Preparation of PrFexCo1-xO3/Mt catalyst and study on degradation of 2-hydroxybenzoic acid wastewater by catalytic wet peroxide oxidation
Binxia Zhao,Yijia Gao,Tiancheng Hun,Xiaoxiao Fan,Nan Shao,Xiaoqian Chen
School of Chemical Engineering,Northwest University,Xi'an 710069,China
Keywords: Montmorillonite Perovskite Catalytic wet peroxide oxidation (CWPO) 2-Hydroxybenzoic acid
ABSTRACT In this study,the perovskite nanocomposite PrFexCo1-xO3(Pr(S)) was successfully synthesized by the sol-gel method;PrFexCo1-xO3/Al-pillared montmorillonite (Pr(S)/Mt) catalysts were prepared by impregnation (D) method and solid-melting (G) method,respectively,with Pr(S) as the active component and Al-pillared montmorillonite as the carrier.The catalysts were applied to treat the 2-hydroxybenzoic acid(2-HA)-simulated wastewater by catalytic wet peroxide oxidation (CWPO) technique,and the chemical oxygen demand (COD) removal rate and the 2-HA degradation rate were used as indicators to evaluate the catalytic performance.The results of the experiment indicated that the solid-melting method was more conducive to preparing the catalyst when the Co/Fe molar ratio of 7:3 and the optimal structural properties of the catalysts were achieved.The influence of operating parameters,including reaction temperature,catalyst dosage,H2O2 dosage,pH,and initial 2-HA concentration,were optimized for the degradation of 2-HA by CWPO.The results showed that 97.64% of 2-HA degradation and 75.23% of COD removal rate were achieved under more suitable experimental conditions.In addition,after the catalyst was used five times,the degradation rate of 2-HA could still reach 76.93%,which implied the high stability and reusability of the catalyst.The high catalytic activity of the catalyst was due to the doping of Co into PrFeO3,which could promote the generation of HO∙,and the high stability could be attributed to the loading of Pr(S) onto Al-Mt,which reduced the leaching of reactive metals.The study of reaction mechanism and kinetics showed that the whole degradation process conformed to the pseudo-firstorder kinetic equation,and the Langmuir-Hinshelwood method was applied to demonstrate that catalysis was dominant in the degradation process.
1.Introduction
In the recent decades,the rapid development of the pharmaceutical industry has produced a large amount of pharmaceutical wastewater;they contain large amounts of antibiotics and pharmaceutical intermediates,which have the characteristics of complicated components,hard degradation,and strong toxicity[1].If not treated properly,it will cause serious threats to human beings and the environment [2].Due to the harm of pharmaceutical wastewater,the treatment of pharmaceutical wastewater has received strong attention from many countries.
At present,the common wastewater treatment technologies include adsorption,photocatalysis,microwave catalysis,ozone oxidation,etc.The adsorption method generally uses porous materials such as montmorillonite and kaolin as adsorbents,and the method mainly uses intermolecular forces between the adsorbent and the pollutant to adsorb the pollutant in the wastewater.The technology has low secondary environmental pollution and low equipment requirements [3],but the high cost of adsorbent regeneration has limited its further application.Nowadays,advanced oxidation processes (AOPs) are considered effective methods to treat pharmaceutical wastewater.In the process of AOPs,the generated hydroxyl radicals (HO∙) mineralize pollutants in wastewater,which have strong oxidation activity and nonselectivity [4,5].Photocatalysis is a kind of AOP that uses light to irradiate semiconductors to generate electrons and holes,which in turn mineralizes pollutants [6].It has advantages such as high catalytic activity and environmental friendliness[7],but the use of visible light by semiconductors is limited and is not applicable to the degradation of actual wastewater.Microwave catalysis is also an effective technology for the degradation of pollutants.The thermal and excitation effects of microwaves produce large amounts of reactive oxygen species (ROSs) in the composite material,allowing for efficient and rapid degradation of pollutants[8,9],but the process is more energy intensive.Ozone has a strong oxidation ability and can produce a large amount of HO∙in a short period to achieve complete degradation of pollutants,which is an environmentally friendly AOP,but the application cost is high and may also produce toxic by-products[10].However,the catalytic wet peroxide oxidation (CWPO) technology with strong oxidation ability can overcome these disadvantages.
The CWPO technology is another kind of AOP;heterogeneous catalysts are usually used to catalyze H2O2to produce HO∙with strong oxidizing properties (2.8 V),which can directly oxidize pollutants in water and eventually oxidize into small inorganic molecules such as carbon dioxide and water[11].The total reaction process is carried out under mild conditions and costs less[4].The application of heterogeneous catalysts in CWPO can overcome the disadvantages of homogeneous catalysts such as metal leaching and secondary pollution[12].Nowadays,the development of them with high activity and stability is the key problem of CWPO.
Montmorillonite (Mt) is a kind of natural clay with a typical layered structure;they are abundant in nature,have a large specific surface area,and exhibit a porous structure and therefore are often used as an absorbent or a catalyst carrier [13].There are many exchangeable cations between the interlayer space;this feature is beneficial to the modification of montmorillonite by ion exchange.Due to the small interlayer spacing of raw montmorillonite,which needs to be pillared to improve structural and ion exchange performance.Briefly speaking,the pillared process is the polycations produced by the hydrolysis and polymerization of metal cations,which are exchanged with the metal cations between layers,and the stable oxide pillars are formed after being calcined,thus strengthening the structure of montmorillonite [14,15].Among many porous materials,pillared montmorillonite is suitable and can be used as a catalyst carrier because of its large surface area,excellent thermal stability,and high mechanical strength [16].Someone loaded FeOCl on Al-pillared montmorillonite,and the removal rate of fuchsin in wastewater was close to 100%[12].Janjić et al.[17] synthesized cobalt-based catalyst-supported on Alpillared montmorillonite by impregnation method and investigated the degradation effect of tartrazine under extreme conditions.The results showed that tartrazine was completely degraded after 240 min,and the reaction mechanism and resulting derivative were elucidated by LC-MS.In general,pillared montmorillonite has a good application value to be used as a catalyst carrier in the preparation of heterogeneous catalysts.
Perovskite materials are defined as a class of oxides with a crystal structure similar to that of CaTiO3and a chemical structure of ABO3,where A is the alkali metal,alkaline earth metal,or rare earth metal ions with a large radius,and B is the transition metal with a small radius [18].Because of its adjustable structure,good thermal stability,and high redox properties,it has become a research hotspot in the field of materials and catalysts [8].Perovskite structure is stable and flexible,easy for doping modification.Some studies have shown that partial doping of elements into perovskite can introduce lattice defects and oxygen vacancies,they can speed-up the flow of electrons on the surface and improve the redox performance of the crystal,therefore improving the catalytic performance of perovskite catalysts significantly [19-21].Therefore,when reacting at a relatively high calcined temperature,using a transition element with +2 or +4 valency to partially replace elements at the B-site of perovskite will produce oxygen vacancies,forming a perovskite structure of ABxC1-xO3,thereby improving the reaction activity of perovskite [9,22].Cobalt is a common active component of catalysts;it shows high catalytic activity[23,24]and costs less.Some studies [25,26] have shown that intercalating Co into praseodymium-based perovskite can form relatively strong interactions,which can significantly improve the activity and stability of the catalyst.Although perovskite catalysts have broad application prospects,the main problem limiting their application is that the specific surface area is not large enough[27].Therefore,this problem could be solved by loading perovskite onto the pillared montmorillonite [28].
Hence,in this study,Al-pillared montmorillonite loaded Codoped PrFeO3perovskite catalysts Pr(S)/Al-Mt were synthesized by impregnation(D)and solid-melting(G)method,respectively.The structural characteristics of the catalysts were also characterized.CWPO technology was applied to treat 2-hydroxybenzoic acid (2-HA) wastewater to evaluate the catalytic performance.The appropriate preparation method and Co/Fe molar ratio were explored.Radical quenching experiments were carried out to determine the reactive groups.Furthermore,the reaction mechanism,reusability,and kinetics were also studied.This research extends the application of perovskite catalysts in the CWPO technique and provides a potential method to treat pharmaceutical wastewater.
2.Material and Methods
2.1.Reagent and materials
Sodium montmorillonite (purity >98% (mass)) was purchased from Xinhui mineral products Co.(Shijiazhuang,China).Fe(NO3)3∙9H2O was purchased from Aladdin Reagent Co.,Ltd.Pr(NO3)3∙6H2O,and 2-HA came from Tianjin Damao chemical reagent factory,China.Co(NO3)2∙6H2O was supplied by Hongda Chemical Co.(Henan,China).All reagents were analytical grade and were used without any further purification.The experimental water was distilled water.
2.2.Catalyst preparation
The 500-mg montmorillonite was added into 15 ml AlCl3(0.4 mol L-1),the mixture was stirred at 80°C for 10 min,then 2.5 ml polyethylene glycol(PEG-400)and 7.5 mL NaOH(0.4 mol∙L-1)were added.After that,the mixture was stirred continuously at 60°C for 30 min,then stirred at room temperature for 30 min.After aging for 12 h,the precipitate was centrifugally washed and dried at 80°C for 20 h and calcined at 400°C for 2 h to obtain Al-pillared montmorillonite(recorded as Al-Mt).
The Pr-based perovskite was prepared by the sol-gel method.The process is as follows: Pr(NO3)3∙6H2O,Fe(NO3)3∙9H2O,Co(NO3)2∙6H2O,and citric acid were dissolved and mixed evenly in a certain proportion (the molar ratio of nitrate to citric acid was 1:1),then ultrasonic treatment for 30 min.After that,they were heated in a 90°C water bath for 30 min.After drying at 103°C for 24 h,the powder was calcined at 500°C for 2 h,then further calcined at 700°C for 4 h,and the obtained sample was PrFex-Co1-xO3(recorded as Pr(S)).
The catalysts were prepared by impregnation and solid-melting method,respectively.The synthesis procedures of the impregnation method are as follows:Pr(S)was added to distilled water and was fully stirred.The excess impregnation liquid was dropped on montmorillonite,and the mixture was stirred,dried at 80°C,and calcined at 220°C for 2 h,then the catalyst was obtained,which was recorded as Pr(S)/Mt-D.The preparation steps of the solidmelting method are as follows: a certain amount of Al-Mt and Pr(S) was mixed and ground for 10 min to obtain a uniform black powder.The powder was calcined at 220°C for 2 h to obtain the catalyst,which was recorded as Pr(S)/Mt-G.The mass ratio of Pr(S)to Al-Mt was 1:2 in both methods.
2.3.Characterization techniques
The morphologies and distribution of the samples were observed by the scanning electron microscope(SIGMA,Carl Zeiss).The crystal structure and composition of samples were determined by X-ray powder diffraction (Smartlabse,Rigaku).The test conditions were as follows: the powder sample was laid in a glass dish,and Cu-Kαradiation was used at 30 mA and 40 kV,scanning speed 10(°)∙min-1,scanning range 5°-75°.The specific surface area and pore-size analyzer(NOVE 2200e,Quantachrome)was used for the N2adsorption/desorption test,which mainly indicated the structural parameters of the catalyst.The Spectro Spectroflame M-inductively coupled plasma optical emission spectrometer(ICPOES,Optima2000,PerkinElmer company) was used to test the content of Fe and Co leaching in wastewater.The X-ray photoelectron spectrometer from Thermo Electron company was used to analyze the valency and composition of the surface elements of the catalyst,model Alpha,under the following conditions:a voltage of 12.5 kV,Al Kα rays,and reference to the standard binding energy of C 1s.The functional groups in the samples were measured using a Fourier transform infrared spectrometer(IRTffinity-1S,Shimadzu).The test conditions were set to scan the wavenumber at 4000-400 cm-1,and samples need to be completely dried.
2.4.Catalytic activity experiment
Firstly,the catalyst was put into a three-necked flask.Then 100 ml 2-HA solution with a concentration of 100 mg∙L-1was poured into the flask.The pH was adjusted to 5.The three-necked flask was put in the magnetic stirring water bath pot,stirring at 80°C for 30 min,then H2O2(30%)was added,and the reaction time was controlled for 2 h.Samples were taken and filtered at different time,and the COD removal rate and the 2-HA degradation rate were tested and calculated.
2.5.Analytical method
The absorbance of the sample was measured at 300.1 nm(based on a full-wavelength scan of 2-HA)on the ultraviolet-visible diffuse spectrum analyzer,and the degradation rate of 2-HA can be calculated by Eq.(1).The COD of samples at different times can be tested by using the JHR-2 COD quick digestion instrument,and the COD removal rate can be obtained by Eq.(2).
where A0refers to the initial absorbance of 2-HA in the sample,and Atrefers to the absorbance of 2-HA in the sample at time t.
where D0refers to the initial COD value of wastewater,and Dtrefers to the COD value of wastewater at time t.
3.Results and Discussion
3.1.Catalyst characterization
The morphologies of Pr(S)/Mt catalysts prepared via impregnation and solid-melting methods could be observed by scanning electron microscopy(SEM)images with a Co/Fe molar ratio of 7/3.As shown in Fig.1(a),the raw montmorillonite (raw Mt) had an obvious layered structure and curled surface,which was due to the irregular agglomeration of plate-like particles in raw montmorillonite[29].Perovskite nanocomposites were agglomerated rod-like or spherical particles,which could be revealed in Fig.1(b).The magnetic attraction between the particles and the mild sintering caused by the high-temperature calcination result in this agglomerated morphology[30].In Fig.1(c),compared to the impregnation method,perovskite particles were more obvious on the surface of the Pr(S)/Mt-G catalyst,the surface of the catalyst retained a rough appearance,and many microporous structures were formed,which were conducive to the adsorption of reactants,thus improving the catalytic performance [20].The aforementioned analysis indicated that the solid-melting method was more favorable to optimize the structure and morphology of the catalyst.

Fig.1.SEM images of samples with different loading methods: (a) raw Mt;(b) Pr(S);(c) Pr(S)/Mt-G;(d) Pr(S)/Mt-D.
The X-ray diffraction (XRD) patterns of different samples were shown in Fig.2.The samples all had the original structure of raw Mt,which indicated that the layered structure of raw Mt was not damaged by the two preparation methods.The characteristic peaks at 2θ=32.4°,46.3°,and 57.4°were consistent with pure crystalline perovskite PrFeO3(PDF#78-2424),which were found in other samples and shifted to a large angle.This was because the samples were doped with Co,introducing some oxygen vacancies,which also proved that the two preparation methods successfully loaded Pr(S) on the Al-Mt,and the main component of the catalyst was Co-doped PrFeO3perovskite [31].Similarly,characteristic peaks of PrCoO3(PDF#75-0280) were also observed in Fig.2.Due to the close radius of Fe3+and Co3+,PrFeO3and PrCoO3have a similar crystal structure,and the main characteristic peaks appeared to overlap.In the XRD pattern,the catalyst Pr(S)/Mt-G prepared by the solid-melting method had a sharper characteristic peak at 2θ=32.4°,which demonstrated that the Pr(S)/Mt-G sample had a higher crystallinity.
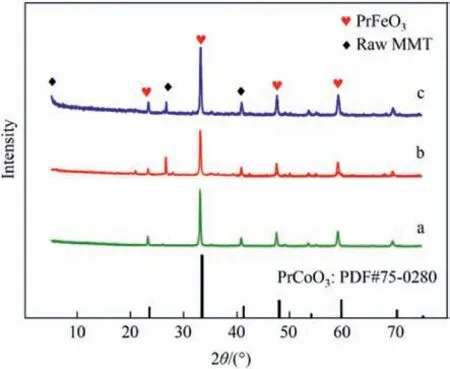
Fig.2.XRD patterns of different samples: (a) Pr(S);(b) Pr(S)/Mt-D;(c) Pr(S)/Mt-G.
To investigate the elemental composition and oxidation status of the samples,X-ray photoelectron spectroscopy(XPS)was used to characterize Pr(S)/Mt-G,Pr(S)and compared with the full spectrum and O 1s spectrum of raw Mt.Fig.3(a) shows the full spectra of samples.The differences in the elemental composition of the different samples could be seen in the full spectra.Montmorillonite was a silica-aluminate mineral,the presence of Si,Al,and O was observed in both of them,and carbon had an obvious characteristic peak because carbon was the base element in the test[32].Fig.3(b)showed the O 1s split-peak-fitted spectra of the different samples.The characteristic peaks at 531.78 eV and 532.53 eV were attributed to the Si-O and Al-O bonds,corresponding to SiO2tetrahedrons and Al2O3octahedrons in the montmorillonite-layered structure,respectively [33].The binding energies of 529.48 eV and 532.1 eV were the characteristic peaks of surface lattice oxygen and adsorbed oxygen [34].In addition,the characteristic peak at 534.63 eV indicated the presence of a small number of hydroxyl species.In the split-peak-fitted spectra of Co 2p,the two main characteristic peaks located at 796 eV and 780.58 eV correspond to the Co 2p1/2and Co 2p3/2orbitals,respectively,while two Co species were also observed,the binding energies of 779.85 eV and 794.77 eV for Co3+and peaks at 781 eV and 796.4 eV indicate the presence of Co2+[35].The Fe 2p spectra of Pr(S)/Mt-G and Pr(S) are shown in Fig.3(d),and the peaks of Fe 2p1/2and Fe 2p3/2could be observed.The characteristic peaks of Fe3+were located at 711.43 eV and 723.9 eV;however,the characteristic peak of Fe2+was not observed at 708.44 eV,which indicated that the Fe3+species were mainly present in the sample [36].In addition,a wider peak area was observed for the characteristic peak of Fe 2p3/2due to the phenomenon of spin-orbit(j-j)coupling of Fe 2p3/2,which had a fourstate simplification,while Fe 2p1/2had only two states [37].
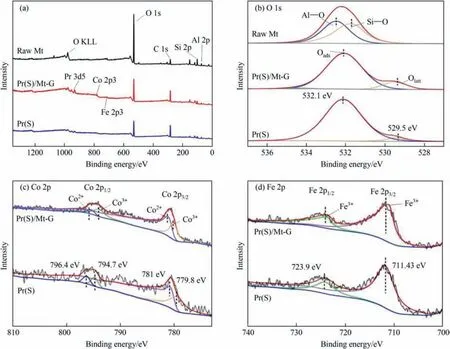
Fig.3.XPS spectra of samples (a) full spectra and (b) O 1s spectra of raw Mt,Pr(S)/Mt-G and Pr(S);(c) Co 2p spectra and (d) Fe 2p spectra of Pr(S)/Mt-G and Pr(S).
The N2adsorption/desorption isotherms of different samples are revealed in Fig.4(a).According to the classification of Brunauer-Deming-Deming-Teller,the raw Mt,active component Pr(S),and the catalysts prepared by different methods all belong to type IV isotherms,indicating that all samples have a mesoporous structure,and the original structure of montmorillonite was not destroyed during the preparation process and the single-layer adsorption phenomenon of capillary condensation occurred[12,38].The isotherm of Pr(S)formed H4 hysteresis loops,indicating that the sample contains micropores and mesopores.H3 hysteresis loops were formed in the catalysts with both preparation methods,indicating that the mesoporous structure was the main structure of the two samples,and the phenomenon of multilayer nitrogen adsorption and capillary condensation occurred [39,40].The transition from H4 to H3 hysteresis loop illustrated the change in the main structure of the perovskite,which was the transition from the microporous to mesoporous structure [41].However,the hysteresis loop of the perovskite component Pr(S) was almost not observed,which means it has the smallest specific surface area[42].The pore size distribution curves of different samples were determined by the Barret-Joyner-Halenda (BJH) method,as shown in Fig.4(b),confirming the presence of mesoporous pores [43,44].The pore size distribution curves of perovskite component Pr(S)showed basically no peaks,indicating that the pore volume varied regularly with pore size.The pore sizes of the other samples were mainly distributed between 3.3 and 6.5 nm,which were typical of mesoporous materials.The pore size distribution of the Pr(S)/Mt-G was wider,showing a double-peak distribution at 3.82 nm and 6.35 nm,respectively,with the first peak being stronger in intensity and the second peak in the form of a shoulder peak.The structural parameters of the samples were presented in Table 1.It could be found that the structural parameters of Pr(S)/Mt-G and Pr(S)/Mt-D were better than those of the raw Mt and the single perovskite component,indicating that the catalytic performance of Pr(S)/Mt may be better than that of the single perovskite catalyst.Pr(S) loaded on the surface of montmorillonite may form new pore structures,which was consistent with the results observed by SEM.Therefore,perovskite could also optimize the overall structure of the catalyst to a certain extent[45].The specific surface area,total pore volume,and average pore size of Pr(S)/Mt-G were larger than those of Pr(S)/Mt-D,this may be because the solid-melting method enhanced the interaction between the perovskite and the carrier,resulting in a more homogeneous distribution on the surface of the montmorillonite,whereas the impregnation method may block the pore structure of the montmorillonite,leading to a decrease in structural properties.Hence,Pr(S)/Mt-G may have higher catalytic performance.
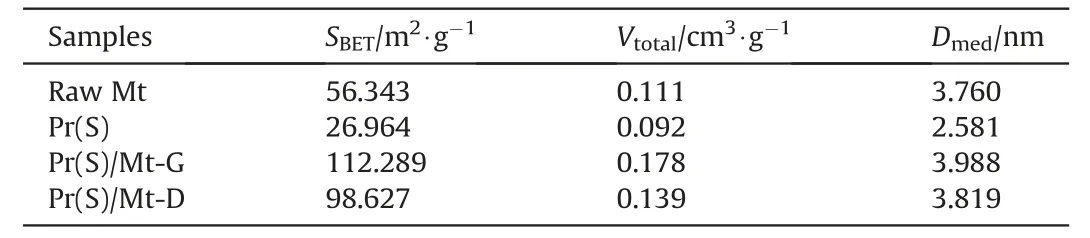
Table 1 Structural parameters of different samples
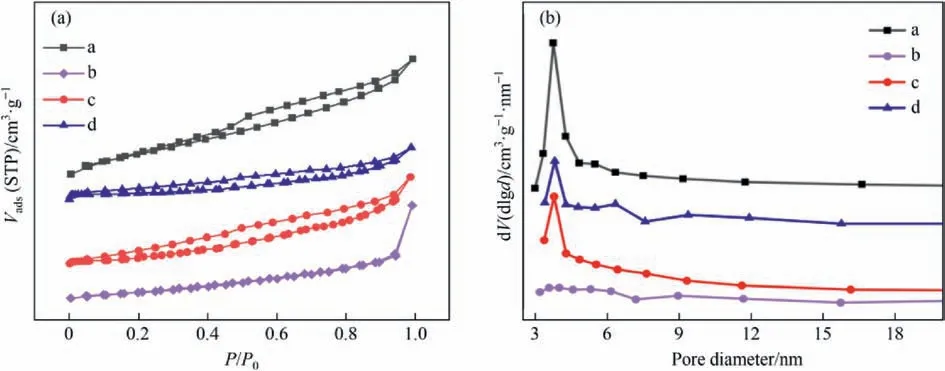
Fig.4.Nitrogen adsorption/desorption isotherms (a),pore size distribution curves (b) of different samples: (a) raw Mt;(b) Pr(S);(c) Pr(S)/Mt-D;(d) Pr(S)/Mt-G.
In general,the Pr(S)/Mt-G catalyst has better structural properties,and the preparation period was shorter;therefore,the solidmelting method was selected to prepare the catalyst.
In perovskite active components,partial substitution of B-site metal elements by transition elements could introduce oxygen vacancies and the enhancement of reactivity.Therefore,catalysts with different Co/Fe molar ratios Pr(S)/Mt-G-x/y were prepared,where x/y was the Co/Fe molar ratio,which were 0/1,2/8,3/7,5/5,7/3,8/2,and 1/0.Through various characterizations and experimental analysis,the appropriate Co/Fe molar ratio was obtained.
Fig.5 shows the XRD patterns of catalysts with different Co/Fe molar ratios.Except for the samples with Co/Fe molar ratios of 0/1 and 1/0,the composition of the remaining samples was not monolithic but the crystalline phase structure with the coexistence of PrFeO3and PrCoO3,which was characteristic of standard perovskite materials[46].It was observed that all samples retained the basic structure of montmorillonite,and with the increase of the Co/Fe molar ratio,the characteristic peak of PrFeO3located at 2θ=32.4°had a tendency to be shifted to a large angle.This may be attributed to part of the Fe in PrFeO3perovskite crystal being replaced by the smaller radius Co,which then led to lattice distortion and torsion [47],resulting in the asymmetric crystal structure,then oxygen vacancies introduced[31].The characteristic peaks of Pr(S)/Mt-G-0/1 and Pr(S)/Mt-G-1/0 located at 2θ=32.4°were sharper for PrFeO3and PrCoO3,respectively,indicating that the doping of the elements may reduce the crystallinity of the samples.

Fig.5.XRD patterns of samples with different Co/Fe molar ratios:(a) Pr(S)/Mt-G-0/1;(b) Pr(S)/Mt-G-2/8;(c) Pr(S)/Mt-G-3/7;(d) Pr(S)/Mt-G-5/5;(e) Pr(S)/Mt-G-7/3;(f) Pr(S)/Mt-G-8/2;(g) Pr(S)/Mt-G-1/0.
The textural properties and parameters of different Co/Fe molar ratio samples were also determined.It could be observed from Fig.S1(a) in Supplementary Material that all samples exhibited type IV isotherms and H3 hysteresis loops,which were typical for montmorillonite materials.From Fig.S1(b),it could be observed that the pore size distribution of all samples was mainly concentrated between 3 and 4 nm,indicating that mesoporous pores were mainly presented in each sample.In addition,it could be seen in Table S1 that the specific surface area and pore volume were the largest when the Co/Fe molar ratio was 7:3,reaching 112.289 m2∙g-1and 0.178 cm3∙g-1,respectively,which was more favorable for the CWPO reaction [48].
The Fourier-transform infrared (FTIR) spectra of samples with different Co/Fe molar ratios are shown in Fig.6;the characteristic peak at 3622 cm-1could be observed,attributed to the stretching vibration of the hydroxyl group between the layers of montmorillonite.The characteristic peak located at 1028 cm-1belonging to Si-O-Si corresponds to the silica-oxygen tetrahedral structure in montmorillonite,which was different from the Pr(S)/Mt-G-0/1,indicating that the interlayer structure of the montmorillonite in the undoped Co samples may be destroyed.While the sample Pr(S)/Mt-G-0/1 has a more obvious peak at 529 cm-1,which was due to the vibrations of Fe-O in the FeO6structure caused by Co doping in other samples,resulting in the weakening of the absorption peak at 529 cm-1[49].The samples has an absorption peak at 2366 cm-1,which indicated that the Fe in the catalyst was mainly in the form of Fe3+[50].
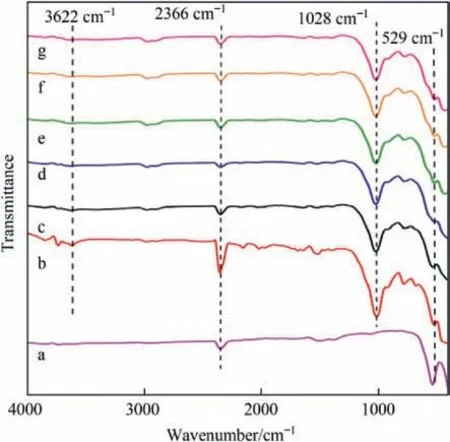
Fig.6.FT-IR spectra of samples with different Co/Fe molar ratios: (a) Pr(S)/Mt-G-0/1;(b) Pr(S)/Mt-G-2/8;(c) Pr(S)/Mt-G-3/7;(d) Pr(S)/Mt-G-5/5;(e) Pr(S)/Mt-G-7/3;(f) Pr(S)/Mt-G-8/2;(g) Pr(S)/Mt-G-1/0.
3.2.Catalytic activity evaluation
3.2.1.Effect of Co/Fe molar ratios
The samples with different Co/Fe molar ratios were used for CWPO degradation of 2-HA wastewater.The results are shown in Fig.7.The number of oxygen vacancies of samples and specific surface area prepared with different Co/Fe molar ratios were different;therefore,the degradation effect on 2-HA wastewater was different.The intercalation of Co introduced oxygen vacancies,thus increasing its catalytic activity [41].Each sample could degrade 2-HA to some extent.It could be seen that the best degradation effect was achieved when the Co/Fe molar ratio was 7:3.Although Co can also activate H2O2to produce HO∙,it was much less effective than Fe [51],and the pure PrCoO3crystal structure was complete and did not introduce oxygen vacancies;therefore,Pr(S)/Mt-G-1/0 has the worst degradation performance.In conclusion,Pr(S)/Mt-G-7/3 was selected as the appropriate catalyst.
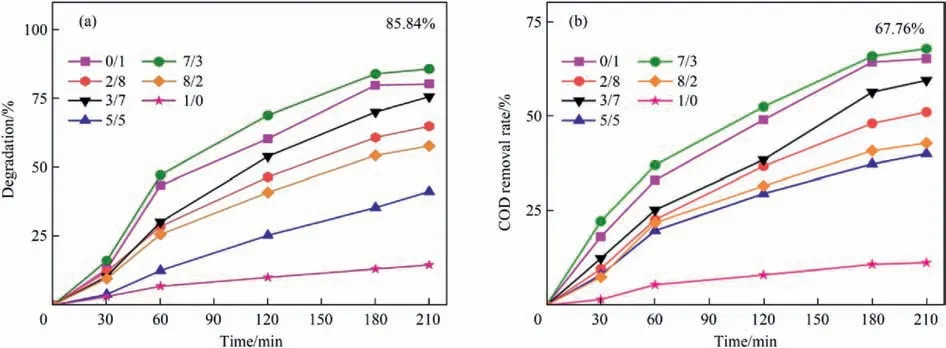
Fig.7.Variation trend of 2-HA degradation rate (a) and COD removal rate (b) with different Co/Fe molar ratios.Experiment conditions: Ccat=0.5 g∙L-1,C2-HA=100 mg∙L-1,=20.56 mmol∙L-1,pH=5.0,T=80 °C,pH=5.0.
3.2.2.Effect of temperature
Temperature was one of the important factors for CWPO,which could significantly affect the reaction rate and energy costs.Therefore,a more suitable reaction temperature was investigated in the range of 50°C-90°C.As can be seen in Fig.8(a),the lowest catalytic activity was observed at(50°C);this was probably due to the lower temperature decelerated the HO∙ and reduced the chance of collision with organic molecules,which was not conducive to the mineralization of pollutants [52].As expected,the COD removal rate increased and then decreased with the increase in temperature,and the optimum degradation performance was achieved when the temperature was 80°C.However,the COD removal rate tended to decrease when the temperature was further increased to 90°C.This was because the high temperature could cause the direct decomposition of H2O2into H2O and O2,and therefore,insufficient HO∙was produced [53].The difference between the catalytic performance at 70°C and 80°C was not significant.From the perspective of energy saving,70°C was the more suitable reaction temperature.

Fig.8.Influencing parameters in CWPO experiments performed with Pr(S)/MMT-G-3/7 on COD removal rate of 2-HA: (a) temperature (Ccat=0.5 g∙L-1,C2-HA=100 mg∙L-1,=20.56 mmol∙L-1,pH=5.0),(b) catalyst dosage (T=70 °C,C2-HA=100 mg∙L-1,=20.56 mmol∙L-1,pH=5.0),(c) H2O2 dosage (T=70 °C,Ccat=0.75 g∙L-1,C2-HA=100 mg∙L-1,pH=5.0),(d) pH (T=70 °C,Ccat=0.75 g∙L-1,C2-HA=100 mg∙L-1,=16.64 mmol∙L-1).
3.2.3.Effect of catalyst dosage
The catalyst dosage affects the efficiency and economy of 2-HA degradation;therefore,it was also an important factor to be considered.Fig.8(b)shows the effect of catalyst dosage on catalytic activity;when the catalyst dosage was 0.025 g,the COD removal rate was only 36.23%,which was because less catalyst provided deficient active sites to produce enough HO∙,and only a small amount of 2-HA could be adsorbed.The catalytic performance also started to decrease when the catalyst dosage reached 0.1 g.This was because the excess catalyst caused more metal ions leaching,which would increase the COD of the reaction system [54].In addition,some studies have shown that when the catalyst dosage was excessive,too much Fe2+will also have a scavenging effect on HO∙(Eq.(3)),thus affecting the degradation effect [20,55].
3.2.4.Effect of H2O2dosage
The CWPO reaction uses H2O2as the oxidant,and the H2O2dosage has a significant effect on the degradation effect.A series of experiments were conducted to investigate the appropriate H2O2dosage,and the results are shown in Fig.8(c).When the H2O2dosage was too low,it could not produce enough HO∙to mineralize the pollutants.However,when the H2O2dosage reached 0.21 ml,it had an inhibitory effect on the reaction.This was because excessive H2O2would induce the quenching reaction of free radicals,and it could also remove HO∙(Eqs.(4) and (5)),which resulted in ineffective consumption of oxidants [56].The experimental result showed that increasing the H2O2dosage alone cannot further improve the catalytic activity of pollutants.
3.2.5.Effect of pH
In general,CWPO reactions were carried out under acidic conditions.The catalytic performance was tested at pH from 3.0 to 7.0.It can be seen from Fig.8(d)that pH had a significant impact on the catalytic activity.With the increase in pH,the COD removal rate first increased and then decreased.When the pH value was 7.0,the degradation effect was the worst because H2O2is a binary weak acid that undergoes partial ionization,as shown in Eqs.(6)and(7),and the increase in pH would accelerate its ionization process,failing the oxidation performance of H2O2[57].However,when the reaction system was too acidic,the excess H+would scavenge HO∙[58].At the same time,it would also aggravate the acidic leaching effect,which could destroy the active site of the catalyst and lead to the deterioration of catalytic performance.
3.2.6.Effect of 2-HA concentration
In addition,the effect of the initial 2-HA concentration on the degradation effect was also explored,as shown in Fig.S2;when the concentration of 2-HA was increased from 50 mg∙L-1to 150 mg∙L-1,the degradation rate decreased from 98.6% to 66.4%.When the dosage of the catalyst and H2O2was constant,the amount of HO∙that could be produced was also certain.If the concentration of pollutants was low,the excess HO∙would spontaneously undergo a quenching reaction,resulting in the waste of oxidants.On the contrary,when the pollutant concentration was high,the amount of HO∙was not enough to further improve the 2-HA degradation rate[59].
3.3.The stability and reusability of the catalyst
The stability and reusability will directly determine the practical application value of the catalyst.After the optimization of the process conditions,the Pr(S)/Mt-G-7/3 sample was used to carry out the repeated experiment of CWPO degradation of 2-HA wastewater.Fe was the active metal that played a major catalytic role in the CWPO reaction,and Co doping could promote the catalytic performance[60].The activity was judged by the degradation rate of 2-HA,and stability was evaluated by the leaching amount of mental ions.The experimental results are shown in Fig.9.After five repeated experiments,the degradation rate of 2-HA only decreased by 8.91%,and the average degradation rate reached 81.97%,indicating that the catalyst has good reusability.The average leaching amount of Fe was 0.184 mg∙L-1,which complied with the standard that the content of Fe in drinking water was less than 0.3 mg∙L-1,and the average leaching amount of Co was 0.493 mg∙L-1.In the first reaction,the maximum amount of Co and Fe were leached,and as the number of reactions increased,the content of active components on the surface decreased,and Pr(S) between the Al-Mt interlayers became more stable;therefore,the amount of mental leaching gradually decreased.Due to the leaching of the active metal,the number of active centers decreased,resulting in a decrease in the degradation rate of 2-HA [57].Therefore,the leaching of any metal was not conducive to the catalytic performance;it will also lead to the occurrence of secondary pollution[61].
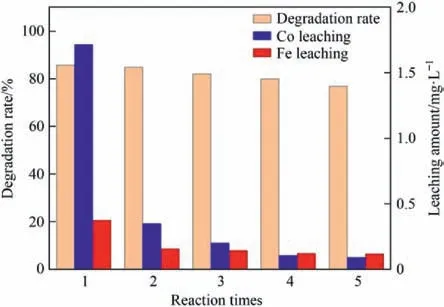
Fig.9.The stability and reusability study of Pr(S)/Mt-G-7/3.

Fig.10.2-HA degradation rate in different systems.
3.4.Characterizations of the catalysts before and after the reaction
To evaluate the change of physical and chemical properties of the catalyst after the reaction,the catalyst after the reaction was characterized,which was recorded as Pr(S)/MMT-G(R).From the SEM images of Fig.S3,it could be seen that although the perovskite particles on the surface were reduced,the catalyst still maintained the layered structure,which confirmed that the pillared montmorillonite as the support has high mechanical strength and stability,indicating that the catalyst may have good reusability.Energy Dispersive Spectrometer(EDS)analysis in Table S2 showed that the content of Pr,Fe,and Co decreased slightly after the reaction,which indicated the prepared Pr-based perovskite catalyst has good stability.However,the decrease in the content of active metal Fe,Co was one of the main reasons for the gradual decrease in catalytic activity.
In addition,the N2adsorption/desorption isotherms of the catalysts before and after the reaction were shown in Fig.S4.Pr(S)/MMT-G(R) still had the characteristics of a type IV isotherm,while an H3 hysteresis loop was also observed,indicating that Pr(S)/MMT-G(R) has a slit-pore structure [62].Consistent with the SEM results,the basic structure of the catalyst has not changed and has high stability.It can be seen from Table S3 that the structural parameters of the catalyst also decreased slightly after the reaction,which may be caused by the blockage of pore channels by intermediates generated in the reaction [63].The decrease in these structural parameters could adversely affect the catalytic performance.
3.5.Study on reaction mechanism and kinetics of 2-HA wastewater
To explore the active groups that played a major role in CWPO reaction,radical quenching experiments were carried out.Benzoquinone (BQ) was added to inhibit ∙O2-,and tert-butyl alcohol(TBA)was added to inhibit HO∙.Potassium iodide(KI)was added to inhibit the free ∙O2-and HO∙in the solution [64].A total of six groups of different reactions were set up,and the experimental results are shown in Fig.10.
It could be seen from Fig.10 that no free-radical-quenching agent was added in the fourth group,and the 2-HA removal rate was as high as 85.84%.In the first group,only H2O2was added,and the removal rate after the reaction was only 27.36%.This was because H2O2decomposed itself to produce a certain amount of HO∙,but the amount was so small that the effect of 2-HA degradation was limited.In the second group,the degradation rate of 2-HA decreased to 5.28% after only adding the catalyst.This was because there was no oxidant in the reaction,the catalyst only has adsorption.However,the removal rate of 2-HA in the third group was only 31.88%,which was because most of HO∙in the reaction was captured by TBA;their reaction rate constant was large,and the inert substances generated prevented the degradation of 2-HA[65].However,the weak degradation of 2-HA was attributed to other ROSs,such as HO2∙or ∙O2-.In the fourth group,the removal rate of 2-HA did not decrease significantly,indicating that ∙O2-was not the free radical that played a major role in the CWPO reaction.Through comparison,it could be found that HO∙was the main group in the CWPO reaction.In the sixth group,after adding KI,the removal rate of 2-HA decreased significantly,which indicated that free HO∙played a role in the CWPO reaction [64].
In this study,a good degradation of 2-HA was achieved by the synergistic effect of the classical Fenton-like surface catalytic reaction and the special structural properties of the doped perovskite catalyst.In the CWPO reaction,the surface reaction process was dominant,and the pollutant was adsorbed on the surface of the catalyst by electrostatic action.H2O2was catalyzed to produce HO∙at the active site on the catalyst surface or by leached Fe3+,which played a major role in CWPO degradation [57].The active component of the catalyst was continuously circulated until the end of the reaction.The reaction mechanism could be described by Eqs.(8)-(12)[66].H2O2was firstly adsorbed by the active site of Fe(III)on the catalyst surface to form a complex,which was unstable and was subsequently decomposed into Fe(II).Fe(II)could catalyze the generation of HO∙from H2O2and was oxidized to Fe(III),which in turn enabled the degradation of pollutants and the recycling of the catalyst.Similarly,Co also could activate H2O2,which allowed for simultaneous cycling with Fe during the reaction,resulting in good catalyst performance [51].
The doping of Co did not only change the perovskite structure but also introduced oxygen vacancies into the perovskite lattice,which was important for the structural properties and catalytic activity of the catalyst.When Fe or Co was present in the B-site of the perovskite,it promoted the production of oxygen vacancies,which could activate some of the oxygen species,which had a positive effect on the catalytic process due to their high catalytic activity[67].When H2O2came into contact with oxygen vacancies on the catalyst surface,the oxygen vacancies could contribute to the breaking of the O-O bond in the H2O2,resulting in the production of HO∙(Eq.(13)).Furthermore,due to the strong chemical activity,oxygen vacancies could activate H2O to produce HO∙(Eq.(14)),which indicated the great importance of oxygen vacancies for perovskite catalysts in CWPO reactions [68].In addition,lattice defects,a common structural defect,had a significant impact on the performance of perovskite catalysts.Partial doping of the B-site of perovskite using transition metals may lead to lattice defects,which could provide more active sites to speed up the reaction process and also act as a pathway for electron migration,accelerating the rate of electron migration[69].Therefore,the synergistic effect of lattice defects and oxygen vacancies was essential for excellent catalytic performance.
Previous studies have shown that the process of pollution degradation by the CWPO technique could be described by pseudofirst-order kinetic equations [Eq.(15)] [70].Fig.11 shows the degradation process of 2-HA wastewater with different concentrations,it could be seen that the degradation of 2-HA using Pr(S)/Mt-G-7/3 was very efficient and that there was no induction period.The reaction rate constant k could be obtained by fitting the concentration of 2-HA wastewater at different times through the pseudo-first-order kinetic model.The relevant parameters were listed in Table 2.The high linear correlation(R2>0.99)between the different initial concentrations of 2-HA-simulated wastewater indicated that the degradation process of 2-HA conformed to the pseudo-first-order kinetic model.It could be seen that although the initial reaction rate increases with the increase of the initial concentration,the higher reaction rate was constant with the lower initial concentration of 2-HA wastewater,indicating that it takes longer to degrade the high concentration of 2-HA wastewater.

Table 2 Relevant parameters of kinetic analysis

Fig.11.Degradation process of 2-HA wastewater with different initial concentrations.
In this study,Al-Mt,as a carrier for the catalyst,was a porous material with a layered structure,so that it could both adsorb pollutants and degrade them through catalysis during the CWPO reaction.As shown in Eq.(16),the Langmuir-Hinshelwood (L-H)model could usually be used to analyze which reaction was dominated by catalysis or adsorption [71].Therefore,the L-H model could be used to calculate the data in Table 2,and the specific mechanism of action of the prepared Pr(S)/Mt-G-7/3 catalyst for the degradation of the simulated 2-HA wastewater could be derived.
where t is the reaction time,C0is the initial concentration of 2-HA,k is the rate constant,and C(t)is the concentration of 2-HA at time t.
where r0is the initial reaction rate;KLis the adsorption constant,which describes the degree of adsorption of the 2-HA molecules to the catalyst;and kris the reaction rate constant.
The data of the initial concentration C0of 2-HA and the initial reaction rate r0are plotted and fitted to the curve.The results are shown in Fig.12.The data in the figure conformed to the L-H model,and the initial reaction rate r0was listed in Table 2.According to the fitting result,kr=1.25 mg∙L-1∙min-1,KL=0.02 mg-1∙L,and kr≫KL,which indicated the catalysis was dominant in the degradation of 2-HA by CWPO.This was consistent with the results of previous radical quenching experiments,and the same conclusions were also found in related studies[72].
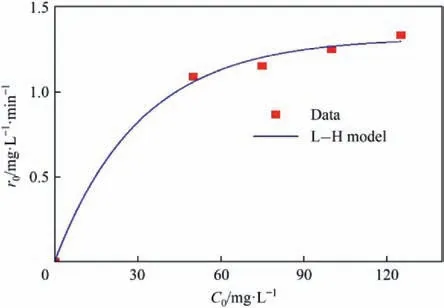
Fig.12.Relationship between initial concentration and initial reaction rate.
4.Conclusions
The heterogeneous CWPO technique was applied to treat 2-HA wastewater.PrFexCo1-xO3/Mt was used as the catalyst,and H2O2was used as an oxidant to provide HO∙.After the optimization of prepared conditions,the catalyst Pr(S)/Mt-G-7/3 prepared by the solid-melting method displayed the best degradation performance with a 97.64% 2-HA degradation rate and a 75.23% COD removal rate under the optimized conditions.The high activity of the catalyst can be attributed to the large specific surface area and the uniform dispersion of the perovskite,which facilitates the adsorption of 2-HA molecules and HO∙production.It still has high catalytic performance after five uses.The study of the reaction mechanism showed that HO∙is the main active group.Kinetic analysis revealed that the degradation process of 2-HA wastewater conformed to the pseudo-first-order equation,and catalysis played a dominant role in the degradation process.The catalyst has the characteristics of low cost,high catalytic activity,and less metal leaching,which opens a new path for the development of perovskite catalyst and provides a new opportunity for the treatment of pharmaceutical wastewater by the heterogeneous CWPO technique.
Declaration of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Key Research and Development Program of Shaanxi,China (2018GY-067).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.08.006.
 Chinese Journal of Chemical Engineering2024年1期
Chinese Journal of Chemical Engineering2024年1期
- Chinese Journal of Chemical Engineering的其它文章
- Flower-like tin oxide membranes with robust three-dimensional channels for efficient removal of iron ions from hydrogen peroxide
- Experimental study on the activation of coal gasification fly ash from industrial CFB gasifiers
- Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol
- Solubility of iron(III) and nickel(II) acetylacetonates in supercritical carbon dioxide
- Filtration performance and modeling of granular bed for dust removal from coal pyrolytic vapors
- Copper slag assisted coke reduction of phosphogypsum for sulphur dioxide preparation
