Sequential experimental observation on the curative effect of Yingbupu decoction of Zhuang medicine on stage I and II acute kidney injury
ZHOU Yan, HUANG Guo-dong, XIE Zhang-qing, ZHOU Chang-yan, LIANG Jing-yan,LUO Jia
1. Guangxi University of Chinese Medicine, Nanning 530000, China
2. Guangxi lnternational Zhuang Medicine Hospital, Nanning 530000, China
3. Nanning Traditional Chinese Medicine Hospital, Nanning 530000, China
Keywords:
ABSTRACT Objective: To observe the clinical efficacy of the Zhuang medicine Yingbupu decoction on stage I and II acute kidney injury through sequential test.Methods: The open one-way qualitative response sequential design of experiments was adopted, and the patients with AKI in phase I and II who met the inclusion criteria were divided into the treatment group and the control group according to the order of hospitalization by random number table.On the basis of basic treatment, the treatment group was treated with Zhuang medicine Yingbupu decoction,and the control group was treated with Jinshuibao tablet.The clinical efficacy, TCM syndrome score, 24 h urine volume, serum creatinine (Scr), microalbumin in urine (mAlb), neutrophil Gelatinase related lipid delivery albumin (NGAL) of the two groups were compared, and the adverse reactions and complications of the two groups were observed.Results: After 14 d of treatment, when the treatment group reached the 10th case, the experimental line contacted the upper bound U-line and reached the experimental standard to terminate the experiment.The effective hypothesis was accepted, and it was believed that the Zhuang medicine Yingbupu decoction had a therapeutic effect on stage I and II AKI.The conclusion was drawn that the treatment group received the Zhuang medicine Yingbupu.The clinical effective rate and improvement days were similar between the two groups, and there was no significant difference (P>0.05).However, the integral value of traditional Chinese medicine syndrome in the treatment group was lower than that in the control group (P<0.05), After treatment, the Scr,mAlb, and NGAL levels of patients in both groups were lower than before treatment(P<0.05).After treatment, the Scr, mAlb, and NGAL values in the treatment group were significantly lower than those in the control group(P<0.05).After treatment, the 24-hour urine volume in both groups was higher than that before treatment, and the values in the treatment group were significantly higher than those in the control group (P<0.05).During the treatment period,there were no significant adverse reactions or complications in either group.Conclusion:The Zhuang medicine Yingbupu decoction is effective in treating stage I and II AKI, and the Zhuang medicine Yingbupu can significantly improve the symptoms and quality of life of patients with stage I and II AKI.Its improvement of renal function is better than that of Jinshuibao tablets, and its safety is good.
1.Introduction
Acute renal injury (AKI) is a clinical syndrome caused by rapid decline of renal function in a short time caused by various causes[1].Its main manifestation is the decrease of glomerular filtration rate(GFR), the retention of nitrogen products such as creatinine (Scr)and urea nitrogen (BUN), and the disturbance of water, electrolyte and acid-base balance, and even multiple organ and multi-system complications.AKI is one of the diseases seriously harmful to human health, and its incidence is increasing year by year.It is directly related to the development of chronic kidney disease (CKD),other organ dysfunction and mortality[2].It has become an important public health problem in the 21st century.Modern medical treatment of AKI mainly includes fluid resuscitation, drug prevention and treatment and renal replacement[3].Renal replacement therapy is one of the important methods, but it has the disadvantages of timeconsuming and high cost, and the curative effect is not satisfactory.At present, the progress of AKI is reversible.Unfortunately, no drugs for the prevention and treatment of AKI have been released at home and abroad.In contrast, traditional Chinese medicine (TCM) and ethnic medicine have a long history in the treatment of renal diseases such as nephrolithiasis, chronic glomerulonephritis, nephrotic syndrome and acute and chronic renal injury.In this study, the use of Zhuang medicine Yingbupu decoction in the treatment of stage I and II AKI by sequential trial has a considerable clinical effect, which is reported below.
2.Objects and methods
2.1 Diagnostic criteria
Diagnostic criteria of Western medicine : according to the diagnostic criteria and staging of AKI clinical practice guidelines issued by KDIGO[4] in 2012.The standard of diagnosis and disease differentiation of Zhuang medicine : in line with the ‘Benfu’ in‘Zhuang medicine internal pediatrics’ [5] : the main symptoms: eyelids, local edema of limbs or whole body edema, and the concurrent symptoms can be seen.Dysuria, fever, fear of wind, limb pain, body weight loss, chest tightness, irritability, thirst, constipation and so on.TCM diagnosis and syndrome differentiation criteria :in line with the 2012 Wu Mianhua editor-in-chief of the ‘Chinese medicine internal medicine’ [6] and the 2012 State Administration of traditional Chinese medicine compiled the ‘TCM syndrome diagnosis and efficacy standards’ [7] of lung and kidney deficiency and damp heat and blood stasis syndrome, symptoms of low back pain, edema, nocturia, fatigue, abdominal distension, dry mouth,dry throat, yellow urine, skin nails, pale red or dark red tongue, thin white or thin yellow moss, fine pulse, smooth pulse or fine pulse astringent, and combined with clinical.
2.2 Inclusion criteria
(1) Western medicine in line with the diagnosis of AKI clinical practice guidelines developed by KDIGO and stage I and II AKI patients; (2) Zhuang medicine disease diagnosis in line with the ‘Benfu’ diagnosis, TCM syndrome differentiation in line with the syndrome type of lung and kidney deficiency and damp heat and blood stasis patients; (3) Patients aged 18-70 years old, male or female; (4) Could insist on taking Zhuang medicine decoction 14 days; (5) Voluntary participation in this study, patients and their families to sign the relevant informed consent, high compliance.
2.3 Exclusion criteria
(1) AKI caused by prerenal and postrenal AKI; (2) pregnant and lactating women; (3) patients with severe cardiovascular and cerebrovascular diseases or malignant tumors; (4) patients with severe liver dysfunction; (5) Patients who were allergic to the drugs used in this study; (6) According to the actual situation of the patients, the researchers believe that they can not complete all clinical trials, affecting the data statisticians.
2.4 Research methods and steps
2.4.1 Standard for designing sequential trials
According to the 2012 KDIGO AKI diagnostic criteria and 2002‘Chinese medicine new drug clinical research guidelines ( Trial )’[8], Scr was used as a sequential test measurement index.According to the relevant clinical experience and the current literature report[9-11], the open one-way qualitative reaction sequential test was adopted, and the test criteria were as follows : 1 After 2 weeks of medication, Scr decreased to normal P1> 95 %, and it was considered that the drug was effective in the treatment of stage I and II AKI, and the conclusion of accepting the drug was made.2 After 2 weeks of medication, Scr decreased to normal P0< 60 %, and it was considered that the drug was ineffective in the treatment of stage I and II AKI, and the conclusion of rejecting the drug was made.3 The allowable false positive rateα when the positive conclusion is obtained ; ( 4 ) The false negative rate β which is allowed when the negative conclusion is drawn is stipulated.The test standard : P1=95 %, P0= 60 %, false positive rate α = 0.01, false negative rate β= 0.01.
2.4.2 Find out the boundary line and sequence diagram drawing process
According to the four values of the test standards P1, P0,α , and β,the upper bound equations of the effective and ineffective drugs are known.The original equation is as follows : U : y = a + b · n ;l : y = -a + b · n where n is any number of samples.The two linear equations are obtained by looking up the table : U ( upper bound) : y = 1.81 + 0.819n ; l ( lower bound ) : y = -1.81 + 0.819 n.The determined efficacy of each Zhuang medicine Yingbupu Decoction or Jinshuibao Tablets in the treatment of AKI patients was tested on the figure.In case of effective cases, the upward diagonal line is drawn, and the invalid horizontal line is drawn.The sequential test line is drawn with the number of patients as the abscissa and the number of effective cases as the ordinate.
2.5.General information
A total of 25 patients with stage I and II AKI with lung and kidney deficiency and damp heat and blood stasis syndrome were included in this study.The patients included in the study came from the Guangxi international Zhuang Medicine Hospital and the Ruikang Hospital affiliated to Guangxi University of Traditional Chinese Medicine.According to the order of hospitalization, 25 patients were divided into treatment group and control group by querying the random number table method.There were 10 cases in treatment group and 15 cases in control group.The baseline data ( gender, age,course of disease ) of the two groups were compared.There were 7 males and 3 females in the treatment group, with an average age of( 50.90 ± 12.64 ) years and an average course of disease of ( 4.14 ±1.38 ) days.In the control group, there were 11 males and 4 females,with an average age of ( 47.81 ± 13.86 ) years and an average course of disease of ( 4.23 ± 2.06 ) days.There was no significant difference between the two groups ( P > 0.05 ).The baseline characteristics were similar and comparable.See Table 1.
Tab 1 Comparison of general information between two groups of patients before treatment(±s)
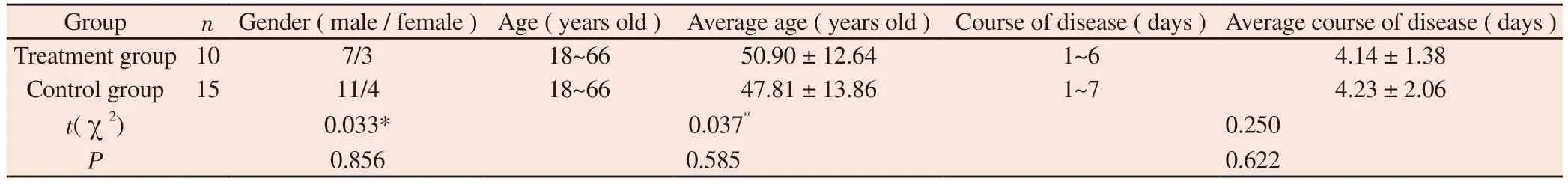
Tab 1 Comparison of general information between two groups of patients before treatment(±s)
The ‘a’ means χ2.
Group n Gender ( male / female ) Age ( years old ) Average age ( years old ) Course of disease ( days ) Average course of disease ( days )Treatment group 10 7/3 18~66 50.90 ± 12.64 1~6 4.14 ± 1.38 Control group 15 11/4 18~66 47.81 ± 13.86 1~7 4.23 ± 2.06 t(χ2) 0.033* 0.037* 0.250 P 0.856 0.585 0.622
2.6 Treatment methods
2.6.1 Basic treatment
(1) Health education was given to the selected AKI patients;patients were advised to pay attention to weather changes, moderate exercise, avoid smoking and drinking, and take drugs for liver and kidney damage; diet control: adjust according to the condition;(2) Other symptomatic treatment: active prevention of infection,regulation of blood pressure, blood lipids, anti-platelet aggregation and other symptomatic treatment.
2.6.2 The treatment group
Was treated with Zhuang medicine Yingbupu Decoction ( Yingbupu 25 g, decocted into 50 mL, vacuum packaging bag, provided by Zhuang Yao Medicine Preparation Center of Guangxi International Zhuang Medical Hospital, refrigerated or stored in dark at room temperature ) for intervention treatment, 2 times a day, 1 bag each time ( 50 mL / bag ), warm clothes after meals, for 14 consecutive days.
2.6.3 The control group
Used Jinshuibao tablets ( Specification : 0.42g / tablet, Jiangxi Jimin Credibility Co., Ltd., Guozi No.Z20003207 ).Validity : 5 years, preserved in a cool and dry place ) treatment.Take 4 tablets at a time, 3 times a day, after meals, continuous treatment for 14 days.
2.7 Observation indicators
2.7.1 Evaluation of efficacy
The treatment group and the control group before and after treatment Scr, TCM syndrome score changes.
2.7.2 Evaluation of laboratory indicators
24 h urine volume, creatinine ( Scr ), urinary microalbumin ( mAlb), neutrophil gelatinase-associated lipocalin ( NGAL ).
2.7.3 Observation of safety indicators
Routine detection of two groups of patients with vital signs, three routine ( blood routine, urine routine, stool routine ), liver function and electrolyte, 14 d / time, and the occurrence of adverse reactions in the two groups of patients during treatment.
2.8 Efficacy evaluation criteria
2.8.1 Evaluation standard of comprehensive curative effect of disease
According to KDIGO‘s AKI diagnostic criteria : Scr was used as a sequential test measurement index.1 After 2 weeks of medication,Scr decreased to normal P1> 95 %, and it was considered that the drug was effective in the treatment of stage I and II AKI, and the conclusion of accepting the drug was made.2 After 2 weeks of medication, Scr decreased to normal P0< 60 %, and it was considered that the drug was ineffective in the treatment of stage I and II AKI, and the conclusion of rejecting the drug was made.
2.8.2 TCM syndrome score
According to the ‘Guiding Principles for Clinical Research of New Chinese Medicines’ , the severity of symptoms was divided into normal ( 0 points ), mild ( 1 points ), moderate ( 2 points ), and severe ( 3 points ).According to the four levels of related symptoms,the scores were calculated, and the total score of TCM syndromes was finally calculated[12].
2.9 Statistical methods
All the data of this study were recorded on the table, the Excel database was generated, the equation was calculated, the equation was substituted into the formula for analysis and the open oneway quality sequential test diagram was drawn.At the same time, SPSS25.0 statistical software was used for analysis.The measurement data were expressed as mean ± standard deviation (±s).If they conformed to the normal distribution, the t test was used in the statistical analysis group, and the two independent samples t test was used between the groups.If they did not conform to the normal distribution, the rank sum test was used, and the count data were tested by χ2test.P < 0.05 was considered statistically significant.
2.10 Ethical statement
This study was approved by the ethics committee of the hospital.All patients understood the details of this study and signed the relevant informed consent, ethical batch number ( 2022-071-01 ).
3.Results
3.1 Sequential test results of two groups
The specific situation of Scr in the treatment group and the control group after treatment is shown in table 2.The determined efficacy of each drug in the treatment of AKI patients was tested on the figure.In case of effective cases, the upper diagonal line is drawn,otherwise the horizontal line is drawn, with the number of patients as the abscissa, the effective number as the ordinate, and according to the linear equation U ( upper bound ) : y = 1.81 + 0.819 n, L (lower bound ) : y = -1.81 + 0.819 n, the sequential test line is drawn.According to the results of Scr treatment in table 2, the treatment curve of the treatment group and the control group is drawn one by one.The 10 th patient in the treatment group touched the upper bound U, and the 15 th patient in the control group touched the upper bound U, and the test was terminated.See Figure 1 and Figure 2.
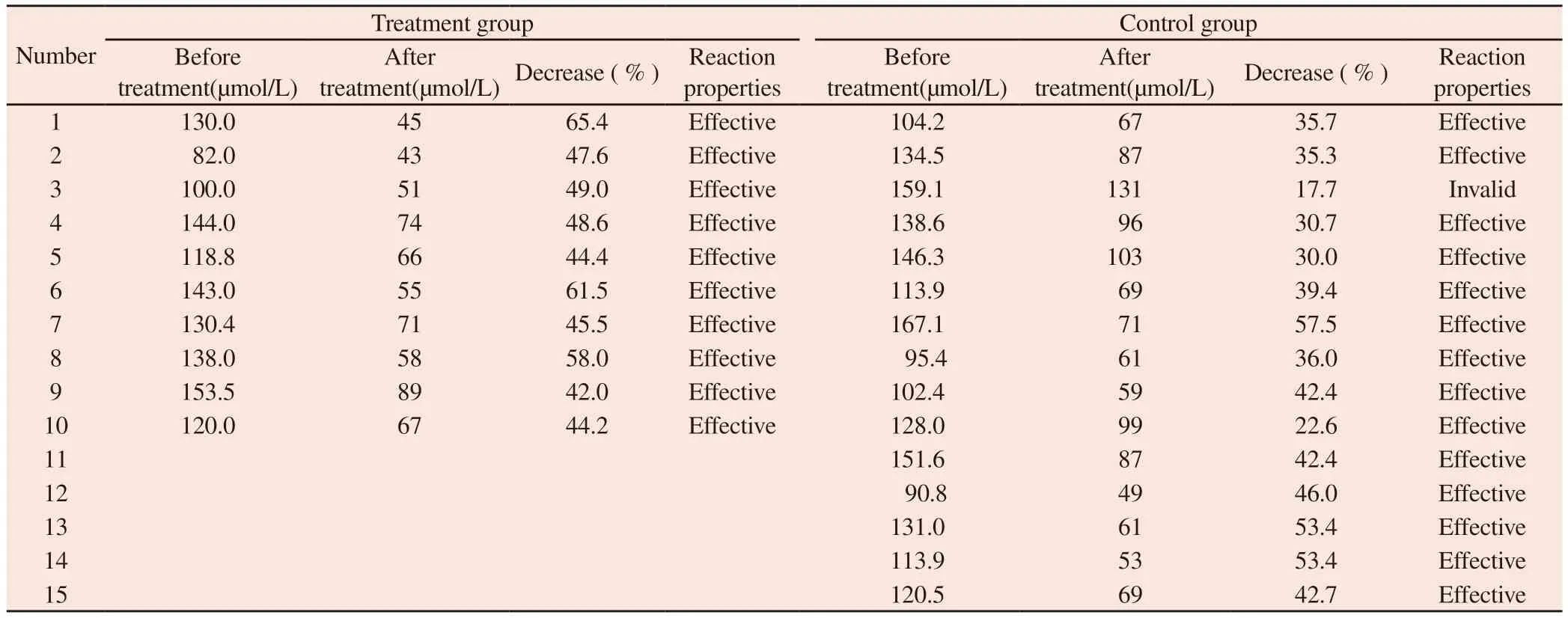
Tab 2 Changes in Scr after treatment in the treatment group and control group
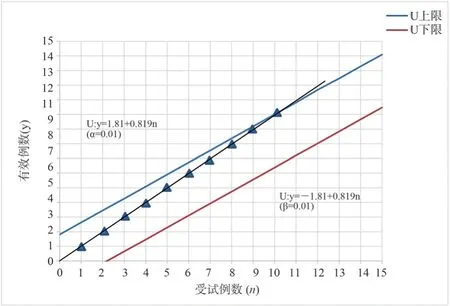
Fig 1 Sequential Test of Scr Changes in Patients in the Treatment Group
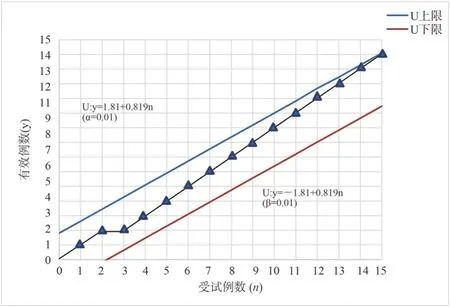
Fig 2 Sequential test of Scr changes in control group patients
3.2 Changes in clinical efficacy of the two groups
After treatment, 10 cases in the treatment group were effective,Ptreatmentgroup= 100 % ; there were 15 cases in the control group, 14 cases were effective, 1 case was ineffective, Pcontrolgroup= 93.33 %.The clinical efficacy of the treatment group and the control group was similar, and there was no statistical significance ( P > 0.05 ), as shown table 3.
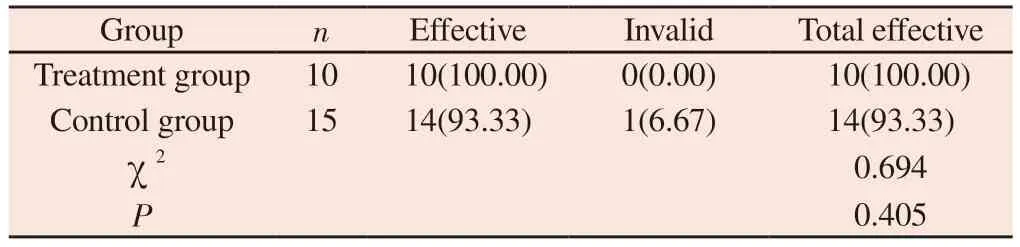
Tab 3 Comparison of clinical efficacy between two groups of patients(n, %)
3.3 Scr changes before and after treatment in the two groups
There was no significant difference in Scr level between the two groups before treatment ( P > 0.05 ).After treatment, the Scr level in the treatment group was ( 61.90 ± 14.26 ) μmol / L, and the control group was ( 86.63 ± 23.83 ) μmol / L.Both groups were lower than before treatment, and the Scr level in the treatment group was lower than that in the control group.The difference was statistically significant ( P < 0.05 ), as shown in table 4.
Tab 4 Comparison of Scr between two groups of patients before and after treatment(±s)
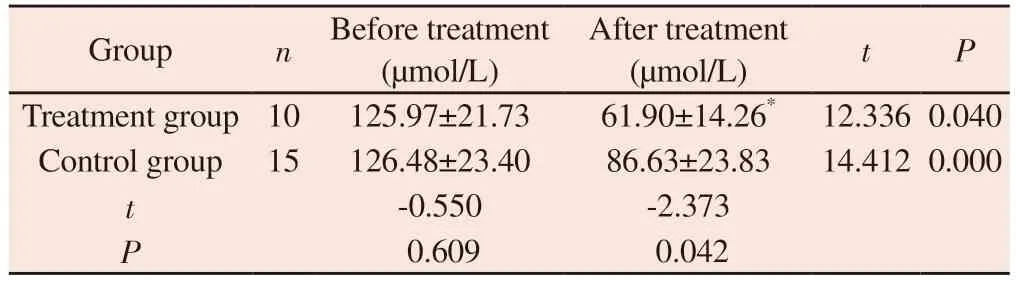
Tab 4 Comparison of Scr between two groups of patients before and after treatment(±s)
To compared with control group, *P<0.05.
Group n Before treatment(μmol/L)After treatment(μmol/L) t P Treatment group 10 125.97±21.73 61.90±14.26* 12.336 0.040 Control group 15 126.48±23.40 86.63±23.83 14.412 0.000 t-0.550 -2.373 P 0.609 0.042
3.4 Changes in the number of days of improvement in the two groups
After treatment, the number of days of improvement in the treatment group was ( 9.00 ± 2.81 ) d, and the control group was (9.55 ± 2.74 ) d.The number of days in the two groups was similar,and the difference was not statistically significant ( P > 0.05 ), as shown table 5.
Tab 5 Comparison of improvement days between two groups of patients(±s)

Tab 5 Comparison of improvement days between two groups of patients(±s)
To compared with control group, P>0.05.
Group n Improvement days ( d) P Treatment group 10 9.00±2.81 0.068 Control group 15 9.55±2.74
3.5 The changes of TCM syndrome scores before and after treatment in the two groups
Before treatment, there was no significant difference in TCM syndrome scores between the two groups ( P > 0.05 ).After treatment, the TCM syndrome score of the treatment group was (1.30 ± 0.49 ) points, and that of the control group was ( 4.40 ± 1.50 )points.Both groups were improved compared with the group before treatment, and the TCM syndrome score of the treatment group was significantly better than that of the control group.The difference was statistically significant ( P < 0.05 ), as shown in table 6.
Tab 6 Comparison of Traditional Chinese Medicine Syndrome Scores between Two Groups of Patients before and after Treatment(±s)

Tab 6 Comparison of Traditional Chinese Medicine Syndrome Scores between Two Groups of Patients before and after Treatment(±s)
To compared with control group, *P<0.05.
Group n Before treatment(scores)After treatment(scores) t P Treatment group 10 7.40±1.90 1.30±0.49* 11.158 0.000 Control group 15 6.73±1.80 4.40±1.50 11.068 0.000 t 0.880 -6.273 P 0.778 0.009
3.6 The changes of mAlb before and after treatment in the two groups
Before treatment, there was no significant difference in mAlb level between the two groups ( P > 0.05 ).After treatment, the mAlb level in the treatment group was ( 18.30 ± 2.91 ) mg / L, and that in the control group was ( 27.80 ± 6.20 ) mg / L.After treatment, both groups were lower than those before treatment, and the mAlb level in the treatment group was lower than that in the control group.The difference was statistically significant ( P < 0.05 ), as shown table 7.
Tab 7 Comparison of mAlb between two groups of patients before and after treatment (±s)
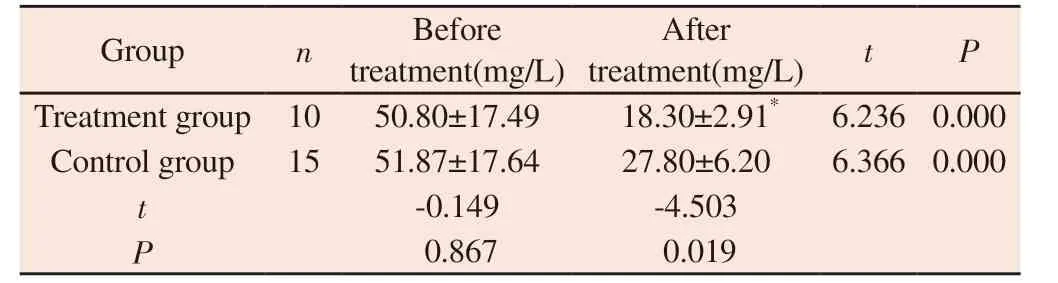
Tab 7 Comparison of mAlb between two groups of patients before and after treatment (±s)
To compared with control group, *P<0.05.
Group n Before treatment(mg/L)After treatment(mg/L) t P Treatment group 10 50.80±17.49 18.30±2.91* 6.236 0.000 Control group 15 51.87±17.64 27.80±6.20 6.366 0.000 t-0.149 -4.503 P 0.867 0.019
3.7 The changes of blood NGAL before and after treatment in the two groups
Before treatment, there was no significant difference in NGAL values between the two groups ( P > 0.05 ).After treatment, the NGAL level in the treatment group was ( 54.10 ± 21.65 ) μmol / L,and that in the control group was ( 77.07 ± 56.30 ) μmol / L.Both groups were lower than those before treatment, and the decrease of NGAL in the treatment group was more obvious than that in the control group.The difference was statistically significant ( P < 0.05), as shown table 8.
Tab 8 Comparison of blood NGAL between two groups of patients before and after treatment (mol/L, ±s)
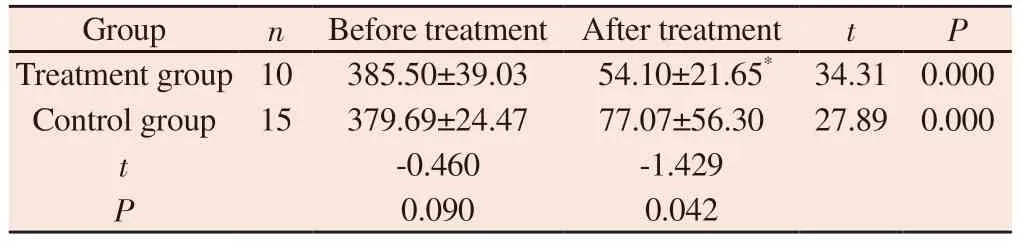
Tab 8 Comparison of blood NGAL between two groups of patients before and after treatment (mol/L, ±s)
To compared with control group, *P<0.05.
Group n Before treatment After treatment t P Treatment group 10 385.50±39.03 54.10±21.65* 34.31 0.000 Control group 15 379.69±24.47 77.07±56.30 27.89 0.000 t-0.460 -1.429 P 0.090 0.042
3.8 The changes of 24h urine volume before and after treatment in the two groups
Before treatment, there was no significant difference in 24 h urine volume between the two groups ( P > 0.05 ).After treatment, the 24 h urine volume of the treatment group was ( 2140.00 ± 418.20 ) mL,and that of the control group was ( 1713.33 ± 220.77 ) mL.The two groups were significantly increased compared with the group before treatment, and the treatment group was more than the control group.The difference was statistically significant ( P < 0.05 ), as shown table 9.
Tab 9 Changes in 24-hour urine volume before and after treatment in two groups(mL, ±s)
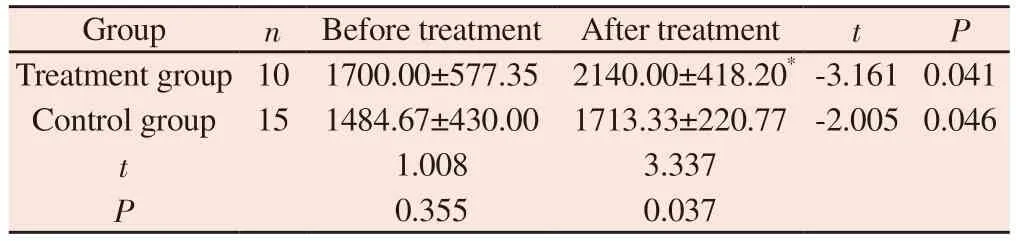
Tab 9 Changes in 24-hour urine volume before and after treatment in two groups(mL, ±s)
To compared with control group, *P<0.05
Group n Before treatment After treatment t P Treatment group 10 1700.00±577.35 2140.00±418.20* -3.161 0.041 Control group 15 1484.67±430.00 1713.33±220.77 -2.005 0.046 t 1.008 3.337 P 0.355 0.037
3.9 Safety observation
During the treatment, the vital signs of the two groups were stable,and no obvious adverse reactions occurred.
3.10 Summary of test results
Two groups of patients were exposed to the upper U line, the treatment group in the test to the 10 th case, the test line contact the upper U line, the control group in the test to the 15 th case, the test line contact the upper U line.According to the provisions of the termination of the test, accept the effective hypothesis, it can be considered that the Zhuang medicine Yingbupu decoction has a therapeutic effect on stage I and II AKI, and the conclusion of accepting the Zhuang medicine Yingbupu is made.Among them, 10 cases were effective in the treatment group, 0 cases were ineffective,the total effective rate was 100 %, the probability of false positive was 0.1 %, and the improvement days were 9.00 ± 2.81 days.In the control group, 14 cases were effective, 1 case was ineffective,the total effective rate was 93.33 %, and the improvement days were 9.55 ± 2.74 days.The clinical efficacy and improvement days were similar between the two groups, and there was no significant difference ( P > 0.05 ).However, for TCM syndrome scores, Scr,mAlb and NGAL values, after treatment, the treatment group was lower than the control group, and the difference was statistically significant ( P < 0.05 ).
4.Discussions
Sequential test ( sequentialtral ), also known as sequential analysis,is an experimental method that analyzes one by one and immediately stops the test when it can be concluded.It was first used for the inspection of arms quality during the Second World War[13].Sequential test is mostly used in clinical drug evaluation, drug screening or drug verification.Its use is not as extensive as that of general clinical controlled trials, but it can quickly obtain observation results and has certain advantages.The open sequential design is not limited to a fixed sample size.Each sampling test is analyzed once, and each time is determined by the results of the previous step.Once a conclusion is reached, the test is stopped immediately.If the drug has no obvious clinical effect, it can be stopped in time to avoid subsequent patients receiving ineffective drugs.If the drug is effective, it can also be put into use better and faster to benefit patients.In this study, Zhuang medicine Yingbupu was used to treat AKI.The course of disease was short, the treatment period was short, and the test results could be obtained quickly.The sequential test method was particularly suitable.
AKI is a major public health problem worldwide due to its high incidence and high risk.Epidemiological studies have shown that there are about 13.3 million people with AKI each year in the world,and nearly 1.7 million people die from AKI and its complications[14].It is a common kidney disease in the world, and the incidence rate is also high in China.Retrospective analysis of multiple single centers in China[15] showed that the overall incidence of AKI was about 11.6 % and the mortality rate was about 8.8 % in about 660,000 adult inpatients.Compared with similar patients without AKI, the risk of in-hospital death in hospitalized patients with AKI increased by 2.5 times, and the long-term mortality risk of CKD patient subgroups also increased.It can be seen that AKI is harmful.The pathogenesis of AKI is very complex and has not yet been fully clarified.According to the different causes of renal ischemia, nephrotoxic drugs, sepsis and urinary obstruction and the different parts of the renal tissue, the pathological mechanism can be divided into prerenal, postrenal and renal[16].The pathogenesis mainly involves oxidative stress, mitochondrial dysfunction, immune response, vascular endothelial cell injury, inflammatory response,apoptosis and autophagy.
At present, there is no established drug therapy for AKI.Domestic and foreign scholars have done a lot of research on AKI treatment.Modern medical treatment of AKI is mainly to correct the etiology, change the reversible factors of the body ‘s internal environment and symptomatic supportive treatment.Maintaining hemodynamic stability, early identification of AKI complications,determination of its causes and treatment are particularly important.In addition, stopping or reducing drugs with nephrotoxicity, early use of antibiotics for sepsis patients is of certain significance for preventing AKI[17].In view of the risk factors of AKI, including dehydration status or insufficient volume, hypoproteinemia, anemia,CKD foundation and other chronic diseases of organs ( heart,lung, liver, etc.), as well as the injury factors of nephrotoxic drugs such as sepsis, shock, burn, trauma, surgery, contrast agent and aminoglycoside antibiotics, early prevention and treatment should be carried out to determine the etiology as soon as possible and actively treat the primary disease[18].There is no specific name for AKI in traditional Chinese medicine.According to its clinical symptoms such as oliguria, anuria, edema and nausea, modern physicians [19-20] believe that AKI can be called ‘guange’ , ‘edema’, ‘uroschesis’ and other diseases, mainly exogenous six evils and epidemics, dietary intake discomfort, physical deficiency, fatigue, as well as blood loss, dead fluid, insect bite or drug toxicity.‘Suwen ·Shuirexue Lunpian’ About : ‘ The kidney reaches Yin, and the Yin reaches water...It is believed that the root of water disease is in the kidney, and the lung is the source of water, so the end is in the lung,and the lung dominates the gas, and the main channel is to regulate the water channel.If the lung and kidney are deficient, and the six evils are invaded, the water disease is easy to occur[21].Therefore,the pathogenesis of edema disease is based on the deficiency of lung and kidney, and the accumulation of blood stasis and water, and the accumulation of dampness and toxin.The main pathogenesis of AKI disease is deficiency, blood stasis, turbidity and toxin, which generally belongs to the deficiency of the standard.It can be caused by deficiency, or by deficiency.However, damp-heat toxin is easy to invade the human body, sweat more, and is easy to damage the body fluid and consume qi.Qi and Yin deficiency, Qi deficiency has no blood circulation, Yin deficiency blood flow is slow, easy to form blood stasis[22], so AKI is more common in lung and kidney deficiency and damp-heat and blood stasis.For stage I and II AKI,it is still mainly based on deficiency and excess.The treatment should be clearing heat and dampness, promoting blood circulation and removing blood stasis.At the same time, it should also protect healthy qi and tonify lung and kidney.
In the theory of Zhuang medicine, the healthy operation of human body needs the coordination of three ways and two ways[23].In the internal medicine of Zhuang medicine, the pathogenic factors mainly include six categories : Sha, miasma, gu, toxin, wind and dampness.The pathogenesis is mainly ‘toxin’ and ‘ deficiency’ ,and the theory of toxin deficiency is the core content of the etiology and pathogenesis of Zhuang medicine[24].Drug evil invades the body through three ways and two ways, endangering the functions of zang-fu organs and three ways and two ways, resulting in the pathogenesis of the body.For example, under the action of various pathogenic factors, or the two ways of zang-fu organs and organs of the human body are disordered, and the ‘deficiency’ of the human body arises from the inside, which can lead to the failure of synchronous operation of heaven, earth and human body.The qi of the upper, middle and lower parts of the human body is not harmonious and becomes a disease[25].AKI in zhuang medicine can be attributed to ‘Benfu’ disease.In the theory of zhuang medicine,water is the source of life, and all living things between heaven and earth depend on the nourishment of water.The water inlet and outlet channels of the human body are waterways, and people communicate with nature through waterways.Waterway hub in ‘Miyao’ ( kidney )and ‘ Mixiaodu ‘ ( bladder ), if the evil poison invasion, waterways,or mediation imbalance, can cause ‘Benfu’, ‘Youka’ , ‘ Youzhuan’a class of diseases[26].The early ‘Benfu’ disease is ‘poison’ , which is formed by wetting the waterway to the three qi can not be synchronized.The treatment principles of Zhuang medicine for AKI are expelling pathogenic toxin, removing dampness toxin, regulating waterways and tonifying deficiency.
Godungjcanz, also known as Aralia elata, Quebuzhan, Niaobusu,Leigongmu, is the root, root bark or branch of Aralia elata.It is mainly produced in Guangxi, Guangdong and Hunan.It is rich in Guangxi Zhuang region and has a long history of clinical application.From the theory of Zhuang medicine, Yingbupu has the effect of regulating ‘three channels and two ways’ , dispelling wind evil, removing dampness and toxin, promoting qi and relieving pain,promoting blood circulation and removing blood stasis.The main indications are : flourishing ( rheumatism arthralgia ), heart headache( stomach pain ), white frost ( diarrhea ), Benfu ( edema ), Youdui (prostatitis ), etc.[27].In modern research, it has been confirmed that eagle puff mainly contains triterpenoid saponins mainly composed of oleanolic acid.The extract of eagle puff has anti-inflammatory,anti-cancer, anti-atherosclerosis and anti-diabetes effects.In addition,it can also repair intimal hyperplasia after renal vascular injury[28].Phytochemical studies have also proved that the main compounds isolated and identified from eagle puff are proteins, polysaccharides,triterpenoid saponins, flavonoids and other compounds.These extracts have multiple effects and have good effects in hypoglycemic,antibacterial, anti-inflammatory, anti-oxidation and liver protection.Triterpenoid saponins have a magical effect, which can inhibit the superoxide reaction caused by inflammatory stimulation[29], mainly through the NADPH oxidase family gene chromosome, serine,tyrosine phosphorylation translocation.Zhuang medicine is less and more refined, and often uses a single herb to treat diseases.This study shows that the sequential test observation, the treatment group in the test to the 10 th case, the test line contact the upper U line,according to the provisions of the termination of the test, to accept the Zhuang medicine yingbupu treatment of AKI conclusion.After 14 days of treatment, the indexes of Scr, mAlb and NGAL in the treatment group were lower than those in the control group.It can be seen that Zhuang medicine Yingbupu can reduce the Scr, mAlb and NGAL of patients with stage I and II AKI lung and kidney deficiency and damp heat and blood stasis syndrome, and promote the recovery of renal function.Its effect may be related to the anti-inflammatory effect, anti-atherosclerotic effect and repair of renal vascular injury and renal interstitial damage.At the same time, Zhuang medicine Yingbupu has the effect of promoting blood circulation and removing blood stasis.It can regulate blood microcirculation, expand blood vessels, improve local microcirculation of glomeruli and renal tubulointerstitium, and achieve the effect of alleviating renal injury.In summary, Zhuang medicine Yingbupu Decoction is effective in the treatment of stage I and II lung and kidney deficiency and damp heat and blood stasis syndrome AKI, and can significantly improve the symptoms of stage I and II lung and kidney deficiency and damp heat and blood stasis syndrome AKI patients.By reducing Scr, mAlb and NGAL levels, renal function is improved, and the quality of life of patients is improved, and the safety is high.The sequential test uses a single indicator Scr as a measurement index, but Scr is affected by many factors such as age, gender, muscle content and protein intake, and cannot reflect changes in renal function in a timely and accurate manner.Therefore, its sensitivity and specificity have certain limitations in the early stage of AKI.In general, due to the limited experimental methods in this study, the number of clinical samples observed is small and the observation period is short ; there are differences in the dosage forms of the test drugs,which may have a certain deviation in the therapeutic effect, and the mechanism of the test drugs has not been further studied.Therefore,in the future research, we hope to continue to carry out large-scale clinical research, increase the sample size to explore the efficacy of the treatment of AKI, and further explore its drug mechanism.
Authors’ contribution
Zhou Yan, Huang Guodong, Zhou Changyan : research design ;Zhou Yan, Xie Zhangqing, Luo Jia : research and implementation ;Zhou Yan and Liang Jingyan : data collection and collation ; Zhou Yan : thesis writing ; Zhou Yan, Xie Zhangqing, Luo Jia : Thesis revision ; Zhou Changyan, Huang Guodong : Proofreading.
All authors declare that there is no conflict of interest.
 Journal of Hainan Medical College2024年4期
Journal of Hainan Medical College2024年4期
- Journal of Hainan Medical College的其它文章
- Advances of lncRNA MAFG-AS1 in cancer
- Research progress on dynamic monitoring of ctDNA and drug resistance related concomitant mutations in non-small cell lung cancer
- Prognostic characterization of copper death-related immune checkpoint genes and analysis of immunologic and pharmacologic therapy in bladder cancer
- Recent research progress from biological perspective on the mechanism of formation of osteoarthritis after anterior cruciate ligament injury
- Meta-analysis of the efficacy and safety of Bushen Huoxue decoction in the treatment of Osteoporosis
- To explore the mechanism of Fuyang Jiebiao granules against viral pneumonia based on network pharmacology and pharmacodynamics
