Preparation and properties of high-energy-density aluminum/boroncontaining gelled fuels
Yi Chen ,Kang Xue ,Yang Liu ,Lun Pan ,2,3,4,* ,Xiangwen Zhang ,2,3,4 ,Ji-Jun Zou ,2,3,4,*
1 Key Laboratory for Green Chemical Technology of the Ministry of Education,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
2 Collaborative Innovative Center of Chemical Science and Engineering (Tianjin),Tianjin 300072,China
3 Haihe Laboratory of Sustainable Chemical Transformations,Tianjin 300192,China
4 Zhejiang Institute of Tianjin University,Ningbo 315201,China
Keywords: Gelled fuels Energetic aluminum/boron Low-molecular-mass organic gellant Fuel property
ABSTRACT Energetic nanofluid fuel has caught the attention of the field of aerospace liquid propellant for its high energy density(HED),but it suffers from the inevitable solid-liquid phase separation problem.To resolve this problem,herein we synthesized the high-Al-/B-containing(up to 30%(mass))HED gelled fuels,with low-molecular-mass organic gellant Z,which show high net heat of combustion(NHOC),density,storage stability,and thixotropic properties.The characterizations indicate that the application of energetic particles to the gelled fuels obviously destroys their fibrous network structures but can provide the new particle-gellant gelation microstructures,resulting in the comparable stability between 1.0%(mass)Z/JP-10+30% (mass) Al or B and pure JP-10 gelled fuel.Moreover,the gelled fuels with high-content Al or B exhibit high shear-thinning property,recovery capability,and mechanical strength,which are favorable for their storage and utilization.Importantly,the prepared 1.0% (mass) Z/JP-10+30% (mass) B (or 1.0%(mass) Z/JP-10+30% (mass) Al) shows the density and NHOC 1.27 times (1.30) and 1.43 times (1.21)higher than pure JP-10,respectively.This work provides a facile and valid approach to the manufacturing of HED gelled fuels with high content of energetic particles for gel propellants.
1.Introduction
With the advancement of aerospace technology,advanced vehicles put more urgent demands on higher performance of power systems.The propulsive capability of a vehicle is closely related to the property of fuel,whose energy density is essential to flight range and speed [1,2].Compared to conventional liquid hydrocarbon fuels such as aviation kerosene,high-energydensity (HED) fuels have better volumetric calorific value and density,allowing for the reduced tank volume or increased flight range of aerospace vehicles under the same conditions [1,3-5].
Adding energy-containing particles can significantly increase the net heat of combustion (NHOC) of HED fuels.Typical energycontaining particles include aluminum [6-8],boron [9-11],magnesium [7,10,12],and carbon [13],in which boron and aluminum are most widely studied [14].Boron is an excellent energy-containing material with very high gravimetric NHOC(58.74 MJ∙kg-1) and volumetric NHOC (137.45 MJ∙L-1),while aluminum is easy to ignite and has low oxygen consumption,which is widely used in various aerospace vehicles for its high density and volumetric NHOC [15].In recent years,many research studies have reported the synthesis and application of B-or Al-added nanofluid fuels with HED and combustion efficiency [14,16,17],which are very promising for the design of advanced aerospace vehicles.
However,the stable dispersion of energy-containing particles in fuels is the key to inhibiting their agglomeration and sedimentation for the actual application.As one of the most effective methods,the gelation of particle-containing HED fuels with non-Newtonian fluid properties can achieve the stable state of the fuel [14].Related metalized gel propellants have been reported in different gel systems and tested for atomization,rheological,and combustion properties.Varghese et al.[18]investigated the performance of metalized gel propellants of dimethylhydrazine-N2O2fuel and showed that the addition of aluminum powder could reduce the minimum gel concentration and effectively increase the specific impulse of the gel,but the addition of silicone oil as a surfactant was required to stabilize the particles.Jyoti et al.[19] investigated the rheological properties of ethanol gels containing aluminum,and the structure showed that the addition of metal particles could enhance the elasticity of the gel and maintain thixotropy,but the study was only positive for conventional ethanol-based fuels.Liu et al.[20]investigated the gelling process of hydroxypropyl cellulose on aviation kerosene,but there is no further research on metalized aviation kerosene.The gellant process of HED fuels is mainly achieved through the interaction of the fuel molecules and gellant molecules,which is primarily a physically cross-linked gel formed with weaker noncovalent interactions [21].Recently,several low-molecular-mass organic gellants (LMOGs),i.e.,organic gellant Gn [22] and mannitol-derivative gellant LMWG [23],have been applied to prepare the gelled fuels of JP-10,HD-03,QC,and RP-3 [24-31].The previous studies on gel propellants include gel formulation,rheological properties,ignition and combustion performance,and engine test.However,there are very less research studies on the preparation and performance of energy-particles-containing gelled fuels,which restricts the development and application of energetic nanofluid fuels.The details about their rheological behavior and basic physical properties (viscosity density and calorific value,etc.)must be precisely understood before their use in future missions.
In this work,the high-Al-/B-containing HED gelled fuels (up to 30% (mass)) with JP-10 as the base liquid fuel were fabricated by using 0.5% (mass) and 1.0% (mass) LMOG Z (synthesized by our previous work [31]).The rheological properties of the gelled fuel were investigated by shear thinning,destroy and recovery,and strain sweep and frequency sweep tests,and the physical properties of gelled fuel,i.e.,density,viscosity,and centrifugal stability,were determined.The as-prepared high-Al-/B-containing gelled fuels show high density,NHOC,storage stability,and thixotropic properties.The rheological method to study the flow and deformation law of this new gel propellant will lay the foundation for the further study of their pipeline transport,atomization,combustion,and other characteristics and may have important theoretical significance for its component process control and engine design.
2.Experimental
2.1.Materials
The JP-10 fuel was synthesized based on the Ref.[24].Since the boric acid coating on the surface of nano-boron causes the poor performance of the gelled fuel and makes it difficult to gel at high solid content,we chose the most commonly used nano-aluminum (50 nm) and micron-boron (0.5-2 μm) as energetic materials.The nano-aluminum (50 nm) and micronboron (0.5-2 μm) were purchased from Aladdin.Acetic acid,phenol,hydrochloric acid,cyclohexanone,and mercaptoacetic acid were purchased from Shanghai Macklin Biochemical Corporation.
2.2.Synthesis of gelled fuels
The LMOG gellant Z was synthesized via our previous work[31].The gelled fuels are prepared using a cross-linking method,which involves first suspending metal particles in the uncrosslinked gelled fuel and then trapping the particles in the gelled fuel matrix in the presence of a gellant.Typically,gellant Z with a mass fraction of 0.5% (mass) or 1.0% (mass) was added to the JP-10 fuel,and the solution was heated and stirred at 140°C for 0.5 h to clear the solution.Then,Al/B particles were added and stirred at 1000 r∙min-1for 10 min.After that,the Al-/B-containing gelled fuels were obtained by standing at room temperature for 1 h.The high-energy gelled fuels are named as Z/JP-10+Al and Z/JP-10+B.Fig.1 shows the preparation process of high-energy gelled fuels.

Fig.1.The preparation process of high-energy gelled fuel.
2.3.Characterization of gelled fuels
A specific gravity method was applied to test the density of gelled fuels by a JA3003 instrument.The gelled fuel stability test was conducted in a laboratory centrifuge(with a rotational speed of 2500 r∙min-1,3500 r∙min-1,and 5000 r∙min-1).The viscosity of gelled fuels was measured by using a rotary viscometer (with a rotational speed of 500 r∙min-1for 10 min to transform into a liquid state).Scanning electron microscopy (SEM) was performed with a Hitachi S-4800.The mass calorific value was measured by the oxygen bomb calorimeter ZDHW-300A.The tress-controlled rheometer (HAAKE Instruments,DHR) was used to determine the rheological properties.A 40 mm-diameter parallel plate rotor with a gap of 1 mm was used,and the sample mass was about 0.5 g during the test to ensure full contact with the parallel plate rotor.The ignition delay time was measured by dropping a 15 μl droplet onto a corundum ark at a high temperature by a droplet ignition test [31].
3.Results and Discussion
3.1.Morphology of Al-/B-containing gelled fuels
The Al-/B-containing gelled fuels show the same stable gel state with pure JP-10 gelled fuel,but the gel color changes from white to gray or black(Fig.2(a)-(c)).Notably,the prepared Al-/B-containing gelled fuels are thermally reversible and thixotropic,which can change from a gel state to a liquid state after heating or stirring(Fig.2(d)-(g)).

Fig.2.Photographs of(a)1.0%(mass)Z/JP-10,(b)1.0%(mass)Z/JP-10+10%(mass)Al,and(c)1.0%(mass)Z/JP-10+10%(mass)B.(d)Liquid state and(e)solid state of 1.0%(mass)Z/JP-10+10% (mass) B gelled fuel.(f) Liquid state and (g) solid state of 1.0% (mass) Z/JP-10+10% (mass) Al gelled fuel.
Different from the Al-/B-containing nanofluid fuels,the gellant Z-mediated (with molecule structure shown in Fig.3(a)) gel fuels can effectively stabilize the energy-containing particles in JP-10.The prepared gelled fuels can be stored stably at the room temperature for at least 1 year without particles deposition.The morphology of xerogels was tested by SEM.From Fig.3(b),it can be seen that the gellant can build up a threedimensional (3D) network structure,via hydrogen bonding,van der Waals forces,and π-π stacking [8,31] to fix fuel molecules during the formation of gelled fuel.From Fig.3(c)-(f),the length of xerogel fibers was reduced significantly by adding energetic particles,among which,the particles are uniformly dispersed.When particles are added to the system,the adsorption of tiny particles and the cross-linking of the gelling agent make the 3D network structure,which is established by interactions such as hydrogen bonding,π-π stacking has more entangled and linked area,and the free-flowing liquid phase is further confined inside the network structure,resulting in a denser structure and higher mechanical strength of the gelled fuels (such as 10% (mass) Al or B in Fig.3(d),(f)) [32].
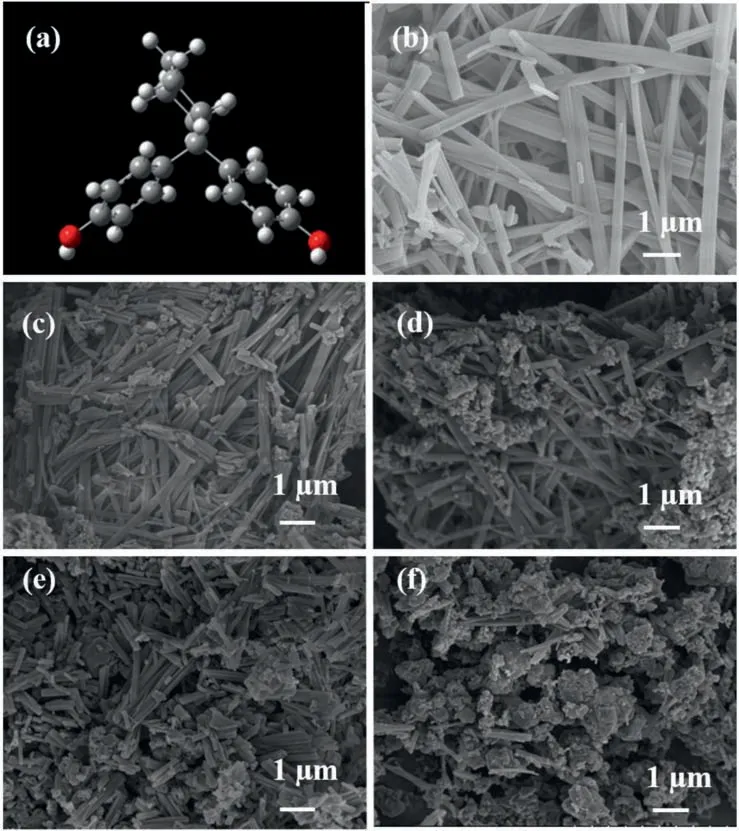
Fig.3.(a) Molecular structure of gellant Z (the carbon,hydrogen,and oxygen atoms correspond to the gray,white,and red spheres,respectively).SEM images of (b) 1.0% (mass)Z/JP-10,(c)1.0%(mass)Z/JP-10+5%(mass)Al,(d)1.0%(mass)Z/JP-10+10%(mass)Al,(e)1.0%(mass)Z/JP-10+5%(mass)B,and(f)1.0%(mass)Z/JP-10+10%(mass)B gelled fuels.
3.2.Physicochemical properties of Al-/B-containing gelled fuels
The density,volumetric NHOC,viscosity,and centrifugal stability of gelled fuels were tested.As depicted in Fig.4(a),(b),pure JP-10 shows the density of 0.937 g∙ml-1,which is increased to 0.956 g∙ml-1and 0.971 g∙ml-1with the Z gellant concentration increasing to 0.5% (mass) and 1.0% (mass),respectively,because of the higher density of Z (1.2 g∙ml-1).Meanwhile,the density of Al-/B-containing gelled fuels increases almost linearly with Al or B concentration increasing from 5% (mass) to 30% (mass).Notably,1.0% (mass) Z/JP-10+30% (mass) Al and 1.0% (mass) Z/JP-10+30% (mass) B show the highest density of 1.215 g∙ml-1and 1.195 g∙ml-1among the samples in Fig.4(a) and (b),respectively,which are 1.30 and 1.28 times higher than that of JP-10 liquid fuel.

Fig.4.Properties of JP-10+Al/B nanofluid fuel,Z/JP-10+Al,and Z/JP-10+B gelled fuels with different particle concentrations:(a,b)density and(c,d)viscosity at 20 °C(tested at 60 r∙min-1).
The gelled fuels can be stored in a gel state.After shear thinning,they can be delivered and atomized in liquid form.The fabricated gelled fuels were stirred at 500 r∙min-1for 10 min to transform into a liquid state,whose viscosity was then measured by the rotary viscometer (with the test speed of 60 r∙min-1).As seen in Fig.4(c),(d),the rise of Al or B content significantly increases the viscosity of the fuels,especially when their contents are above 20%(mass).For example,pure JP-10 has a low viscosity of 3.2 mPa∙s,which significantly increases to 118 mPa∙s with the addition of 30%(mass)Al particles(nanofluid fuel).Moreover,the addition of Z gellant also increases the viscosity of sheared gel fuels.Specifically,1.0% (mass) Z/JP-10+30% (mass) Al shows the viscosity of 830 mPa∙s,which is 1.91 and 7.03 times higher than those of 0.5% (mass) Z/JP-10+30% (mass) Al gelled fuel and JP-10+30% (mass) Al nanofluid fuel,respectively.However,for Bcontaining gelled fuel,the Z gellant can mitigate the viscosityincreasing trend caused by high B content.For instance,for the JP-10+B nanofluid fuel,the rise of B content leads to the exponential increase of viscosity value (similar to previous work[33]),and JP-10+30% (mass) B shows ca.700 times higher viscosity than pure JP-10.However,for JP-10+B gelled fuels,the viscosity increases linearly with the rise of B content,and 1.0%(mass) Z/JP-10+30% (mass) B shows only 3.31 times higher viscosity than 1.0%(mass)Z/JP-10,which indicates the addition of gellants can decrease the viscosity of the fuels with high content of energetic nanoparticles.
The viscosity was measured with different temperatures and test speeds.As shown in Fig.5,the viscosity decreases with increasing temperature or test speed.The viscosity of 1.0%(mass)Z/JP-10+10% (mass) Al and 1.0% (mass) Z/JP-10+10% (mass) B decreased by 17% and 19%,respectively,when the temperature was increased from 20°C to 50°C at a constant shear rate of 60 r∙min-1.For JP-10+10%(mass)Al and JP-10+10%(mass)B,their viscosity values are decreased by 42% and 21% when the temperature was increased from 20°C to 50°C,respectively.At a constant temperature of 20°C,the viscosity of 1.0% (mass) Z/JP-10+10% (mass) Al,1.0% (mass) Z/JP-10+10% (mass) B,JP-10+10% (mass) Al,and JP-10+10% (mass) B decreased by 22%,14%,27%,and 27%,respectively,when the test speed is reduced from 60 r∙min-1to 30 r∙min-1.It indicates that both temperature and shear rate have effects on the internal structure of the gelled fuels,which directly affects the fuel viscosity.

Fig.5.Viscosity of Z/JP-10 gelled fuels with 10% (mass) content of Al/B particles with different (a) temperatures at 60 r∙min-1 and (b) test speeds at 20 °C.
Then,the volumetric NHOC values of gelled fuels are determined (Fig.6(a),(b)).Since Al and B have much higher NHOC values (83.86 MJ∙L-1and 137.45 MJ∙L-1,respectively) than JP-10(39.49 MJ∙L-1),their addition in JP-10 gelled fuels can significantly improve the volumetric NHOC value,which rises linearly with the increase of Al or B amount.Specifically,1.0% (mass)Z/JP-10+30% (mass) Al and 1.0% (mass) Z/JP-10+30% (mass) B show the highest volumetric NHOC value of 47.97 MJ∙L-1and 56.37 MJ∙L-1among the samples,respectively,which are 1.21 and 1.43 times higher than that of pure JP-10 fuel.In addition,the application of Z gellant can also increase volumetric NHOC slightly,for its relatively higher NHOC value of 41.02 MJ∙L-1.Moreover,the pressure rises during the combustion process were tested,and the results are presented in Fig.6(c),(d).The addition of Al and B particles in JP-10 gelled fuels leads to the decrease of maximum pressure peak intensity,and it becomes more obvious as the content of energetic particles increases.For example,1.0%(mass) Z/JP-10+30% (mass) Al and 1.0% (mass) Z/JP-10+30%(mass) B show the smallest value of maximum pressure peak of 4168.95 Pa and 4095.90 Pa among the samples,respectively,which are reduced by 10.94% and 12.51% than that of 1.0% (mass)Z/JP-10 gelled fuel.Meanwhile,the peak combustion pressure decreases with the particle content increasing,which is due to the more serious particle agglomeration and insufficient combustion[34-36].
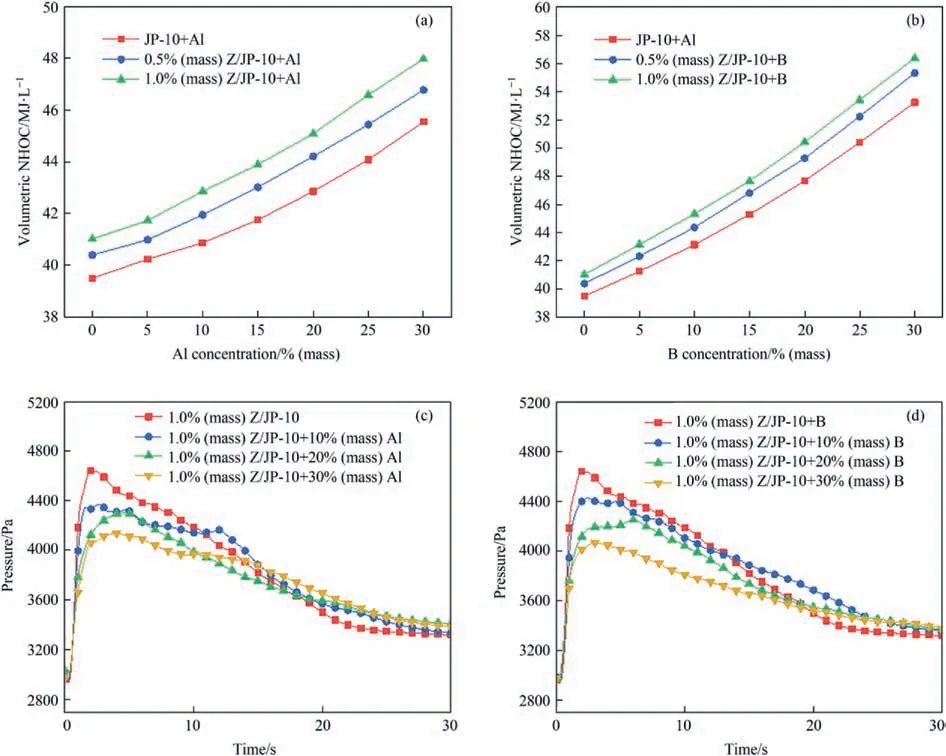
Fig.6.Volumetric NHOC of gelled fuels with different concentrations of (a) Z/JP-10+Al,(b) Z/JP-10+B.Pressure change of oxygen bomb calorimeter for (c) 1.0% (mass) Z/JP-10+Al,(d) 1.0% (mass) Z/JP-10+B.
Then,the mechanical stability of the fabricated gelled fuels was evaluated.The gelled fuels were centrifuged at 2500 r∙min-1,3500 r∙min-1,and 5000 r∙min-1for 10 min,and the ratio of overall gel mass retention (ωrem,residual rate of total gelled fuel mass after centrifugation) before and after centrifugation is evaluated as an index [37].Apparently,the centrifugal stability of the gelled fuel system decreased after the addition of energycontaining particles.At a speed of 2500 r∙min-1(Fig.7(a),(b)),the ωremvalue after centrifugation for 1.0% (mass) Z/JP-10+10%(mass) Al and 1.0% (mass) Z/JP-10 +10% (mass) B are 80.02% and 74.55%,respectively,which are less than that of 1.0% (mass) Z/JP-10 gelled fuel (96.81 %),but with the increase of particle concentration,the ωremvalue of the gelled fuel gradually increases.For example,the ωremvalue for 1.0% (mass) Z/JP-10+30% (mass)Al and 1.0% (mass) Z/JP-10+30% (mass) B can reach 95.00% and 93.60%,respectively(at a speed of 2500 r∙min-1).As confirmed in Fig.3,the addition of particles destroys the 3D network structure assembled by gellant Z and decreases the centrifugal stability,especially for relatively low particle contents,such as 10% (mass),but interestingly,as the particle content increases to 20% (mass)or 30% (mass),the particles can co-serve as the gellant and leads to the improved centrifugal stability of the gelled fuels.With the increase of the gellant content or decrease of rotational speed,the centrifugal stability of the system is enhanced.In addition,the overall mass retention of the B-based gelled fuel is similar to that of the Al-based gelled fuel.

Fig.7.Centrifugal stability of Z/JP-10 gelled fuels with different content of Al and B particles at a centrifugal speed of(a),(b)2500 r∙min-1;(c),(d)3500 r∙min-1;and(e),(f)5000 r∙min-1 for 10 min.
3.3.Rheological properties of Al-/B-containing gelled fuels
Fig.8 shows the shear-thinning performance of Al-/B-containing gelled fuels.As the shear rate increases from 0.1 s-1to 100 s-1,the viscosity of the gel system decreases obviously.The initial viscosity of the gelled fuels with 1.0% (mass) gellant Z is about 4 times higher than those with 0.5% (mass) gellant Z,confirming the higher stability of the gelled fuels.Moreover,10%(mass) addition of Al or B particles leads to higher viscosity and worse shear-thinning property,while the higher particle concentration,20% (mass) and 30% (mass),leads to lower viscosity and better shear-thinning properties,indicating the higher particle contents can improve the performance of gelled fuels.In addition,the viscosity is not significantly influenced by the particle type (Al or B).
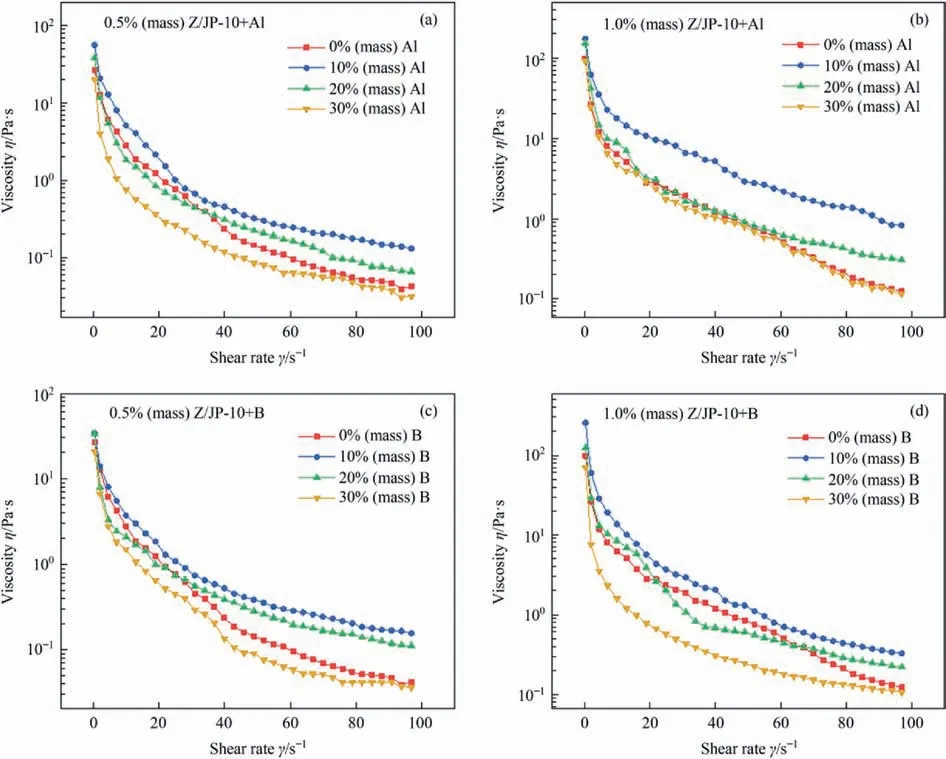
Fig.8.The shear-thinning performances of(a)0.5%(mass)Z/JP-10+Al,(b)1.0%(mass)Z/JP-10+Al,(c)0.5%(mass)Z/JP-10+B,and(d)1.0%(mass)Z/JP-10+B with different Al or B contents.
In order to demonstrate the shear-thinning properties of the gelled fuels more accurately,the curves were fitted using the Power-Law model equation (Eq.(1)) [38],with the detail parameters shown in Table 1.

Table 1 Values of coefficients for gelled fuels fitted with P-L model equation
where η means non-Newtonian viscosity,K means the consistency index,γ means the shear rate,and n means the power index.
The consistency index K reflects the initial viscosity of the fuel system.As can be seen,K increases first and then decreases with the increase of particle content to 10% (mass) and above 20%(mass) for the same gellant content.When the particles reach 20% (mass) and 30% (mass),the particles may slightly agglomerate and decrease the fuel viscosity.The power index(n)reflects shear-thinning performance;the gelled fuels exhibit preferable shear-thinning performance with a lower power index,indicating that the gelled fuel with high particle content has better shear thinning performance.Meanwhile,the K and n values are lower for gelled fuel with lower gellant content,which indicates that the gelled fuel with low gellant content exhibits higher shear-thinning properties,similar to the previous work [39].
Destroy and recovery test was used to explore the thixotropy and recovery capability of the gelled fuels (a step experiment,Fig.9).Stage 1: step for 2 min at 0.01 s-1low share rate;Stage 2:step for 0.5 min at 60 s-1high share rate;Stage 3: step for 2 min at 0.01 s-1low share rate;Stage 4:step for 0.5 min at 60 s-1high share rate.The thixotropy is a time-dependent rheological phenomenon,which can be defined as the property that the apparent viscosity decreases at a constant high shear stress or shear rate and gradually recovers when the shear stress or shear rate is reduced.In this work,the change in viscosity with time at the share rate of 60 s-1(Stage 2) is used to evaluate the thixotropy,while the variation of viscosity before and after shear(Stage 1 and Stage 3) is used to judge the recovery capability.Fig.9 depicts the viscosity of Z/JP-10 gelled fuel with different concentrations of particles as a function of shearing time (with different shear rates),which indicates that Al-/B-containing gelled fuel has thixotropic property.In Stage 2,the viscosity of 1.0%(mass) Z/HD-01 gelled fuel with 10% (mass) Al decreases continuously within 120-150 s,while the gelled fuel with 30%(mass) Al gradually stabilizes at about 140 s.The viscosity of all gelled fuels increased rapidly after the shear rate is reduced from 60 s-1to 0.01 s-1(Stage 3 and Stage 4),indicating their recovery capability.By comparing the viscosity of the gelled fuel around Stage 1 and Stage 3,it is concluded that the recovery capability of the gelled fuel increases first and subsequently decreases as the increase of particle concentration reaches 10% (mass) and above 20% (mass),which are similar to previous work [40]).For example,For 1.0% (mass) Z/JP-10+10% (mass) Al gelled fuel,as the Al content increases from 10% (mass) to 20% (mass) and 30%(mass),the viscosity recovery of gelled fuel decreases from 39.9%to 27.24% and then increases to 32.04%,which is lower than that of 1.0% (mass) Z/JP-10 (66.05%).For 0.5% (mass) Z/JP-10+B fuel,as the boron content increases from 10% (mass) to 20% (mass)and 30% (mass),the viscosity recovery of gelled fuel decreases from 45.71% to 20.06% and then increases to 39.00%,which is lower than that of 0.5% (mass) Z/JP-10 (46.06%).The decreased recovery capability with the addition of particles is because the connection between particles and gellants is weaker than that between the gellants,but when the particles content reaches 30%(mass),the system structure is mainly dominated by particle accumulation,and the recovery capability increases [40].Moreover,as the gellant content increases from 0.5% (mass) to 1.0%(mass) (for Z/JP-10+10% (mass) Al gelled fuel),the viscosity recovery of gelled fuel increases from 24.17% to 39.9%.It shows that with the increase of gellant content,the recovery capability of the gelled fuel increases.

Fig.9.Viscosity of(a)0.5%(mass)Z/JP-10+Al,(b)1.0%(mass)Z/JP-10+Al,(c)0.5%(mass)Z/JP-10+B,and(d)1.0%(mass)Z/JP-10+B with different particle contents vs.shearing time in the four stages.Stage 1:step for 2 min at 0.01 s-1 low share rate;Stage 2:step for 0.5 min at 60 s-1 high share rate;Stage 3:step for 2 min at 0.01 s-1 low share rate;Stage 4:step for 0.5 min at 60 s-1 high share rate.
Strain sweep tests were performed at room temperature to investigate the mechanical stability and strength (with the test frequency of 1 Hz).Gelled fuels exhibit a combination of elasticity and viscosity during deformation,and the elastic behavior is measured by storage modulus (G′),while the viscous behavior is measured by loss modulus (G′′).The linear viscoelastic region refers to the region where the storage modulus (G′) is independent of strain.By determining the strain value at the intersection of G′and G′′,it is possible to obtain the critical strain at which the gelled fuel changes from expressive elasticity to viscosity [41].In the critical strain range,G′is greater than G''.The entire gelled fuel system maintains a homogeneous semi-solid state under resting conditions,which shows that the gelled fuels are elastic semi-solid materials.From Fig.10,the G′of critical strain(G'=G′′)for the Al-/B-containing gelled fuels shows a decreasing trend with the increase of particle concentration.For example,the G′of critical strain of 1.0% (mass) Z/JP-10+B decreases from 1556.80 Pa (10% (mass) B) to 1330.16 Pa (20% (mass) B) and 455.83 Pa (30% (mass) B) with the B content increasing(Fig.10(d)).Nevertheless,all of them are higher than the strain values without adding metal particles,indicating that the mechanical strength of the gelled fuel improves with the addition of particles.Moreover,the increase in gellant content facilitates the enhancement of the G′value of critical strain.As shown in Fig.10(a),(b),the G′of a critical strain of 1.0% (mass) Z/JP-10 is 341.36 Pa,which is almost 5 times higher than that of 0.5%(mass)Z/JP-10.Specifically,the B-containing gelled fuel has better mechanical strength than Al-containing gelled fuel.1% (mass) Z/JP-10 +10% (mass) B and 1.0% (mass) Z/JP-10+20% (mass) B show the G′values (of critical strain) of 1556.80 Pa and 1330.16 Pa,which are 1.44 and 1.86 times higher than those of 1.0%(mass)Z/JP-10+10% (mass) Al and 1.0% (mass) Z/JP-10+20% (mass) Al,respectively,indicating that larger particles lead to the better mechanical strength [42].
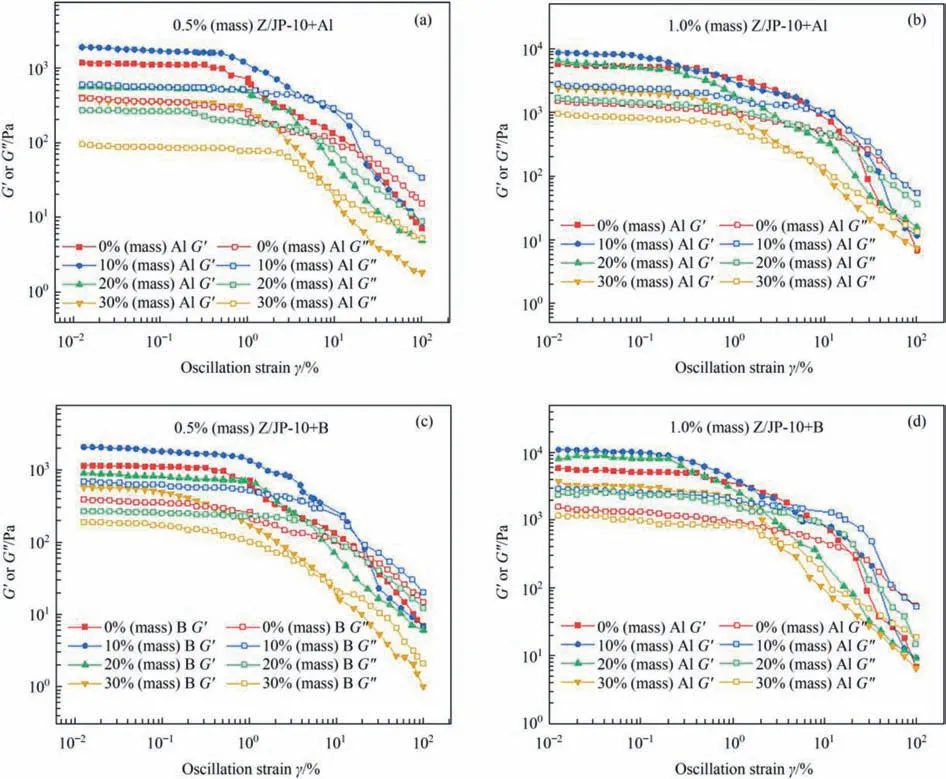
Fig.10.Storage modulus and loss modulus of(a)0.5%(mass)Z/JP-10+Al,(b)1.0%(mass)Z/JP-10+Al,(c)0.5%(mass)Z/JP-10+B,and(d)1.0%(mass)Z/JP-10+B as functions of oscillation strain at 25 °C and 0.1% strain.
Then,the relationship between modulus and angular frequency for the aforementioned gelled fuels was tested at 25°C and 0.1% strain(with a frequency sweep range of 1-100 rad∙s-1),with the results shown in Fig.11.For all gelled fuels,their corresponding loss modulus (G′′) is lower than their storage modulus(G′),confirming their solid-like characteristics.The G′of the gelled fuel with Al or B particles does not change significantly with angular frequency,but the G′′values increase gradually with the rise of frequency.The increase rate of G'' (with a frequency sweep range of 1-100 rad∙s-1) reflects the frequency dependence of the gelled fuel,which indicates that the gelled fuel is more likely to be destroyed by external forces at high frequencies.With the increasing of particle content,the frequency dependence of the G′′tends to be strong first and then weak with the increase of particle content to 10% (mass) and above 20%(mass).For example,the G′′increase rates of 1.0% (mass) Z/JP-10 with 10% (mass) Al,20% (mass) Al,and 30% (mass) Al are 57.87%,164.61%,and 96.33%,respectively.The gelled fuel with 20%(mass) Al or 20% (mass) B is the most severely affected by frequency,which is due to the abundant interactions between highcontent particles and gellant molecular chains.However,too many particles (such as 20% (mass)) will destroy the stable interactions and lead to interface sliding and then decrease the fuel stability.When the particle content is 30% (mass),the architecture of the gelled fuels is mainly dominated by the particle accumulation and the stability is improved [32].The frequency dependence of G′′becomes weaker as the gellant concentration increases,indicating that the gelled fuels have greater stability.
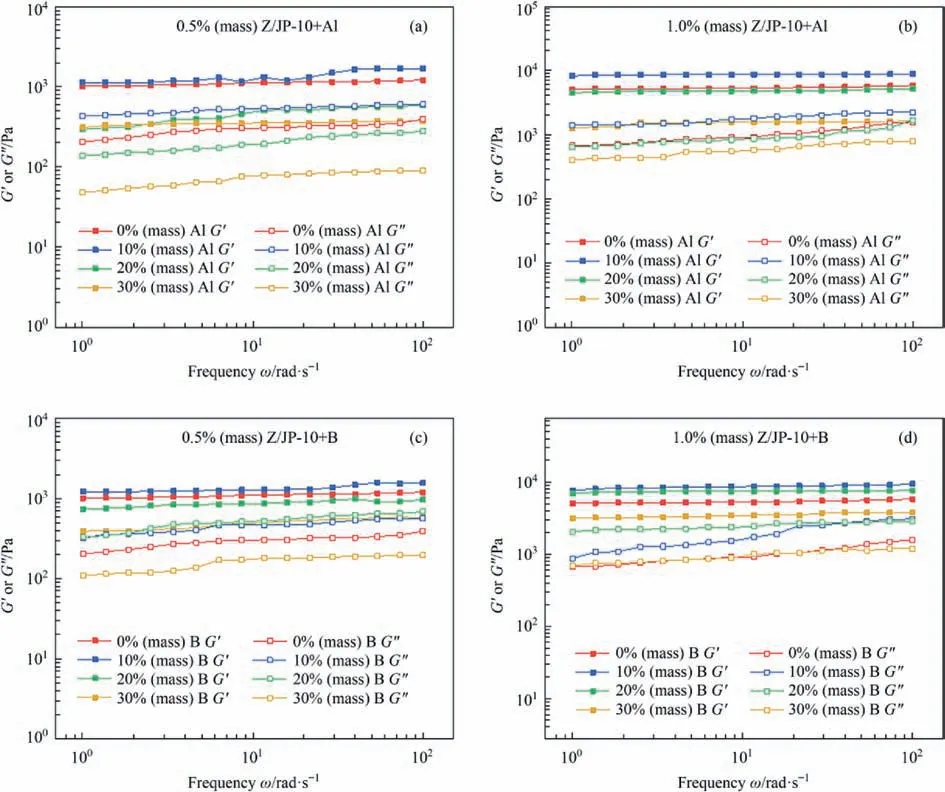
Fig.11.Storage modulus and loss modulus of(a)0.5%(mass)Z/JP-10+Al,(b)1.0% (mass)Z/JP-10+Al,(c)0.5%(mass)Z/JP-10+B,and(d)1.0%(mass)Z/JP-10 +B as functions of angular frequency.
3.4.Ignition and combustion properties of Al-/B-containing gelled fuels
The ignition and combustion properties of the gelled fuels were measured by using the flat-plate ignition device [31],and Fig.12(a)-(d) show the typical ignition and combustion process.The time interval between the initial droplet with contacting hot plate and the presence of flame is the ignition delay time[43,44].As illustrated in Fig.12(e),(f),the ignition delay time of pure JP-10 is 2128 ms (430°C),which increases to 2262 ms and 2495 ms for the gelled JP-10 fuels with 0.5% (mass) and 1.0%(mass) gellant Z,respectively,probably for the increased viscosity,reduced volatilization of the fuel,and the relatively worse heat transfer process [45].

Fig.12.Images for fuel combustion flame of(a)JP-10+10%(mass) Al,(b)1.0% (mass) Z/JP-10+10% (mass)Al,(c) JP-10+10%(mass) B,and (d) 1.0% (mass)Z/JP-10 +10%(mass)B gelled fuel at 430 °C.Ignition delay time of (e) Z/JP-10+Al and (f) Z/JP-10+B with different particle contents.
For the energetic particle added gelled fuels,the high contents of gellant Z also lead to a larger ignition delay time(Fig.12(a)-(d)).Under the same gellant-Z content(0.5%[mass]or 1.0%[mass]),their ignition delay time first decreases when the Al or B addition content is 10% (mass) because the particles can accelerate the heat transfer of fuel droplet.However,the ignition delay time of the gelled fuels further increases rapidly for the fuels with higher particle contents of 20% (mass) and 30% (mass),owing to the reduced volatilization rate of the fuel inside the droplet [45].The ignition delay time of the Al-containing gelled fuel with the same particle content is lower than that of the B-containing gelled fuel.For example,1.0%(mass)Z/JP-10+30%(mass)Al shows the ignition delay time of 3098 ms,which is lower than that of 1.0%(mass)Z/JP-10+30% (mass) B (3397 ms).This is because Al nanoparticles are easier to ignite than B particles (with surface inert boron-oxygen compounds) [14].This indicates the Al-containing gelled fuel with high particle content can realize excellent ignition and combustion properties.
4.Conclusions
In this work,we applied LMOG (Z) to prepare high-Al-/Bcontaining (up to 30% (mass)) HED gelled fuels based on JP-10,which are elastic semi-solid materials.The high-content addition of Al or B particles can significantly increase the density and NHOC of gelled fuel.Specifically,the prepared 1.0% (mass) Z/JP-10+30%(mass)B(or 1.0%(mass)Z/JP-10+30%(mass)Al)shows the density and NHOC 1.27 times (or 1.30 times) and 1.43 times(or 1.21 times) higher than those of pure JP-10,respectively.The viscosity increases with increasing particle content and gellant concentration.The characterization of xerogel samples indicates the addition of Al or B particles in gelled JP-10 destroys the fibrous network structure but provides a new particle-gellant gelation structure,which leads to the relatively high stability of high-content Al or B gelled fuels.By analyzing the rheological properties,the gelled fuels with high-content Al or B exhibit high shear-thinning property and recovery capability,and the mechanical strength of the gelled fuel can be improved by adding energetic particles.Moreover,the high-Al-/B-containing HED gelled fuels exhibit excellent ignition and combustion performance.
Declaration of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
Data will be made available on request.
Acknowledgements
The authors appreciate the support from the National Natural Science Foundation of China(22222808,21978200) and the Haihe Laboratory of Sustainable Chemical Transformations for financial support.
 Chinese Journal of Chemical Engineering2024年1期
Chinese Journal of Chemical Engineering2024年1期
- Chinese Journal of Chemical Engineering的其它文章
- Flower-like tin oxide membranes with robust three-dimensional channels for efficient removal of iron ions from hydrogen peroxide
- Experimental study on the activation of coal gasification fly ash from industrial CFB gasifiers
- Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol
- Solubility of iron(III) and nickel(II) acetylacetonates in supercritical carbon dioxide
- Filtration performance and modeling of granular bed for dust removal from coal pyrolytic vapors
- Copper slag assisted coke reduction of phosphogypsum for sulphur dioxide preparation
