PD-1 antibody in combination with chemotherapy for the treatment of SMARCA4-deficient advanced undifferentiated carcinoma of the duodenum: Two case reports
Yi-Nan Shi,Xiao-Rui Zhang,Wei-Yu Ma,Jing Lian,Yan-Feng Liu,Yi-Fan Li,Wen-Hui Yang
Abstract BACKGROUND SMARCA4 is a component of chromatin remodeling of SWItch/sucrose-nonfermenting (SWI/SNF) complexes and plays an essential role in oncogenesis.SMARCA4-deficient malignancies arising from the gastrointestinal tract are rare and have a poor prognosis.There is no standard treatment for advanced and undifferentiated SMARCA4-deficient duodenal malignancies.Programmed death 1 (PD-1) antibodies,known as immune checkpoint inhibitor antibodies,potentially play a role in treating gastrointestinal tract malignancies.CASE SUMMARY We present two patients with SMARCA4 deficiency and TP53 gene mutation in advanced undifferentiated carcinomas of the duodenum.For both patients,SMARCA4 deficiency was confirmed by immunohistochemical staining for the BRG1 protein,while TP53 gene mutations were observed via next-generation sequencing.Both patients were administered chemotherapy in combination with an anti-PD-1 antibody.The two patients exhibited completely different responses to treatment and had different prognoses.Case 1 experienced rapid progression after PD-1 infusion and chemotherapy,case 2 experienced a remarkable response after treatment,and the progression-free survival was more than 6 months.CONCLUSION This study described our clinical and pathological observations of SMARCA4-deficient advanced undifferentiated carcinoma of the duodenum.PD-1 combined with chemotherapy showed a certain efficacy in select patients,providing options for treating these highly malignant tumors.Patients with liver metastases had a worse prognosis than did those with only lymph node metastasis.
Key Words: SMARCA4 deficiency;Undifferentiated carcinomas;Chemotherapy;Programmed death 1;Immune checkpoint inhibitors;Case report
INTRODUCTION
The small intestine accounts for only 1%-1.4% of all gastrointestinal (GI) malignancies.The most common primary malignancy in the small bowel is adenocarcinoma,with the duodenum being the most frequently affected site.Various histological subtypes of duodenal malignancies,including adenocarcinoma,sarcoma,lymphoma,and neuroendocrine tumors,have been identified.Undifferentiated carcinoma of the duodenum,characterized by rhabdoid features,is a rare form of malignancy.A previous study revealed that,in most cases,patients with undifferentiated/rhabdoid carcinoma in the gastrointestinal tract lacked expression of at least one of the four SWItch/sucrose-nonfermenting (SWI/SNF) complex subunits,namely,SMARCB1,SMARCA2,SMARCA4,and ARID1A[1,2].The SWI/SNF complex is an ATP-consuming multisubunit cellular machine that modulates chromatin compaction,thereby regulating DNA-related processes such as transcription,replication,and DNA repair and oncogenesis[3,4].The absence of the key proteins INI1 and BRG1,which are encoded by SMARCB1 and SMARCA4,respectively,is commonly observed in various malignancies,including atypical teratoid/rhabdoid tumors,epithelioid sarcoma,and ovarian small-cell carcinomas of the hypercalcemic type[3,5].
Recently,investigations into SMARCA4 inactivation as a driver event in malignancies have been performed.Mutations leading to the loss of expression of these protein components have also been identified in a subset of poorly differentiated or undifferentiated carcinomas at many sites throughout the body.These include the sinonasal tract[4],lung[5],gastrointestinal tract[6,7],and uterus[8].
In this case report,we documented two patients diagnosed with duodenal SMARCA4-deficient undifferentiated carcinoma who underwent immunohistopathological tests combined with next-generation sequencing and multiplex immunofluorescence analysis.There is no current standard treatment for advanced and undifferentiated SMARCA4-deficient duodenal malignancies.Both patients were administered chemotherapy in combination with programmed death 1 (PD-1) antibody,known as immune checkpoint inhibitor (ICI).Patients with high tumor mutational burden(TMB) responded well to PD-1 antibodies in combination with chemotherapy,and those with liver metastases had a worse prognosis.
CASE PRESENTATION
Chief complaints
Case 1:A 51-year-old male patient presented to our hospital with increasing upper abdominal pain for more than one month.
Case 2:A 43-year-old female complained of intermittent upper abdominal pain for 4 months and enlargement of the left supraclavicular lymph nodes.
History of present illness
Case 1:The patient’s premorbidity was good,with an Eastern Cooperative Oncology Group performance status score of 0.Gastroscopy revealed an ulcer in the descending part of the duodenum,approximately 3 cm × 3.5 cm,with irregular protrusions around the perimeter.A biopsy from the descending part of the duodenum was performed by endoscopy,and an ulcerative mass was found.
Case 2:Gastroenteroscopy was also conducted,and an ulcer in the duodenal papilla was found.Biopsy revealed undifferentiated carcinoma of the duodenum,and contrast-enhanced computed tomography (CT) indicated multiple lymph node metastases involving the retroperitoneal lymph nodes.
History of past illness
Case 1:He had no history of smoking or drinking or a significant medical history.
Case 2:She had no history of smoking or drinking or a significant medical history.
Personal and family history
Case 1:The patient had no personal or family history.
Case 2:The patient had no personal or family history.
Physical examination
Case 1:The patient had mild upper abdominal tenderness.
Case 2:A mass in the left neck was palpated.
Laboratory examinations
Case 1:All of the tumor marker results in the blood sample were negative.
Case 2:An elevated CEA level of 5.14 μg/L was observed.CA199,CA242,and CA724 were all negative.
Imaging examinations
Case 1:An initial CT scan and magnetic resonance imaging (MRI) with enhanced contrast agent injection showed abnormal wall thickening in the duodenal ampulla and multiple liver nodular masses revealing malignant metastases.Retroperitoneal lymphadenopathy was also considered (Figure 1A and B).
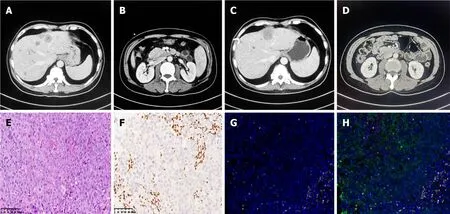
Figure 1 No response to immunochemotherapy or disease progression was confirmed by contrast computed tomography in case 1. A and B: A mass in the descending part of the duodenum and multiple liver metastases at the end of 1st-line chemotherapy;C and D: Disease progression was confirmed by enlargement of liver metastases and infusions and impaired liver function with persisting mixed jaundice even after percutaneous transhepatic cholangial drainage;E: H&E: The undifferentiated tumor cells grew in cords,nests,and diffuse sheets,and the nucleus of the tumor was vacuolated with obvious nucleoli.Mitotic images and scattered multinucleated giant cells were also observed.Extensive areas of necrosis were observed within the tumor;F: The SMARCA4 protein was negative for BRG1 according to immunohistology;G: CD8+T-cell infiltration was assessed by multiple immunofluorescence assays;H: Tertiary lymphoid structures were not intact,and case 1 was classified as an acquired immune tolerance type by microenvironment analysis.
Case 2:Gastroenteroscopy was also conducted,and an ulcer in the duodenal papilla was found.Biopsy revealed undifferentiated carcinoma of the duodenum.Contrast-enhanced CT indicated multiple lymph node metastases involving the retroperitoneal lymph nodes (Figure 2A and B).

Figure 2 Major response to immunochemotherapy and disease regression in case 2. A and B: A mass in the descending part of the duodenum and multiple retroperitoneal lymph node metastases initially observed;C and D: Significant disease regression after the administration of immunochemotherapy;E: H&E:The undifferentiated tumor cells were diffusely distributed,and patchy necrosis was observed;F: The SMARCA4 protein was negative for BRG1 according to immunohistology.
Further diagnostic work-up
Case 1:Histology was performed,and the epithelioid or undifferentiated tumor cells grew in cords or nests.hematoxylineosin staining is shown in Figure 1E.Extensive areas of necrosis were observed within the tumor.The immunohistological analysis revealed the following results: Ki-67 (approximately more than 90% positive),CK7 (-),SMARCA4 (-),and INSM1 (-) (Figure 1F).Multiplex immunofluorescence staining was also analyzed.PD-L1 expression was positive,with a CPS of 5,and microsatellite stabilization (MSS) was observed.The tumor immune microenvironment was classified as an acquired immune tolerance type according to the presence of both CD8+tumor-infiltrated lymphocytes TILs and PD-L1-expressing cells.Notably,tertiary lymphoid structures were not found in the tumor area (Figure 1G and H).Nextgeneration sequencing of biopsy tissue was subsequently performed.The results revealed TP53 and CTNNB1 mutations(Table 1).However,the SMARCA4 mutation variant was not observed in this patient.

Table 1 Genomic profile of somatic alterations for the patients
The final pathological diagnosis was duodenal SMARCA4-deficient undifferentiated carcinoma,clinical stage IV with multiple liver metastases and retroperitoneal lymph node metastases.
Case 2:Immunohistopathological staining revealed the following results: Ki-67 (90%+) and SMARCA4 (-) (Figure 2E and F).Next-generation sequencing revealedTP53,NCOR1,MYH9,ERBB3,RAD52andCTNNB1mutations (Table 1),and the TMB increased to 10.56/Mb,with a score of 6 for PD-L1 expression.MSS was observed.However,the SMARCA4 mutation variant was not observed in this patient.
FINAL DIAGNOSIS
The final pathological diagnosis was duodenal SMARCA4-deficient undifferentiated carcinoma,clinical stage IV with multiple lymph node metastases.
TREATMENT
Case 1
First,two cycles of bevacizumab in combination with the XELOX regimen were initiated on March 10,2023(bevacizumab,7.5 mg/kg D1+oxaliplatin,130 mg/m2D1+capecitabine,1250 mg/m2D1-14;Q21D).However,an enlargement of liver metastases was detected,and disease progression was considered.Given the positive expression of PD-L1 and the acquired immune tolerance environment,we introduced a second-line combination therapy comprising a PD-1 antibody,nab-paclitaxel and gemcitabine (nab-paclitaxel 220 mg/m2D1+gemcitabine 1000 mg/m2D1 and D8;Q21D),and the patient initially exhibited good tolerance.After two cycles of second-line immunotherapy-chemotherapy combination therapy,progressive disease was observed as liver-targeted lesions increased significantly after infusion(Figure 1C and D).Disease progression was considered.However,the patient refused subsequent immunochemotherapy.The timeline of the summarizing events is shown in Figure 3A.
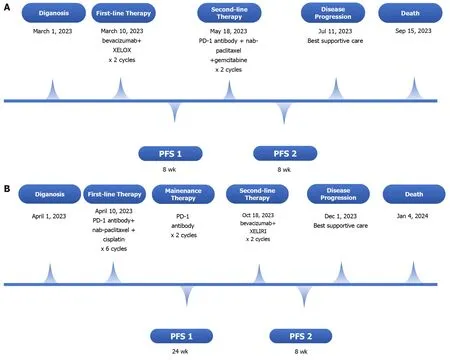
Figure 3 Timeline summarizing. A: Case 1;B: Case 2.PFS: Progression-free survival;PFS1: PFS time during first-line therapy;PFS2: PFS time during secondline therapy.
Case 2
This patient received PD-1 antibody in combination with chemotherapy comprising nab-paclitaxel and cisplatin for six cycles (nab-paclitaxel 220 mg/m2D1+cisplatin 80 mg/m2D1;Q21D).The patient responded positively to this combination therapy.Disease regression with extensively shrunken retroperitoneal lymph nodes was observed(Figure 2C and D),with an improvement in the general condition of the patient.After six cycles of combination therapy,the patient started PD-1 antibody maintenance treatment,and the disease was in remission.Patients showed disease progression with liver metastases at 6 months after diagnosis,and second-line therapy was administered.The second-line therapy consisted of the XELIRI regimen and bevacizumab (bevacizumab,7.5 mg/kg D1+irinotecan,180 mg/m2D1 +capecitabine,1250 mg/m2D1-14;Q21D).The patient was administered two cycles of second-line treatment and started receiving the best supportive care for cachexia and obvious weight loss.The timeline of the summarizing events is shown in Figure 3B.
OUTCOME AND FOLLOW-UP
Case 1
The patient suffered from significant mixed jaundice and showed no improvement after supportive care.Finally,the patient died from liver failure on September 15,2023,with an overall survival period of 6 months.The progression-free survival (PFS) times for first-line and second-line therapy were both 8 wk.
Case 2
The patient died from this malignancy on January 4,2024.
DISCUSSION
Duodenal malignancies are uncommon but highly fatal,and undifferentiated duodenal malignancies are extremely rare.As extensive immunohistochemical profiling has been performed for malignancies such as atypical rhabdoid tumors or epithelioid sarcomas,SMARCA4-deficient malignancies are frequently observed and can be identified[1,9].Undifferentiated carcinomas with rhabdoid cells are characteristic diagnostic clues,and SWI/SNF complex deficiencies are complex molecular events of undifferentiated carcinoma.It is highly malignant and has a short survival time,with no standard treatment at present.Several case reports and retrospective studies have analyzed SMARCA4-deficient malignancies arising from the gastrointestinal tract[1,6,7,9].A previous study demonstrated that a small proportion of gastroesophageal carcinomas exhibit loss of SMARCA4 expression separately or with coinactivation of other subunits of SWI/SNF complexes[1].Among the two patients,not all SMARCA4-deficient tumors harbored SMARCA4 pathological genomic variants.Although these tumors exhibit poorly differentiated and undifferentiated morphologies,they exhibit a broad range of genomic variant features[7,9,10].First,compared with gastroesophageal adenocarcinomas,SMARCA4-deficient gastroesophageal carcinomas exhibit a similar range of somatic mutations,including enrichment of TP53,KRAS,ARID1A,and APC mutations.The most common cooccurring mutations in pathogenic SMARCA4 were identified in TP53,APC,ARID1A,CDKN2A,and CTNNB1[7].On the one hand,this difference may be attributed to the limitations of NGS in accurately detecting large deletions.Additionally,epigenetic modifications,including DNA methylation,histone modifications,and noncoding RNAs,may also contribute to deficiencies in gene expression.Therefore,some investigators have proposed that most SMARCA4-deficient gastroesophageal carcinomas are considered undifferentiated or dedifferentiated gastroesophageal adenocarcinomas rather than distinct biological entities[9,10].
Several factors,such as PD-L1 expression,tumor mutational burden,body mass index,and laboratory parameters(including the neutrophil-to-lymphocyte ratio),may predict the response to immunotherapy as well as immune chemotherapy.
As somatic mutations generate neoantigens,a high TMB is expected to induce a positive antitumor response.However,it can serve as a biomarker for predicting favorable responses to ICIs.The ability of high TMB to predict the efficacy of immunotherapy was not affected by the expression of PD-L1.Hence,some patients benefit from immunotherapy and have prolonged survival[10,11].The infiltration of CD8+lymphocytes and positive PD-L1 expression are also considered potential predictive biomarkers of immunotherapy response[12].
In previous studies,immunochemotherapy has been shown to have various anticancer effects,including immunogenic cell death and a reduction in the number of tumor cells that are engaged in the production of immunosuppressive substances[13,14].Furthermore,the combination of chemotherapy and immunotherapy results in the depletion of myeloid-derived suppressor cells and regulatory T cells.Building upon these principles,the use of chemotherapy combined with immune checkpoint inhibitors (ICIs) has demonstrated synergistic effects.Over the past few years,several trials investigating combination strategies of chemotherapy and ICIs have been presented and published,leading to their approval for diverse solid tumors.Examples of these approved combinations can be found in non-small cell lung cancer,gastric cancer and urothelial carcinoma[15].As mentioned above,in our second patient,a high TMB was associated with a major partial response to PD-1 antibodies during first-line therapy.
The clinical significance of recognizing SWI/SNF complex-deficient undifferentiated carcinoma/rhabdoid carcinoma lies in its aggressive clinical behavior and poor response to traditional chemotherapy.SMARCA4-deficient undifferentiated tumors originating from the gastrointestinal tract might have a worse prognosis than those originating from the thorax[11,16].Adding immunotherapy to conventional chemotherapy may improve the treatment efficacy and increase the response rate.This study was the first to describe the response of SMARCA4-deficient undifferentiated duodenal tumors to immunochemotherapy.According to our observations,case 1 did not benefit from immunotherapy as a second-line chemotherapy,while case 2 benefited from immunochemotherapy combination therapy.The differences in the response of the two patients were partially due to differences in metastatic target organs,differences in mutation genes and differences in the tumor microenvironment,which resulted in differences in tumor mutation burdens.A study reported a median progression-free survival time of 7.2 months in patients who initially presented with metastatic disease,whereas the median overall survival was 13.6 months in patients with malignancies involving the esophagogastric junction and stomach[9].
CONCLUSION
In brief,this case report presented the histopathological and clinical responses of two patients with SMARCA4-deficient advanced undifferentiated carcinoma of the duodenum.PD-1 combined with chemotherapy showed a certain efficacy in select patients,providing options for treating these highly malignant tumors.Patients with liver metastases had a worse prognosis than did those with only lymph node metastasis.Potential molecular mechanisms need to be further studied to elucidate this phenomenon.
FOOTNOTES
Author contributions:Shi YN and Zhang XR analyzed and interpreted the patient data regarding the disease and the diagnosis;Lian J performed the histological examination and diagnosis;Shi YN,Zhang XR,Ma WY and Liu YF handled the therapeutic management of the patient;Liu YF and Yang WH participated in the acquisition,analysis and drafting of the manuscript;all the authors read and approved the final manuscript.
Informed consent statement:The number of ethics approval points was JC2023012.The patients provided written informed consent to participate in this study.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016),and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Yi-Nan Shi 0009-0007-5660-4654;Yi-Fan Li 0000-0002-6378-7635;Wen-Hui Yang 0000-0002-1452-6789.
S-Editor:Gong ZM
L-Editor:A
P-Editor:Zhao S
 World Journal of Clinical Oncology2024年3期
World Journal of Clinical Oncology2024年3期
- World Journal of Clinical Oncology的其它文章
- Approaches and challenges in cancer immunotherapy pathways
- Role of targeting ferroptosis as a component of combination therapy in combating drug resistance in colorectal cancer
- High-dose methotrexate and zanubrutinib combination therapy for primary central nervous system lymphoma
- Mechanisms and potential applications of COPS6 in pan-cancer therapy
- Leveraging electrochemical sensors to improve efficiency of cancer detection
- Transarterial chemoembolization plus stent placement for hepatocellular carcinoma with main portal vein tumor thrombosis: A meta-analysis
