Discovering Cathodic Biocompatibility for Aqueous Zn-MnO2 Battery: An Integrating Biomass Carbon Strategy
Wei Lv, Zilei Shen, Xudong Li, Jingwen Meng, Weijie Yang, Fang Ding, Xing Ju, Feng Ye, Yiming Li, Xuefeng Lyu, Miaomiao Wang, Yonglan Tian, Chao Xu
ABSTRACT Developing high-performance aqueous Zn-ion batteries from sustainable biomass becomes increasingly vital for large-scale energy storage in the foreseeable future.Therefore, γ-MnO2 uniformly loaded on N-doped carbon derived from grapefruit peel is successfully fabricated in this work, and particularly the composite cathode with carbon carrier quality percentage of 20 wt% delivers the specific capacity of 391.2 mAh g-1 at 0.1 A g-1, outstanding cyclic stability of 92.17% after 3000 cycles at 5 A g-1, and remarkable energy density of 553.12 Wh kg-1 together with superior coulombic efficiency of ~ 100%.Additionally, the cathodic biosafety is further explored specifically through in vitro cell toxicity experiments, which verifies its tremendous potential in the application of clinical medicine.Besides, Zinc ion energy storage mechanism of the cathode is mainly discussed from the aspects of Jahn-Teller effect and Mn domains distribution combined with theoretical analysis and experimental data.Thus, a novel perspective of the conversion from biomass waste to biocompatible Mn-based cathode is successfully developed.
KEYWORDS Aqueous Zn-ion batteries; Biocompatibility; Jahn-Teller effect; Mn domains; γ-MnO2
1 Introduction
With the continuous global carbon emission, biomass energy as a kind of green renewable, biodegradable and nontoxic energy resource has attracted considerable attention [1-8].In other words, the recycling of biomass resources as a feasible way to achieve carbon neutrality shows tremendous potential in the field of energy [9-16], materials, health care, and so on [17-24].In particular, the development of high safe and green large-scale energy storage technology using biomass is of great significance for building a clean and low-carbon modern energy system [25-32].Lithium-ion batteries have been widely used in new energy electric vehicles [33-46], but the utilization of flammable organic electrolytes plus high manufacturing costs for lithium batteries are not conducive to the application in large-scale energy storage [47-57].Therefore, the inexpensive and eco-friendly aqueous Zn-ion batteries (AZIBs) with high-safety are considered to have great potential in massive-scale energy storage [58-65].There are many factors affecting the properties of AZIBs, among which the development of stable cathodic materials becomes the key [66-73], and thereinto, MnO2with the characteristics of diverse structures (α-, β-, γ-, and δ-, etc.), low price, and environmental friendliness has been widely used as cathodic materials of AZIBs [74-81].Nevertheless, the repeated insertion/extraction of Zn2+during charge-discharge process leads to the structural deformation for MnO2[82-88].Meanwhile, Mn4+is prone to be reduced to Mn3+so as to induce the Jahn-Teller effect and lattice distortion [89, 90].In addition, the disproportionation reaction of Mn3+also promotes the dissolution of MnO2[91-96].Thus, exploring sustainable and renewable biomass resources to improve the structural stability of MnO2would be a “Win-Win” strategy.
The recombination of carbon materials and MnO2has been identified as an important way to optimize the cathodic performance of aqueous Zn-MnO2batteries [97-99].Chen et al.synthesized carbon nanofiber@δ-MnO2with a facile method combining solid-grinding and wet-chemical reaction, and achieved a capacity of 277 mAh g-1and capacity retention of 79.78% after 700 cycles at 200 mA g-1[100].Li et al.prepared N-doped carbon nanowires incorporated with δ-MnO2by hydrothermal method, the discharge capacities of which were 325 mAh g-1at 100 mA g-1and 90 mAh g-1at 2 A g-1respectively, and its cycle life after 2500 cycles was 95% at 2 A g-1[101].Huang et al.electrochemically deposited α-MnO2onto carbon nanotube as cathode, which achieved the specific capacities of 292.7 and 105.6 mAh g-1at 0.2 and 3 mA cm-2respectively, and its cycling life remained 88.5% after 300 cycles at 0.3 mA cm-2[102].Moreover, Kim et al.reported a carbon-coated α-MnO2cathode, which exhibits a discharge capacity of 272 mAh g-1and cycle life of 69.49% after 50 cycles at 66 mA g-1[103].Overall, carbon materials used to be compound with MnO2are mainly based on the conventional commercial materials such as carbon nanofiber, carbon nanotube, etc.Therefore, the research on how to improve Zn-ion storage performance by constructing Mn-based cathode compounded with carbon materials derived from biomass, especially the inexpensive and renewable biomass waste, is still rare.
In this work, the abundant grapefruit peel as a natural biomass carbon source is adopted to synthesize N-doped carbon carrier powder (CP) through a simple calcination in N2atmosphere, and γ-MnO2prepared by electro-deposition is uniformly loaded onto CP to obtain the composite cathode (γ-MnO2@CP).A systematic study about the improving mechanism of Zn-ion storage efficiency via compounding CP is conducted from multiple perspectives of Jahn-Teller effect, Mn valence, and Mn domains, etc.Besides, the in vitro cytotoxicity experiments of pure γ-MnO2and γ-MnO2@CP are carried out to investigate the application prospect in the field of biomedicine.Therefore, the above research provides a valuable guidance for the comprehensive utilization of wasted biomass to design high-performance MnO2-biomass carbon cathode.
2 Experimental Section
2.1 Sample Preparation
The grapefruit peel was washed three times by deionized water, then heated at 80 °C for 3 h and crushed into yellow powder, which was heated at 650 °C for 2 h under a nitrogen atmosphere to obtain CP.A three-electrode system composed of a stainless steel working electrode, a saturated calomel reference electrode, and a platinum plate counter electrode was used for MnO2electro-deposition under the current density of 5 mA cm-2for 30 min with electromagnetic stirring, and the electrolyte was made up of 0.5 M Mn(CH3COO)2and 0.5 M Na2SO4, the chemical reaction occurred on the surface of stainless steel working electrode is described as: Mn2++ 2H2O → MnO2+ 4H++ 2e-.Finally, the thin films of MnO2electrochemically deposited on the stainless steel electrode were scrapped off.The mixtures of as-prepared MnO2and CP (Quality percentage of CP: 10, 20, 30, and 40 wt%) were magnetically stirred in deionized water for 1 h, and then ultrasonically dispersed for another 1 h respectively, the above solutions were centrifuged and then dried at 80 °C (Heating rate: ~ 2 °C min-1) in air for 3 h to obtain the cathodic active materials, which were labelled as CP-10, CP-20, CP-30 and CP-40, respectively.Besides, the as-prepared MnO2mentioned above was labelled as CP-0.
2.2 Materials Characterization
The X-ray diffraction (XRD) profiles were measured with Bruker-D8 Advance X-ray diffractometer (Cu Kα radiation, 2θ step: 0.02°) and analyzed with Jade 5.0 software.The spectroscopic property was tested though PerkinElmer Spectrum 100 FTIR.The micro morphology was observed through JEOL JEM-2100F TEM and Nova Nano SEM 450.The composition and valence state were tested by Thermo ESCALAB 250XI XPS.
2.3 Computational Calculation
2.4 Electrochemical Properties
The cathodic active materials (CP-0, CP-10, CP-20, CP-30, and CP-40), acetylene black and poly-vinylidene fluoride were mixed together in accordance with the gravimetric ratio of 7:2:1, and N-methyl-2-pyrrolidone was added into the above mixtures to produce black slurries, which were then painted on 5 stainless steel webs (Diameter: ~ 14 mm) and then heated at 80 °C (Heating rate: ~ 2 °C min-1) in air for 8 h to obtain the cathodic current collectors (Load quality of active substance: ~ 2 mg cm-2).A CR2032 button battery configuration with a Whatman glass-fiber diaphragm (Grade GF/D), Zn anode, and the electrolyte of 2 mol L-1ZnSO4and 0.1 mol L-1MnSO4was used to estimate the cathodic active materials, the prepared button batteries shared the same numbers as CP-0, CP-10, CP-20, CP-30, and CP-40 respectively.The galvanostatic charge/discharge performance was tested using LANHE CT3002A equipment (Voltage: 0.8-1.8 V) based on the active material mass.The cyclic voltammetry (CV) curves were recorded using a CHI660E electrochemical work station (Scan rate: 0.1, 0.2, 0.4, 0.6, and 0.8 mV s-1), thenbvalue was obtained according to the equation log(i) = log(a) +blog(v) (i: Peak current;v: Scan rate;aandb: Adjustable values), the pseudocapacitive fitting was calculated on the basis of the relational expressioni(V) = k1v+ k2v0.5(k1v: Non-diffusion controlled contribution; k2v0.5: Diffusion controlled contribution).
2.5 In Vitro Cytotoxicity
3T3 mouse embryonic fibroblast cells were cultured in DMEM medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA) and 1% penicillin/streptomycin (Gibco, USA) at 37 °C.In vitro cell viability was evaluated by CCK-8 assay against 3T3 cells.Briefly, cells were seeded in 96-well plates and incubated with the as-synthesized materials, including CP-0, CP-10, CP-20, CP-30, and CP-40.10 μL CCK-8 solution was added to each well and the absorbance was recorded on a microplate reader (EnSpire, USA) at 490 nm.Cell apoptosis assay was conducted by using an Annexin V-FITC/PI apoptosis detection kit (Beyotime, China).For apoptosis assay, 3T3 cells were seeded into 6-well plates and incubated with the above-mentioned materials for 48 h.Thereafter, cells were stained with Annexin V FITC/PI for 30 min and the apoptosis percentage was detected by a flow cytometer (BD FACS Calibur, USA).Calcein AM/PI Co-staining was performed to detect the live/dead cells under different treatments.Following incubation cells were stained with calcein AM and PI for 30 min, and the stained cells were imaged by a fluorescence microscope (NIKON, Japan).For the above experiments, cells in PBS were set as the control group.
3 Results and Discussions
3.1 Microstructure
The preparation detail of CP is schematically illustrated in Fig.1a, and the N-doped biomass carbon is expected to be obtained.As shown in Fig.1b, all the samples display five characteristic peaks indexed to (120), (131), (300), (160), and (421) crystal planes, which means that γ-MnO2is successfully synthesized by electro-deposition corresponding to JCPDS No.14-0644 [104], and the broad peaks at around 26° belonging to (002) crystal plane of carbon for CP-10, CP-20, CP-30, and CP-40 are also observed [100].Compared with CP-0, a new peak at around 1025 cm-1corresponding to Mn-O-C bond is observed in the FTIR spectrum of CP-20 (Fig.1c), which once again demonstrates the successful recombination of CP and γ-MnO2for CP-20.The characteristic planes of CP and γ-MnO2are also observed in SAED pattern (Fig.1d), and the HRTEM images of CP-20 indicate that the interplanar spacings of 0.162 and 0.241 nm are well indexed to the lattice planes of (160) and (131) for γ-MnO2(Fig.1e, f), which further confirms the accuracy of the analytical result from Fig.1b, d.Figure 1g shows a successful loading of γ-MnO2nanoparticles on the surface of irregular flower-like CP.The EDS result shows a uniform element distribution of Mn, O, C, and N for CP-20 (Fig.1h-k), and the XPS result once again affirms the coexistence of Mn, O, C, and N on the surface of CP-20 (Fig.1l-o).Besides, Fig.S1 certifies the existence of Mn and O elements for CP-0, and Figs.S2, S3, and S4 also reveal the existence of Mn, O, C, and N elements for CP-10, CP-30, and CP-40 respectively, meanwhile the coexistence of Mn3+and Mn4+is revealed for all the samples.
The Jahn-Teller effect primarily due to high-spin Mn3+with (t2g)3(eg)1electron configuration in 3dorbital usually leads to detrimental structural disorder for MnO2, so MnO2with a higher Mn valence is promising to exhibit excellent long-term cycling stability [105].Therefore, the compound strategy of γ-MnO2with CP is beneficial to reduce the content of Mn3+, and it’s worth noting that CP-20 exhibits the highest percent of Mn4+(93.68%) and the lowest percent of Mn3+(6.32%), which foreshadows the weakest Jahn-Teller effect in CP-20 (Figs.1l and S1a, S2a, S3a, S4a).
3.2 Theoretical Calculation
As shown in Fig.2a, the calculatedDvalues of Zn2+in the internal structures of pure γ-MnO2and γ-MnO2@CP are 70 × 10-6and 174 × 10-6Å2fs-1respectively, and it can be seen that the CP composite strategy effectively improves Zn2+kinetics.TheEadsvalues of Zn2+on the tunnel-shaped surface of pure γ-MnO2and γ-MnO2@CP are - 2.968 and - 2.353 eV respectively, which demonstrates that Zn2+is more liable to migrate smoothly inside γ-MnO2@CP (Fig.2b).Bader charge (1.11e) of Zn2+and γ-MnO2@CP group is less than that (1.323e) of Zn2+and pure γ-MnO2group, and this implies a more obvious electron transfer tendency and a stronger binding interaction between Zn2+and pure γ-MnO2, which hinders the diffusion of Zn2+(Fig.2c).Besides, the nano-sized Mn-based cathode effectively reduces the migration time of Zn2+according to the equationτeq=L2/2D(τeq: Diffusion time;L: Material size;D: Diffusion coefficient) [69], so the combination of nanocrystallization for γ-MnO2(Fig.1g) and CP composite strategy would be helpful to boost Zn storage efficiency theoretically.
3.3 Electrochemical Property

Fig.1 a Schematic diagram for the preparation of CP.b XRD patterns of CP-0, CP-10, CP-20, CP-30, and CP-40.c FTIR spectra of CP-0 and CP-20.d SAED, e and f HRTEM, g SEM, h-k EDS of CP-20.XPS high-resolution patterns of l Mn 2p, m O 1s, n C 1s, and o N 1s of CP-20.
It is worth noting that the cathodic peaks shift toward higher values and the anodic peaks shift toward lower values for CP-10, CP-20, CP-30, and CP-40 compared with those of CP-0 from the CV curves at 0.4 mV s-1, which indicates the reduced inherent voltage polarization plausibly related to CP composite, and especially the smallest polarization is seen in CP-20, and CP-20 also shows the highest peak current density implying its largest electrochemical capacity (Fig.3a).CP-20 with a medium potential of ~ 1.4 V displays a maximum discharge capacity of 391.2 mA g-1and a minimum voltage hysteresis when the current density is 0.1 A g-1among the samples (Fig.3b).The high rate discharge ability of γ-MnO2mixed with CP are better than that of pure γ-MnO2when the current density increases from 0.1 to 5 A g-1and then reduces to 0.1 A g-1, besides CP-20 also exhibits the optimal discharge capability of 189.8 mAh g-1at 5 A g-1compared with other samples (Fig.3c).The cyclic stability of CP-0 is only 24.54% after 200 cycling times at 0.1 A g-1, while CP-20 demonstrates the best cycling performance (89.47%) and coulombic efficiency (~ 100%) (Fig.3d).In particular, the 3000 times cycling life of CP-20 achieves even high up to 92.17%, and its coulombic efficiency still maintains at ~ 100% at 5 A g-1(Fig.3e).Furthermore, the specific energy density of CP-20 at 0.1 A g-1reaches 553.12 Wh kg-1, which is superior to that of CP-0 (250.32 Wh kg-1), CP-10 (383.08 Wh kg-1), CP-30 (427.00 Wh kg-1) and CP-40 (307.21 Wh kg-1).More importantly, the energy density of CP-20 in our work is also superior to those reported in literatures (Fig.3f).To sum up, the recombination strategy with CP is of benefit to the electrochemical performance enhancement for γ-MnO2.

Fig.2 Theoretical calculated results of a MSD, b Eads, and c bader charge about the models of Zn2+ intercalating into the tunnel structures of pure γ-MnO2 and γ-MnO2@CP
As shown in Figs.S5a, S6a, S7a, S8a, and S9a, the phenomenon that the cathodic and anodic peaks of CV curves for all the samples shift toward negative and positive potentials respectively as the scan rate increases reveals a distinct insertion/deinsertion energy storage behavior of Zn2+[74].The CP-20 sample, by contrast, has a stronger redox peak and smaller gap between cathodic and anodic peak, which indicates that CP-20 is promising to have faster dynamics, less polarization and better long cycle performance among the samples.The calculated pseudocapacitive proportion results (Figs.S5b, S6b, S7b, S8b, and S9b) show that CP-20 exhibits the maximum pseudocapacitance contribution, and it is again verified that the reinforced pseudocapacitance behavior by CP composite strategy is beneficial to ameliorate the electrochemical characteristics of γ-MnO2.Usually, a largerb-value (close to 1.0) is deemed to be advantageous for strengthening pseudocapacitance behavior so as to improve energy storage dynamics, obviouslyb-value of CP-20 is lager than that of other samples according to Figs.S5c, S6c, S7c, S8c, and S9c, and this also reveals the effectively promoted pseudocapacitance behaviour of CP-20.Furthermore, The fact that the peak1 and peak2 off-set values of CP-20 are the smallest demonstrates again its enhanced intercalation pseudocapacitance (Figs.S5d, S6d, S7d, S8d, and S9d), which strongly supports the above fitting conclusions about pseudocapacitive andb-value.More significantly, the presence of pyridinic-N and pyrrolic-N is considered to be responsible for boosting the pseudocapacitance behavior of electrode materials [106], and this might be another key factor to improve the pseudocapacitance characteristics of γ-MnO2via CP composite.(Figs.1o and S2d, S3d, S4d).
3.4 Mechanism Analysis
Under the current density of 0.1 A g-1, the byproduct Zn4SO4(OH)6·4H2O (JCPDS No.44-0673) emerges at the fully discharged (F-D) state and almost completely fades away at the fully charged (F-C) state on the 10th cycle for CP-20, and the reversible reaction is represented by the following equation:γ-MnO2+SO2-4+4Zn2++2OH-+6H2O+2e-↔Mn2++Zn4SO4(OH)6·4H2O , while Zn4SO4(OH)6·4H2O always exists at F-D and F-C states for CP-0 (Fig.4a).Noticeably, the calculatedEadsvalues of OH-with the surfaces of pure γ-MnO2and γ-MnO2@CP are - 3.166 and - 1.568 eV respectively (Fig.4b), this obvious difference illustrates that OH-is more liable to be adsorbed on the surface of pure γ-MnO2to promote the formation of Zn4SO4(OH)6·4H2O, which reconfirms the conclusion from Fig.4a.Herein, the CP composite strategy effectively inhibits the side effect and ultimately increases the reversibility and efficiency of Znion energy storage.
The O 1sXPS spectra of CP-0 and CP-20 are fitted into tetravalent Mn-O-Mn bonds, trivalent Mn-OH bonds, and H-O-H bonds for residual water, correspondingly the average Mn valences of CP-0/CP-20 are estimated to be 3.6 +/3.8 + at F-D state and 3.75 +/3.95 + at F-C state on the 10th cycle according to the area contributions of Mn-O-Mn and Mn-OH components respectively (Fig.4c).More precisely, the average oxidation state (AOS) of Mn can be computed based on the equation AOS = 8.95-1.13 ΔEMn3s, where ΔEMn3sis the energy difference between the main and satellite peaks in Mn 3sXPS spectra [107], so the AOS of Mn in CP-0 (F-D), CP-0 (F-C), CP-20 (F-D), and CP-20 (F-C) on the 10th cycle are also 3.6 +, 3.75 +, 3.8 +, and 3.95 + respectively (Fig.4d), thus the calculated Mn valence from Mn 3sand O 1sXPS spectra are entirely the same, which further illustrates that the CP composite is beneficial to suppress the Jahn-Teller effect by regulating Mn valence, besides the Zn 3ppeak of CP-0 is also lower than that of CP-20.Meanwhile, the Zn 2ppeaks of CP-20 are apparently stronger than that of CP-0 on the 10th cycle at F-D state (Fig.4e), which reconfirms the conclusion in Fig.4d.Different from the Mn-O bonds of γ-MnO2@CP nearly without distortion, the Jahn-Teller effect induces a geometric distortion with two longer (O5 and O6) Mn-O bonds of pure γ-MnO2according to theoretical calculation (Fig.4f).The formation of Mn domains is supposed to disrupt the cooperativity of Jahn-Teller effect as schematically shown in Fig.4g [108], and the Mn domains with different orientation are also visually identified in CP-20 (Fig.4h) but not in CP-0 (Fig.4i), hence the CP composite strategy promotes the anisotropic Jahn-Teller distortion to improve the structural stability of γ-MnO2.

Fig.3 Electrochemical test results of a CV curves, b constant current charge-discharge profiles, c high rate discharge ability, d 200 times cycling performance, and e 3000 times cyclic stability of CP-0, CP-10, CP-20, CP-30, and CP-40.f Comparison diagram about the energy density calculated based on cathodic active material mass between literatures and CP-20
3.5 In Vitro Cell Toxicity
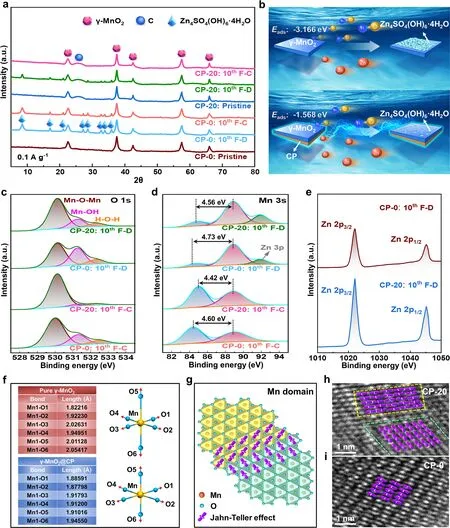
Fig.4 a Ex-situ XRD of CP-0 and CP-20 at F-D/F-C states on the 10th cycle.b Sketch map at F-D state and the calculated Eads values of OH- to the surface of γ-MnO2.XPS high-resolution patterns of c O 1s, d Mn 3s and e Zn 2p of CP-0 and CP-20.f Calculated Mn-O bonding distances of MnO6 octahedra of pure γ-MnO2 and γ-MnO2@CP.g Schematic Mn domains and h, i actually observed Mn domains

Fig.5 a Relative cell viability of 3T3 cells at different time points post treatment.b Fluorescence microscopic images of 3T3 cells by Calcein AM/PI staining at 48 h with various treatments.c Cell apoptosis percentage of 3T3 cells upon different treatments for 48 h by flow cytometry (Ctrl: cells treated with PBS)
To investigate the intrinsic cytotoxicity of the above-mentioned samples against 3T3 cell, CCK-8 assay is performed to determine the relative cell viability after 24, 48, and 72 h of incubation, respectively.Currently, cells treated with PBS are set as a reference.Negligible proliferation inhibition is observed in cells with the treatment of CP-10, CP-20, CP-30, and CP-40 even up to 72 h of incubation compared with the control group, while exposure to CP-0 exhibits notable cytotoxicity to cells as the relative cell viability decreases over 50% after 72 h (Fig.5a), indicating the desirable biocompatibility of the above samples except CP-0.Besides, Calcein AM/PI staining is carried out to assess the live/dead cell after different treatments, and no evident cell death is noticed in cells under these above treatments other than CP-0 as expected (Fig.5b), which is consistent with the results of CCK-8 assay.Furthermore, flow cytometry is performed to investigate the apoptosis profile of 3T3 cells incubated with each sample for 48 h, and no apoptosis is induced by most of the as-synthesized materials with a total apoptotic cell rate less than 5% compared to the control group, but exposure to CP-0 results in severe cell apoptosis as indicated by nearly 40% of total apoptosis percentage (Fig.5c), and it is in good accordance with the cell toxicity results.It is verified that CP-10, CP-20, CP-30, and CP-40 possess better biosafety compared with CP-0, which might be attributed to the loading of biocompatible CP.Therefore, the CP composite strategy shows a great potential not only in the field of large-scale energy storage, but also in clinical applications.
4 Conclusion
The environmental friendly AZIBs with considerable theoretical capacity (820 mAh g-1) and appropriate redox potential (- 0.763 V versus standard hydrogen electrode) attract researchers’ broad concern recently [109-117].Thereinto, biocompatible AZIBs are proposed as candidates for powering biocompatible electronics due to their excellent features of low cost, high-level safety and high-performance [118-120].Hence, the biomass CP derived from waste grapefruit peel is successfully prepared, and the electrochemical properties and biocompatibility for the composite cathode of γ-MnO2loaded on CP are simultaneously investigated in the present work.The considerable electrochemical properties of 3000 times long cycle stability at 5 A g-1(92.17%), energy density (553.12 Wh kg-1), and coulombic efficiency (~ 100%) for the composite cathode with CP quality percentage of 20 wt% are achieved, which is mainly ascribed to the effective regulation of Mn-O bond distance, Mn valence, and Mn domains combined with experimental and DFT computational analysis.Furthermore, the cathodic biosafety is also verified via in vitro test extensively.In brief, this work not only brings forward a feasible countermeasure for structural regulation of multi-function Mn-based cathode with inexpensive biomass-derived carbon, but also paves a novel way for the application of AZIBs in biomedical field.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China [Grant no.51821004].
Declarations
Conflict of InterestThe authors declare no interest confict.Twests or personal relationships that could have appeared to influence the work reported in this paper.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http:// creat iveco mmons.org/ licen ses/ by/4.0/.
Supplementary InformationThe online version contains supplementary material available at https:// doi.org/ 10.1007/ s40820- 024- 01334-3.
- Nano-Micro Letters的其它文章
- Highly Elastic, Bioresorbable Polymeric Materials for Stretchable, Transient Electronic Systems
- Molecular Mechanisms of Intracellular Delivery of Nanoparticles Monitored by an Enzyme-Induced Proximity Labeling
- Proton-Prompted Ligand Exchange to Achieve High-Efficiency CsPbI3 Quantum Dot Light-Emitting Diodes
- A Sustainable Dual Cross-Linked Cellulose Hydrogel Electrolyte for High-Performance Zinc-Metal Batteries
- Optoelectronic Synapses Based on MXene/Violet Phosphorus van der Waals Heterojunctions for Visual-Olfactory Crossmodal Perception
- MXene Hollow Spheres Supported by a C-Co Exoskeleton Grow MWCNTs for Efficient Microwave Absorption

