Hodgkin’s lymphoma: 2023 update on treatment
Sicong Zhang, Xianming Liu, Lanfang Li, Lihua Qiu, Zhengzi Qian, Shiyong Zhou, Xianhuo Wang,Huilai Zhang
Department of Lymphoma, Tianjin Medical University Cancer Institute & Hospital, National Key Laboratory of Druggability Evaluation and Systematic Translational Medicine, National Clinical Research Center for Cancer, Tianjin’s Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, The Sino-US Center for Lymphoma and Leukemia Research, Tianjin 300060, China
Hodgkin’s lymphoma (HL) is a common, malignant hematological tumor of the lymph nodes and lymphatic system,accounting for 10% of all lymphomas.HL comprises 2 main subtypes: classical HL (cHL) and nodular lymphocyte predominant HL.With modern advancements in chemotherapy regimens, long-term survival rates among patients with HL have improved to approximately 80%.However, some patients either do not respond to front-line therapy or experience clinical relapses.A variety of novel therapies for lymphoma have recently rapidly evolved.Immunotherapies,such as immune checkpoint inhibitors, antibody drug conjugates, bispecific antibodies, and cellular therapy, are becoming increasingly important in the development of regimens for HL1.Recent advances in HL management have focused on optimizing treatment strategies to improve outcomes and decrease adverse effects.Herein, we discuss progress in developing novel treatment regimens for HL(Table 1).
Treatment strategies for early-stage HL
The initial treatment strategies for HL are decided upon according to the histology (cHL or nodular lymphocyte predominant HL), stage, and adverse risk factors.The principles of management of HL are that early-stage patients are treated with a combination of chemotherapy followed by radiation therapy, whereas advanced-stage patients undergo longer chemotherapy treatment with or without radiation therapy.
Typically, patients with limited-stage cHL receive chemotherapy with 2-4 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy limited to the involved lymph nodes for consolidation.
For early stage unfavorable HL, debate is ongoing regarding the ideal number of chemotherapy cycles, the most effective chemotherapy regimen, the appropriate radiotherapy dosage,and the optimal size of the irradiation field.Brentuximab vedotin (BV), a CD30-targeted antibody drug conjugate, attaches to CD30-positive cells, thus leading to tumor cell eradication through endocytosis.Additionally, it induces death in CD30-negative tumor cellsviathe bystander effect.A multicenter phase II trial has assessed the efficacy and safety of BV-AVD or ABVD chemotherapy followed by 30 Gy of radiotherapy.In the BV-AVD arm, 93 patients (82.3%) were PET negative(Deauville score 1-3), compared with 43 (75.4%) of 57 in the ABVD arm.The 2-year progression-free survival (PFS) rates were 90.9% in the BV-AVD arm and 70.7% in the ABVD arm for patients with high total metabolic tumor volume,thus indicating that addition of BV into the initial treatment regimen not only enhances safety but also improves survival outcomes2.
Treatment options for advanced HL
PD1 antibody
The primary objective for patients diagnosed with advanced disease (stage IIB-IV) is to enhance the proportion of individuals achieving sustained remission while concurrentlymitigating the occurrence of long-term adverse effects.Several studies have explored the efficacy and adverse effects of the use of PD1 antibody plus chemotherapy.At the 17th International Conference on Malignant Lymphoma(17-ICML), Herrera et al.3have reported the results of the SWOG S1826 study, which has demonstrated that, compared with BV-AVD, nivolumab (N)-AVD prolongs PFS and decreases the incidence of toxic reactions in patients diagnosed with advanced stage HL.This breakthrough treatment method is important for patients with advanced HL.However, notably, the study follow-up time was short, and no overall survival (OS) benefit was observed; therefore,longer follow-up is needed.Another investigation has evaluated the effects of pembrolizumab and AVD (doxorubicin,vinblastine, and dacarbazine; APVD) on newly diagnosed cHL.The 2-year PFS and OS were 97% and 100%, respectively, thus indicating favorable safety and efficacy14.All these studies have demonstrated that anti-PD1 antibodies exhibit notable clinical effectiveness in patients with newly diagnosed HL.
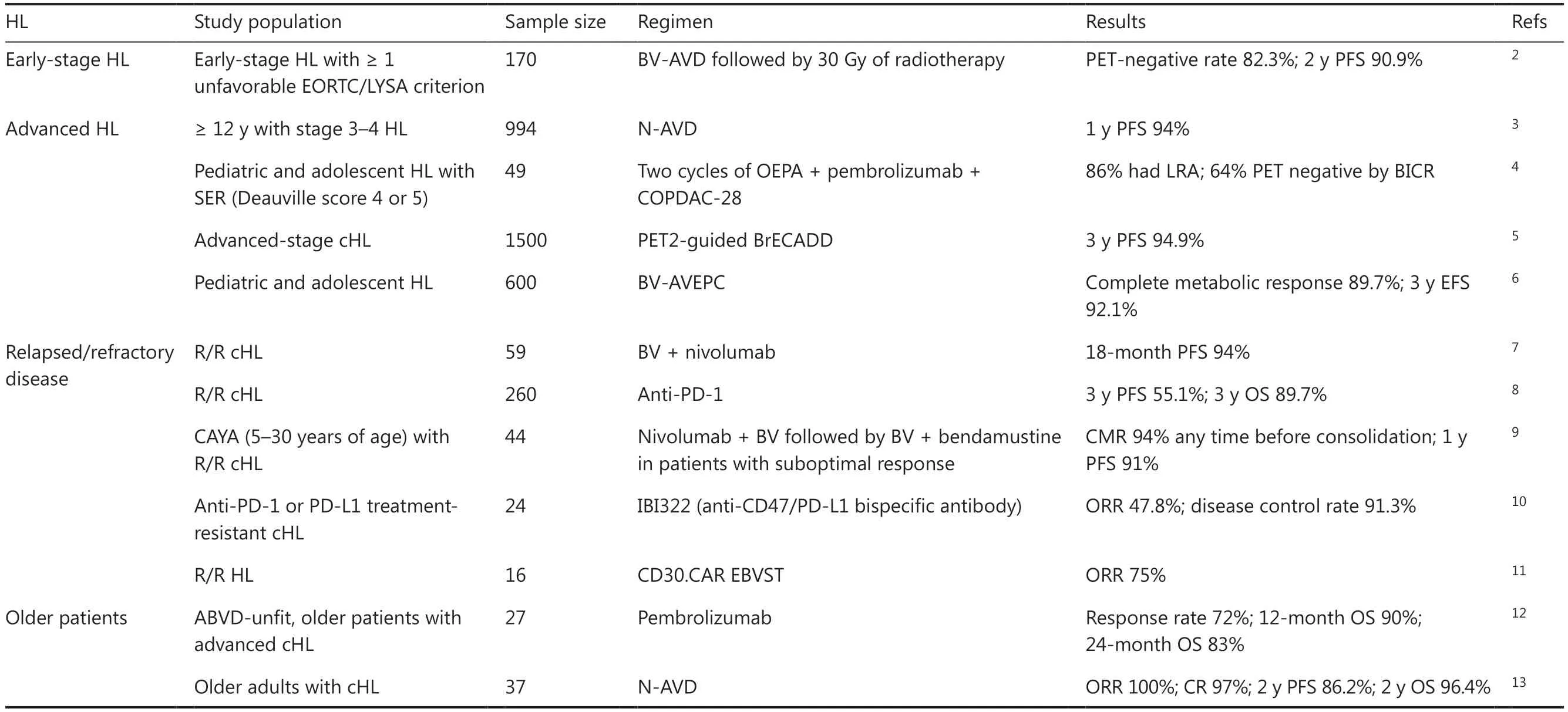
Table 1 Progress in developing novel therapies for HL in 2023
For pediatric and adolescent HL patients, assessing the effectiveness of treatment in relation to the potential longterm consequences is imperative.The Children’s Oncology Group (COG) program has demonstrated a favorable success rate in curing pediatric patients over the past 30 years.However, long-term follow-up studies have revealed that these patients may experience infertility, premature heart failure, and the development of secondary tumors because of the administration of certain alkylating drugs.Therefore,a primary objective of treatment is to achieve a high rate of remission while minimizing the use of radiotherapy and chemotherapy, to mitigate long-term adverse effects.The currently recommended chemotherapy regimen is tailored specifically to children and differs from the regimen used for adults.Recently, at the European Hematology Association 2023 Congress, Vinti et al.4have reported that pembrolizumab and 4 cycles of cyclophosphamide, vincristine, prednisone/prednisolone, and dacarbazine (COPDAC-28) consolidation regimens demonstrate manageable safety and promising antitumor efficacy in the treatment of children and young adults with cHL with a slow early response to first-line chemotherapy (OEPA, vincristine, etoposide, prednisone/prednisolone, and doxorubicin).This study suggests that the pembrolizumab plus COPDAC-28 regimen had the potential to augment the therapeutic response in high-risk cHL populations.
Brentuximab vedotin
eBEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazide, and prednisone) is a personalized PET2-guided initial therapy for individuals diagnosed with advanced cHL.This treatment approach has achieved remarkable survival outcomes but also caused treatment- associated morbidity.At the 17-ICML, Borchmann et al.5have reported the noninferiority of BV, etoposide,cyclophosphamide, doxorubicin, dacarbazine, and dexamethasone (BrECADD) to eBEACOPP in the GHSG phase III HD21 trial.Notably, when comparing the eBEACOPP group to the BrECADD group, a reduction in early PFS events was observed in the latter, thus leading to an impressive 3-year PFS rate of 94.9%.These promising PFS outcomes suggest that personalized treatment with PET2-guided BrECADD is currently the optimal therapeutic approach for adult patients with advanced-stage cHL.
The AHOD1331 study was a large phase III randomized trial aimed at assessing the effectiveness and safety of BV-AVEPC,in pediatric patients with newly diagnosed high-risk HL.The experimental group received BV instead of bleomycin to mitigate long-term toxicity and after the fifth cycle of treatment,radiotherapy was performed on the involved sites of slow-responding lesions or large mediastinal adenopathy, in both the BV group and the standard-care group.Incorporating BV into conventional chemotherapy regimens has been found to enhance efficacy while decreasing adverse events or mortality by 59%, without increasing the occurrence of toxic reactions over a 3-year period6.
Strategies for relapsed/refractory disease
PD-1 plus BV therapy
Multiagent chemotherapy has made substantial advancements in recent years, but as many as 10% of patients with HL do not respond to treatment and have an elevated risk of death.For patients eligible for transplantation, a combination of second-line salvage chemotherapy along with autologous hematopoietic stem cell transplantation is recommended.In the era of new drugs, as treatment advancements continue,the second-line regimen for R/R patients is transitioning from conventional chemotherapy to PD-1 inhibitor treatment, either alone or in conjunction with BV.In a multicenter phase II trial, after undergoing hematopoietic stem cell transplantation, patients initiated treatment with a combination of BV and nivolumab.The PFS rate for 59 patients was 94%(95% CI 84-98), thereby indicating that BV plus nivolumab is highly effective in consolidating patients with R/R cHL7.To explore long-term outcomes in patients with R/R cHL who have responded to PD-1 inhibition, 4 phase II trials have been performed in China.A total of 260 patients were included in the analysis.Nearly one-fifth of patients who achieved complete remission with nonrefractory diseases demonstrated the most favorable survival outcome, with a 3-year PFS exceeding 80%.However, the survival outcome of PR patients was poor,and the 3-year PFS was less than 50%8.Therefore, additional investigations are imperative to investigate the multimodal treatment approach for patients who attain objective remission after PD-1 monoclonal antibody therapy.
The cure rate for HL in the population of children,
adolescents, and young adults (CAYA) is high, exceeding 90%.However, despite this favorable outcome, approximately 10% of patients may experience relapse.Unlike certain non-Hodgkin’s lymphoma subtypes, HL typically exhibits a less aggressive disease progression, thereby offering potential avenues for further therapeutic interventions.For R/R HL in CAYA, the CheckMate 744 study has investigated a riskstratified, response-adapted system using nivolumab plus BV,followed by BV plus bendamustine.Induction with nivolumab plus BV resulted in a CMR rate of 59% and, any time before consolidation with nivolumab plus BV ± BV plus bendamustine, resulted in a CMR rate of 94%.A high CMR rate with low toxicity was observed in CAYA patients with R/R cHL after use of this response-adapted, risk-stratified salvage treatment9.
Bispecific antibody
Because some patients with R/R HL face resistance problems,new therapeutic strategies should be explored.The results of the CD47/PD-L1 bispecific antibody IBI322 in anti-PD-1 or PD-L1 treatment-resistant cHL were orally presented by Yu et al.10at the European Hematology Association 2023 Congress.The results showed an objective response rate (ORR) of 47.8%and a disease control rate of 91.3%.IBI322 monotherapy exhibited encouraging antitumor activity and manageable safety profiles in anti-PD-1 or PD-L1 treatment-resistant cHL.
CAR-T therapy
Cellular therapy is considered a highly promising novel therapeutic approach.Previous studies have demonstrated the antitumor efficacy with minimal observable toxicity of CD30-specific chimeric antigen receptor (CAR) T cells in R/R HL.However, the application of readily available allogeneic T-cell therapies is hindered by substantial obstacles, primarily graft-vs.-host disease15.To address the issue of graft-vs.-host disease, at the 17-ICML, a study was reported promising results for cellular therapies in R/R HL.Epstein-Barr virus-specific T cells (EBVST) were modified by incorporation of a CAR specifically targeting CD30, an antigen upregulated on allo- activated T cells.Consequently, CD30.CAR EBVST cells served as targets for CD30.All 16 patients with R/R HL were assessable for ORR analyses, among whom 12 (75%) had ORRs, thus demonstrating that CD30.CAR EBVST can be considered a safe and effective therapeutic approach for R/R HL11.
Treatment options for older patients
In patients > 60 years of age, in comparison to younger patients,the disease usually presents with aggressive characteristics and unfavorable prognostic factors.Consequently, the management of older patients poses considerable challenges, because of their limited ability to tolerate intensive treatment options.At present, no consensus exists regarding the best treatment options in older patients with HL.At the 17-ICML, Dickinson et al.12presented preliminary results of the effectiveness of pembrolizumab monotherapy as a first-line treatment for older patients with HL in whom ABVD treatment was unsuitable.A total of 72% of patients achieved remission, and 32%achieved CR.However, notably, 52% of patients experienced grade 3 or higher adverse events, although no treatment-associated deaths were reported.Another study has also shown that the N-AVD regimen demonstrates good efficacy and tolerability as a first line treatment option for older adults with cHL13.These findings suggest that pembrolizumab monotherapy may be a viable treatment option for older patients with advanced cHL.
Conclusions
The management principles of HL involve a stratified treatment approach based on the stage and risk factors, involving comprehensive use of chemotherapy and radiotherapy.The treatment objectives encompass enhancing the therapeutic outcome; mitigating toxicity, particularly long-term adverse effects, through low-toxicity chemotherapy regimens; and minimizing the number of chemotherapy cycles.BV or anti-PD1 antibodies exhibit remarkable efficacy in patients with newly diagnosed HL as well as those with R/R HL.Emerging therapeutic approaches, such as bispecific antibodies and cellular therapy, have shown promising antitumor activity and may bring hope for patients with R/R HL.A promising avenue for future advances is incorporating novel agents into combination therapy to enhance the efficacy of treatment outcomes for patients with HL.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed the analysis: Huilai Zhang, Xianhuo Wang.
Collected the data: Sicong Zhang, Xianming Liu, Lanfang Li,Lihua Qiu, Zhengzi Qian, Shiyong Zhou.
Wrote the paper: Sicong Zhang.
 Cancer Biology & Medicine2024年4期
Cancer Biology & Medicine2024年4期
- Cancer Biology & Medicine的其它文章
- Neoantigen cancer vaccines: a new star on the horizon
- Deciphering gastric inflammation-induced tumorigenesis through multi-omics data and AI methods
- Blockade of CD300A enhances the ability of human NK cells to lyse hematologic malignancies
- Influence of sex on outcomes of liver transplantation for hepatocellular carcinoma: a multicenter cohort study in China
