of HPV-related OPSCC care for U.S.veterans is unique and demands innovative and personalized techniques for cancer surveillance.
By many metrics, the quality of cancer care within the U.S.VHA is equivalent to private sector healthcare systems[36,37].Nevertheless, several studies have suggested significantly worse disease-specific survival for veterans with HPV-related OPSCC[32,35].This is perhaps attributable to their higher proportion of“intermediate-risk” diseases with biological behaviors that mimic HPV-negative OPSCC[32].However, other important factors, such as comorbid substance use, psychiatric disorders, and financial barriers, likely contribute[38].For veteran survivors of HPV-related OPSCC, physical and psychological sequelae of treatment are even more amplified[35].A summary of published studies on HPV-related OPSCC specifically in veteran populations is provided in Table 1.
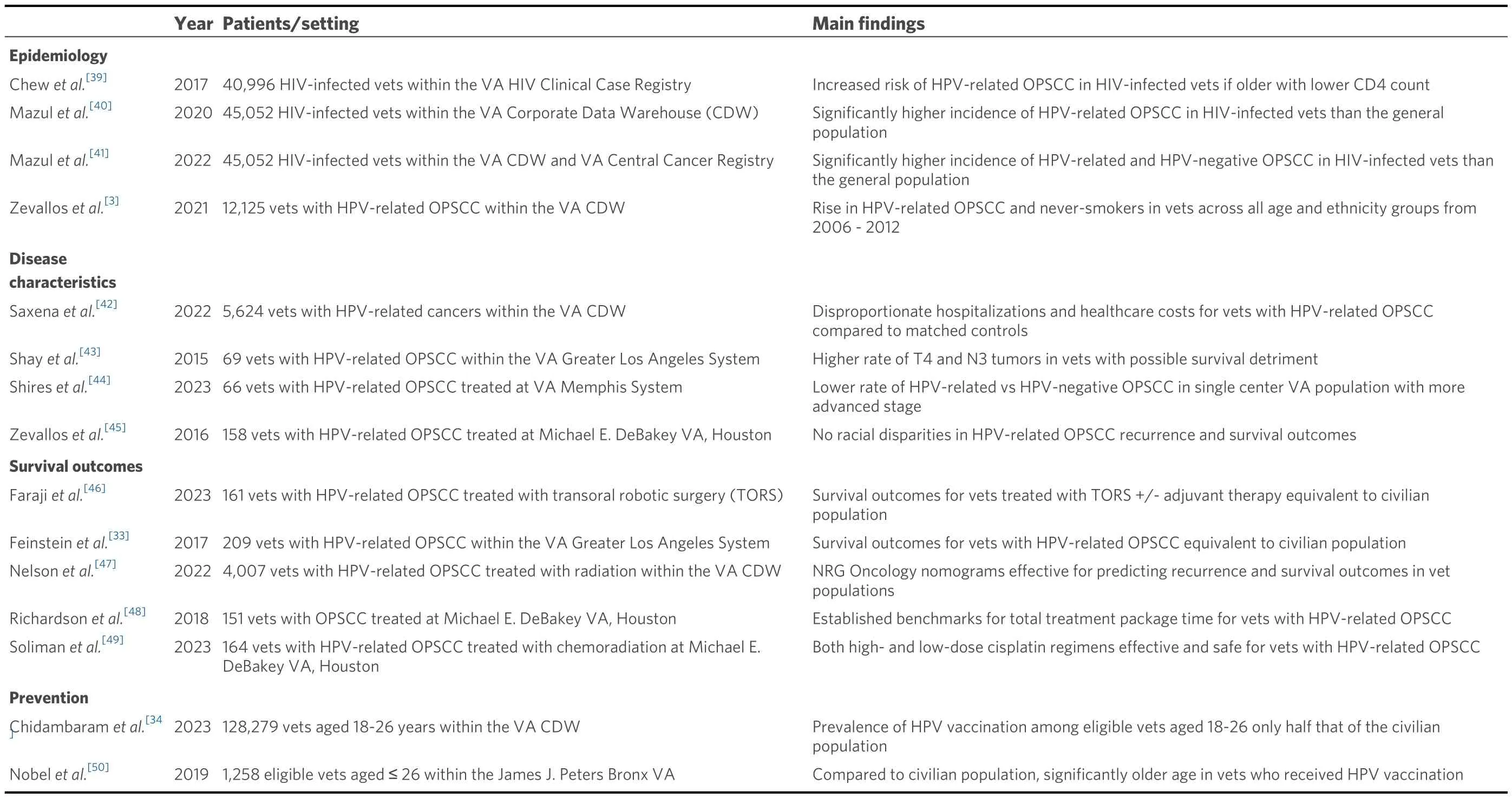
Table 1.Summary of published studies on HPV-related OPSCC in U.S.veterans
PLASMA HPV CTDNA MONITORING FOR HPV-RELATED OPSCC SURVEILLANCE IN VETERANS
Presently, there is a paucity of published literature on the feasibility and efficacy of plasma HPV ctDNA monitoring in the U.S.Veteran population.The advantages of these assays for HPV-related OPSCC survivorship care in civilians, including improved disease surveillance and possibility of enhanced patient convenience and satisfaction, are similarly applicable for veterans with HPV-related OPSCC.However, we posit that HPV ctDNA monitoring may, in fact, be of unique benefit and impact in the survivorship period for these patients, as illustrated in the following three domains and summarized in Figure 1.
A biologically distinct population
In 2019, a “Field-Based Meeting” (FBM) was convened with the goal of identifying unmet needs in the clinical care of veterans with HPV-related OPSCC[35].Participants of the FBM identified a principal need for improved biomarker signature(s) that accurately predict recurrence and survival in intermediate-risk patients.This need was informed by several publications showing a disproportionately high rate of “dual exposed” veterans with HPV-related OPSCC and significant tobacco use history[32,51].Shortly after the FBM,the first prospective HPV ctDNA biomarker study was reported by Cheraet al.[28].
Biologically, intermediate-risk HPV-related OPSCC displays highly variable mutational signatures and distinct tumor-immune microenvironments with characteristics of both carcinogen and virally mediated HNSCC[52].Clinically, these patients may respond less favorably to treatment de-intensification and experience higher rates of delayed recurrences and distant metastases[51].Thus, these patients have a distinct need for robust predictive biomarkers that accurately reflect their unique tumor biology and risk profile.The limitations of current NCCN guidelines for HPV-related OPSCC are particularly evident in the intermediate-risk Veteran population due to their comparative disease heterogeneity and aggressiveness.Clearly, a “one size fits all” approach to surveillance is insufficient in this population.In the future, we envision more personalized surveillance paradigms for patients with HPV-related OPSCC, with plasma HPV ctDNA monitoring as the backbone, with the frequency of visits and additional tests (e.g., imaging)dictated by biological risk profiles.
Plasma HPV ctDNA monitoring has shown robust statistical parameters for prediction and detection of locoregional and distant recurrences across several HPV-related OPSCC subgroups, including those with significant tobacco use history[20,21,25].While no published study has been powered to examine this population specifically, preliminary results suggest that the kinetics of plasma HPV ctDNA levels accurately reflect the unique biology and disease activity of intermediate-risk HPV-related OPSCC[19].In fact, given the higher rate of recurrence and metastases in this population, positive and negative predictive values of these assays may be enhanced, but this requires further study.With a higher pre-test probability for disease recurrence, the likelihood of lead time provided by these assays yielding a tangible survival benefit is only heightened for veterans with HPV-related OPSCC.
Reducing burden of disease surveillance and survivorship care
Beyond cancer surveillance, an ideal survivorship care paradigm for patients with HPV-related OPSCC would maximize cost-effectiveness, convenience, accessibility, and patient satisfaction.Each of these factors is crucial in a veteran population disproportionately faced with unique sociocultural, financial, and psychological challenges.We posit that the incorporation of plasma HPV ctDNA monitoring into routine survivorship care paradigms for veterans with HPV-related OPSCC would yield tangible improvement in each of these metrics.
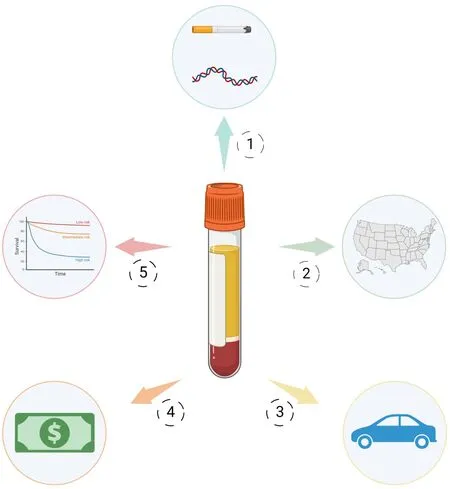
Figure 1.Unique benefits of plasma HPV ctDNA monitoring for survivorship care of veterans with HPV-related OPSCC.1.A biologically distinct population: enhanced utility of plasma HPV ctDNA monitoring in recurrence prediction for intermediate-risk disease;2.Broadened geographic accessibility: ability to surveil a greater number of patients in wide catchment area via “surveillance at a distance” paradigm; 3.Reduction of logistical barriers and enhanced patient satisfaction: reduction of in-person visits and imaging afforded by plasma HPV ctDNA monitoring attenuates the burden of transportation, lodging, and lost work time; 4.Reduced costs for VHA and veterans alike: plasma HPV ctDNA monitoring may allow economic triaging of patients who need in-person care; 5.Equal opportunities for treatment and surveillance de-intensification via clinical trials.Created in BioRender.com.
The U.S.VHA has spent approximately $136 million to treat veterans with HPV-related cancers[34].Such exorbitant costs will only continue to rise in the coming decades.From 2014-2018, Saxenaet al.estimated a total treatment cost of $82,763 per patient with HPV-related OPSCC within the VHA[42].This cost was eight times higher than the average VHA patient, though it notably did not include the longitudinal costs of survivorship care.The prospect of plasma HPV ctDNA monitoring supplanting in-person surveillance visits, imaging, and/or exploratory biopsies is particularly desirable for the VHA to maximize the costeffectiveness of care delivery[29].However, we recognize that prospective clinical trials providing definitive support for such altered surveillance paradigms are critical.
Individual VHA centers differ widely in their facilities, personnel, and resources for multidisciplinary cancer care[35].Those centers equipped for survivorship care of veterans with HPV-related OPSCC service a broad catchment area encompassing urban, suburban, and rural demographics.[35]Numerous studies have shown a detrimental impact of “distance to facility” on metrics such as time to treatment initiation[53],completion of radiation therapy[54,55], and survival[56]for veterans with various cancer types.Undoubtedly,the logistical and financial burdens of transportation and lodging for interval surveillance appointments may prove untenable for many veterans with HPV-related OPSCC.Routine plasma (or urine)[30]HPV ctDNA monitoring may thus be the backbone of a “surveillance at a distance” paradigm in which veterans are seen in person only for new symptomatic concerns noted during a virtual visit or when ctDNA kinetics prompt concern for recurrence.The potential benefits of such a novel paradigm on survivorship care access,convenience, and affordability for veterans with HPV-related OPSCC are myriad.
Equal opportunity for de-intensification of treatment and surveillance
The participants of the 2019 FBM noted that the results of contemporary de-intensification trials (e.g.,E3311 and the PATHOS trial) for HPV-related OPSCC cannot be readily extended to Veteran populations given their unique disease biology and outcomes[5,57,58].Thus, they identified a major goal for clinical trial development specifically for intermediate-risk HPV-related OPSCC within the VHA.Despite their identical need for safe, effective treatment and surveillance de-intensification, veterans lag behind their civilian counterparts in clinical trial access and enrollment.
As a predictive biomarker for safe de-intensification, plasma HPV ctDNA monitoring may significantly advance the care of our nation’s veterans, both by improving survival outcomes and mitigating treatmentrelated toxicities.Validation is urgently needed in this population to permit equal opportunities afforded to civilian populations.Multiple clinical trials examining the utility of plasma HPV ctDNA as a biomarker for de-intensification of definitive treatment and/or surveillance are currently accruing, including the SIRS 2.0[59]and ReACT (NCT04900623) trials.The results of these trials are eagerly awaited, as they may support the conclusion that plasma HPV ctDNA is a robust, reproducible biomarker for safe de-intensification in HPV-related OPSCC.We echo the call of the 2019 FBM for the development of plasma HPV ctDNA-based clinical trials in the U.S.VHA.
CONCLUSION
Veterans with HPV-related OPSCC are a rapidly growing population with comparatively poorer outcomes and unique geographic and socioeconomic barriers compared to the general population.The potential benefit of plasma HPV ctDNA monitoring in the survivorship care of these patients goes beyond prediction of recurrence, but also still requires formal clinical trials to evaluate clinical utility.Such assays, if successful and if incorporated into routine surveillance paradigms, may significantly enhance disease surveillance and alleviate financial, psychological, and social stressors of HPV-related OPSCC care.
DECLARATIONS
Authors’ contributions
Conceptualized and planned, revised the manuscript: Smith JD, Spector ME, Brenner JC, Maxwell JH
Wrote the original manuscript draft: Smith JD
Supervised the study: Spector ME, Brenner JC, Maxwell JH
All authors read and approved the final manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This manuscript was supported by a 2022 AHNS CORE Grant Presidential Award.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
 Journal of Cancer Metastasis and Treatment2024年2期
Journal of Cancer Metastasis and Treatment2024年2期
- Journal of Cancer Metastasis and Treatment的其它文章
- Editorial on “Chinese expert consensus on the clinical practice of non-small cell lung cancer fusion gene detection based on RNA-based NGS” (2023 edition)
- Integration of community pharmacies in an Italian colorectal cancer screening program: insights from the Local Health Authority of Bologna
- Cancer-associated fibroblasts (CAFs) based model reveals potential for predicting bladder cancer patients’ prognoses and immunotherapy responses
