Removal of kathon by UV-C activated hydrogen peroxide: Kinetics,mechanisms,and enhanced biodegradability assessment
Jinzhi Cui,Guiqiao Wang,Xing Rong,Wensu Gao,Yaxin Lu,Yawen Luo,Lichao Zhang,Zhongfa Cheng,Canzhu Gao,*
1 School of Environmental Science and Engineering,Shandong University,Qingdao 266237,China
2 Shandong Taihe Technologies Co.,Ltd.,Zaozhuang 250031,China
Keywords: Kathon UV/H2O2 Biological degradation Circulating cooling water
ABSTRACT Kathon (CMI-MI),a mixture of 5-chloro-2-methyl-4-isothiazolin-3-one (CMI) and 2-methyl-4-isothiazolin-3-one (MI),was extensively used in industry as a nonoxidizing biocide or disinfectant.However,it would show adverse effects on aquatic life when it is discharged into surface water.In this study,the removal performance,parameter influence,degradation products and enhancement of subsequent biodegradation of CMI-MI in UV/H2O2 system were systematically investigated.The degradation rate of CMI-MI could reach 90% under UV irradiation for 20 min when the dosage of H2O2 was 0.3 mmol∙L–1.The DOC (dissolved organic carbon) mineralization rate of CMI-MI could reach 35% under certain conditions ([H2O2]=0.3 mmol∙L–1,UV irradiation for 40 min). kobs was inversely proportional to the concentration of CMI-MI and proportional to the concentration of H2O2.The degradation rate of CMIMI was almost unchanged in the pH range from 4 to 10.Except the presence of inhibited the removal rate of CMI-MI,,Cl-,,and did not interfere with the degradation of CMI-MI in the system.It was found that UV/H2O2 system had lower energy consumption and more economic advantage compared with UV/PS system by comparing the EEO (electric energy per order) values under the same conditions.Two main organic products were identified,namely HCOOH and CH3NH2.There’s also the formation of Cl- and .After UV and UV/H2O2 photolysis,the biochemical properties of CMI-MI solution were obviously improved,especially the UV/H2O2 treatment effect was better,indicating that UV/H2O2 technology is expected to combine with biotechnology to remove CMI-MI effectively and environmentally friendly from wastewater.
1.Introduction
Kathon (CMI-MI),a mixture of 5-chloro-2-methyl-4-isothiazo lin-3-one (CMI) and 2-methyl-4-isothiazolin-3-one (MI),was widely used in industry as a nonoxidizing biocide or disinfectant[1,2].Both in circulating cooling water systems and in cosmetic personal care products (moisturizers,eye shadows,shampoos),CMI-MI is an indispensable ingredient because of its excellent inhibition and killing effect on molds,bacteria and other microorganisms [3–5].In order to inhibit the breeding of microorganisms and prevent the generation of biological sludge,the added concentration of CMI-MI in the reverse osmosis system and circulating cooling water system is 0.04–2.6 mmol∙L–1,100–300 mg∙L–1,respectively [6].The concentration varies with different adding methods.MI concentration in cosmetics is required to be within 0.01 percent in China,which is attribute to its strong allergenic properties[6].The structures of CMI and MI were shown in Supplementary Material (Table S1).
With the rapid development of industry,the water consumption and displacement of circulating cooling water are increasing,contributing to the mass release of the residual isothiazolones in wastewater into the natural environment and or sewage treatment plants.Several studies have reported the presence of CMI-MI in natural water bodies or wastewater treatment plants [5,7,8].The existence of CMI-MI in wastewater treatment plants increased the complexity of water treatment,because of its negative impact on activated sludge activity and nitrification process [9,10].It is known as pseudo-persistent compounds because CMI-MI was continuously released into the environment with circulating cooling wastewater [11,12].
CMI-MI exposed to the environment poses a potential threat to ecology and human health [13,14].Several studies have shown that CMI or MI has irreversible toxic effects on many organisms[15–20].For example,long-term exposure to low levels of MI and related compounds damaged irreparably the immune and nervous systems of mice[15].The hydrophilic substances MI and CMI exhibited pronounced high cytotoxic effects on the human hepatoblastoma cell line Hep G2,the marine bacteriumVibrio fischeri,and the limnic green algaScenedesmus vacuolatus[20].Tanget al.[16]discovered that kathon was highly genotoxicin vitro,which contributed to an increase in the genotoxicity of the reverse osmosis concentrate.In addition,due to the high toxicity of MI/CMI,bactericides lingering in the environment for a long time could induce the development of resistance in bacteria [21].Thus,it is of great significance to take effective measures to remove CMI-MI from wastewater.
Biodegradation is considered as an efficient,environmentally friendly and low-cost approach to eliminate organic contaminants from the aqueous environment[22].Microbial metabolism leads to the breakdown of organic contaminants into small molecules in the biodegradation process.However,when faced with toxic and refractory CMI-MI,microorganisms had no ability to resist,because the active site of the N—S bond on their heterocyclic ring binds to some structure inside the cell,thus damaging the structure and function of the cell [23–25].In the activated sludge process,Amatet al.[9] found that MI inhibited the activity of nitrite oxidizing bacteria,resulting in a 70% loss of efficiency in the nitrification process.In wastewater treatment by biofilm method,isothiazolinone concentrations of 3 mg∙L-1and above could lead to a significant decrease in treatment efficiency.When isothiazolinone reached 15 mg∙L-1,it could inhibit all microbial activities and lead to biofilm death.Hence,a single biological method for the direct degradation of isothiazolinones is complicated.
In recent years,UV-AOPs,which make up for the defect of biodegradation,have presented good efficiency in the removal of refractory and toxic organic pollutants from wastewater,and thus have attracted wide attention[26–31].In particular,UV/H2O2,relying on hydroxyl radicals (∙OH),are considered a fast,clean,and effective technique for detoxifying contaminated water,which can effectively improve the biodegradability of refractory organic compounds [32–34].In addition,compared with other UV-AOPs and fenton-like technologies,UV/H2O2process is more mature[33,35–39].UV/H2O2has been established and fully operational in drinking water treatment and water reuse facilities,making good progress [26].It also had significant advantages in removing refractory contaminants in wastewater,such as pesticides and antibiotics [40].Most importantly,the pollutants could be destroyed without transferring them to another medium,and there is no secondary contamination due to the rapid decomposition of hydrogen peroxide [41,42].However,AOPs technology are difficult to completely mineralize parent compounds,resulting in the presence of intermediates,and the toxicity of intermediates is unknown.Therefore,it is necessary to investigate the toxicity of degradation products.
To the best of our knowledge,no researchers have systematically studied the mechanism and degradation path of UV/H2O2on CMI-MI under different conditions.Therefore,this study has essential reference value for degrading other pollutants by UV/H2O2.In this experiment,the influence of different operating parameters on the degradation process in the process of UV/H2O2was carried out.Meanwhile,the analysis of degradation path andEEOeconomic evaluation were also explored by various means.Considering the toxicity of the products after UV/H2O2degradation,the effluent from the secondary sedimentation tank of a sewage treatment plant was used for biodegradation experiment,which can not only investigate the biochemical effect after UV hydrogen peroxide degradation,but also examine the toxicity assessment.
2.Materials and Methods
2.1.Chemicals and reagents
The chemical reagents used were analytical grade or above,except as noted.The detailed information of chemical reagents used in this work was shown in the Supplementary Material(Text S1).In addition,the inoculum used in the biodegradation experiment came from the effluent of the secondary sedimentation tank of the sewage treatment station of Shandong University Qingdao Campus.
2.2.Degradation apparatus
The photochemical experiments were conducted at ambient temperature (20 ± 2 °C) in a UV disinfection reactor which was equipped with the low-pressure mercury UVC lamp (10 W,Shenzhen,China) emitting monochromatic light at 253.7 nm.The experimental device has the advantages of simple operation,safety,reliability and low maintenance cost.The specific structure of this device has been mentioned in the previous literature [43].The incident light intensity of the UVC lamp was determined by iodide-iodate chemical actinometry [44],and the effective optical path was determined by measuring the photolysis rate of lowconcentration H2O2[45].The calculated value of the average UV intensity is 7.26 mW∙cm-2.See the Supplementary Material for detailed calculation formulas (Text S2).
2.3.Degradation experiment
The UVC light was turned on 15 min ahead of schedule to ensure stable UVC light tensity before the experiments.250 mL solution which was adjusted to pH 7 by phosphate buffer(4 mmol∙L–1) was used for UV/H2O2oxidation.During the experiment,the 250 mL solution prepared in advance was added to the equipment,and the injection and sampling ports were blocked with cork to form a closed system.In addition,shake the device at intervals of one minute to ensure uniform degradation of the solution in the device.At predetermined intervals,a small amount of solution was removed with a syringe and transferred to a sample bottle,and Na2S2O3was added to quench the residual oxidants.In formal experiments,each group of experiments should be conducted at least twice in parallel to avoid accidental errors.
2.4.Determination of isothiazolinone content in kathon
Kathon (CMI-MI,a mixture of CMI and MI,2%) were purchased from Shandong Taihe Water Treatment Technology Co.Ltd(China).Usually,the mass concentration ratio of CMI to MI is 3:1.However,the selling concentration of the industrial fungicide may be slightly different from the actual concentration.In order to obtain a more accurate content of isothiazolinone and isothiazolinone,nuclear magnetic resonance (NMR) was used for quantitative analysis.10% pure acetic acid was selected as the external standard and added to the samples containing CMI-MI.The percentage of CMI and MI in the sample could be obtained according to the peak area corresponding to the hydrogen atoms of HAc,CMI and MI shown by the nuclear magnetic hydrogen spectrum.The total content of CMI-MI was 1.93%,according to the analysis of peak area in the nuclear magnetic hydrogen spectrum (Fig.S3).The content of CMI and MI was 14% and 5.3%,respectively.
In this study,ultraviolet spectrophotometry was used to determine the concentration of CMI-MI in degradation,which has the advantages of simple operation,rapid determination and accurate results.Both CMI and MI are five-membered heterocyclic compounds,which have obvious ultraviolet characteristic absorption at 273 nm.Therefore,the concentration of CMI and MI were determined by UV–visible spectrophotometer (UV-2450,Shimazu,Japan).
2.5.Degradation kinetics of CMI-MI
Based on the experimental research,the photodegradation kinetics of CMI-MI conformed to the pseudo-first-order kinetic model (Eq.(1)).Thekobswas the time-based observed pseudofirst-order rate constant (min-1),which could directly reflect the degradation rate of pollutants in the photolysis system.What’s more,in the actual wastewater treatment process,the influence of some operating parameters (the dose of oxidants and contaminants,pH,and the concentration of ions) onkobscould not be ignored because they may be involved in the production and consumption of free radicals [46].Therefore,it is essential to investigate the effect of those operating parameters on the degradation of hydroxyl radicals.The specific values depended on the quality of circulating cooling water.
2.6.The analysis of mineralization
The degree of CMI-MI mineralization was characterized by the DOC (dissolved organic carbon) value of water sample.The Shimadzu TOC analyzer (TOC-L CPH) was used to determine the DOC value of water samples,mainly based on the difference between DC (dissolved carbon) value and IC (inorganic carbon)value.Ultrapure water (now used and replaced) was used for needle washing before sample determination.The peak area of the needle washing was less than 0.5,and the error of the mapping solution was less than 5%.The volume of the measured sample was 30 mL and the sampling volume was set at 50 μ-L.
2.7.Determination of EEO
EEO(electric energy per order),as defined by Boltonet al.[47],is the number of electrical energy required to reduce the concentration of pollutants by an order of magnitude in 1 m3volume polluted water.Since the UV/H2O2process is electric-energy intensive and electric energy cost is a prominent fraction of operation costs,simple figures-of-merit based on electric energy consumption can be very informative [47,48].EEOis a common index used to evaluate process energy consumption,and its calculation formula is shown in Eq.(2).
wherePis the output power of UV lamp (kW),tis the irradiation time (min),Vis the volume of water sample in reactor (L),c0andctrepresent the initial concentration of organic pollutants and the concentration at reaction timet(mg∙L–1),respectively.Based on Eqs.(1)–(2),EEOcan be written as follows:
2.8.The analysis of transformation products
In addition to CO2and H2O,some inorganic ions and intermediate organic matter may be produced after the photolysis of pollutants.The type and concentration of anions in the solution were measured by ion chromatograph (Qingdao,China).The chromatographic column was SH-AC-3 (250 mm × 4.0 mm).A mixture of Na2CO3(2.0 mmol∙L–1) and NaHCO3(8.0 mmol∙L–1) solutions was used as the eluent.The flow rate was set to 1.0 mL∙min-1and the injection volume was 25 μL.The organic products produced during the degradation of CMI-MI were determined by nuclear magnetic resonance (NMR) spectrometer (Bruker Avance NEO 600,Switzerland).See the Supplementary Material for details(Test S3).
2.9.Biodegradation experiment
Activated sludge process,a mature conventional treatment process widely used in sewage treatment plants.However,isothiazolinone compounds as biocides are inevitably biotoxic,leading to the problem that the single activated sludge process is difficult to degrade fungicide substances [9].In order to solve this problem,UV and UV/H2O2technologies were first used to degrade CMI-MI in this experiment,and then combined with biodegradation to evaluate the overall mineralization trend of CMI-MI,which provided an important basis for advanced oxidation technology combined with biotechnology to degrade fungicide pollutants.
This biodegradation experiment was based on DOC Die-Away(301 A),which was a type of tests for determining the ready biodegradability of chemical compounds from the Organisation for Economic Cooperatoin and Development (OECD) Guidelines for the Testing of Chemicals[49].The experiment mainly included blank control group,reference control group and multiple experimental groups.The blank control group prepared with test medium was supplemented with inoculum but without test substance,which was set up for taking into account the endogenous activity of the inoculum.Sodium acetate was used as a reference,and a reference control group was set up to investigate the biodegradation performance of the inoculum.The inoculum was filtrate obtained from fresh secondary effluent filtered by 0.45 micron filter membrane.In order to explore the effect of high concentration of CMI-MI in pre-oxidation of UV and UV/H2O2technology on biodegradation in the later stage,one group of control and four groups of experimental groups were set up.The control group was 60 mg∙L–1CMI-MI stock solution for direct biodegradation,the experimental group was 60 mg∙L–1CMI-MI solution after preoxidation for biodegradation,mainly divided into experimental group B (UV-3 h),experimental group C (UV-4 h),experimental group D (UV/H2O2-1 h),experimental group E (UV/H2O2-2 h).The selected concentration of CMI-MI was 60 mg∙L–1,which was to make the initial DOC of the solution and the value of DOC after oxidation as large as possible,so as to reduce the error of later determination.Because of the nature of biodegradation and of the mixed bacterial populations used as inocula,determinations should be carried out at least in duplicate.See the Supplementary Material for details (Test S4).
The inoculum was derived from secondary effluent from the school’s domestic sewage treatment plant.Normally,the test lasted for 28 days,and DOC concentrations were measured to observe the trend of biodegradation on day 0,day 10 and day 28.After removal from the conical flask,the samples were filtered through 0.45 μm filter membrane and then determined.The degradation percentage(Dt)in each sampling is calculated using the following equation:
whereDt(%) stands for degradation at timet,C0(mg∙L-1) means starting concentration of DOC in the inoculated culture medium containing the test substance,Ct(mg∙L-1) means concentration of DOC in the inoculated culture medium containing test substance at timet,Cbl(0)(mg ∙L-1) means starting concentration of DOC in blank inoculated mineral medium,Cbl(t)(mg ∙L-1)means concentration of DOC blank inoculated mineral medium at timet.See the Supplementary Material for details (Test S4).
3.Results and Discussion
3.1.Degradation kinetics of CMI-MI in different systems
Preliminary experiments were conducted to examine the degradation kinetics of CMI-MI in different systems (Fig.1),where pH=7 with 20 mmol∙L–1phosphate buffer.The degradation data were in agreement with pseudo first order kinetic model.It is obvious in Fig.1 that the degradation of CMI-MI had no significant change under dark conditions and could be neglected.It turns out that H2O2alone make it difficult to oxidize an electron-rich group like CMI-MI.However,under the conditions of UV direct photolysis and UV/H2O2,the removal efficiency of CMI-MI was significantly improved,attaining 72%,90% within 20 min,especially in the UV/H2O2system.This was because in the UV/H2O2system,in addition to direct photolysis,a large number of free radicals were produced by oxidants under ultraviolet conditions,which attacked CMI-MI and promoted the degradation of CMI-MI [9].The photolysis of CMI-MI in the UV/H2O2system could be described simply by Eqs.(5)–(8) [33].

Fig.1.Comparison of degradation kinetics of CMI-MI in different systems.
Experimental conditions: [CMI-MI]0=30 mg∙L–1,[H2O2]0=0.3 mmol∙L–1,and pH=7 (4 mmol∙L–1phosphate buffer),Iavg=7.26 mW∙cm-2.
3.2.Effect of CMI-MI initial concentration
In order to investigate the effect of the concentration of CMI-MI on the degradation effect,the degradation kinetics of different concentrations of CMI-MI(10,20,30,50 mg∙L–1)were conducted using UV/H2O2process at pH 7(Fig.2(a)).In Fig.2(a),the removal rate of CMI-MI decreased with the increase of its initial mass concentration.The degradation rate of CMI-MI decreased from 100% to 76% within 20 min when the initial mass concentration of CMI-MI increased from 10 to 50 mg∙L–1.It was also observed that CMI-MI had different degradation rate constants at different initial CMIMI concentrations(as evident from the results in Fig.2(b)).The initial concentration of CMI-MI was inversely proportional to the pseudo-first-order rate constant,the equation waskobs=3.8/[CMI-MI].

Fig.2.Effects of initial CMI-MI concentration and H2O2 concentration on CMI-MI degradation.(a)-(b) Effects of CMI-MI concentration on degradation kinetics and rate constants(kobs,CMI-MI)in UV/H2O2 system,[CMI-MI]0=10,20,30,50 mg∙L–1,[H2O2]0=0.3 mmol∙L–1,and pH=7(4 mmol∙L–1 phosphate buffer),Iavg=7.26 mW∙cm-2;(c)-(d)Effects of H2O2 concentration on degradation kinetics and rate constants (kobs,CMI-MI) in UV/ H2O2 system,[CMI-MI]0=30 mg∙L–1,[H2O2]0=0–0.5 mmol∙L–1,and pH=7(4 mmol∙L–1 phosphate buffer), Iavg=7.26 mW∙cm-2.
Similar results were obtained by Caiet al.[43] in the experiment of UV/H2O2degradation fluorescence tracer PTSA (1,3,6,8-pyrene-tetra sulfonic acid,sodium salt)by changing the initial concentration of pollutants.There are three main reasons for explaining this phenomenon: with the increase of the initial mass concentration of CMI-MI,(i) the increased CMI-MI in the solution may compete with H2O2for photons produced by UV light and reduce the yield of ∙OH,thus reducing the removal rate of CMIMI.(ii) The number of hydroxyl radicals allocated to a single CMI/MI molecule decreased,then competition between molecules increased [50].(iii) Excessive substrate molecules reduced the transmission capacity of the solution and the intensity of the transmitted light,thus leading to the reduction of the removal rate[51].
3.3.Effect of H2O2 concentration
In general,the concentration of CMI-MI in circulating cooling water fluctuates within the range of 0.5–10 mg∙L–1.As the circulating cooling water is discharged after 5–8 times concentration,the residual concentration of CMI-MI in the wastewater may reach tens of mg∙L–1.In practical engineering applications,considering that oxidant cost is an important factor affecting process cost,controlling the amount of the oxidant is essential,therefore requiring exploring the impact of oxidant dosage on degradation.The effect of H2O2concentration on the degradation kinetics was shown in Fig.2(c).As expected,when the oxidation dose increased from 0 to 0.5 mmol∙L–1,the degradation efficiency of CMI-MI increased from approximately 72% to 98% and thekobsincreased from 0.0673 to 0.1852 min-1within 20 min.This is due to the fact that in an ideal UV reaction,doubling the H2O2dose results in doubling the formation of ∙OH(Eq.(5)),assuming that the total yield of radical formation and UV flux are constant.
Some previous studies have shown that a self-quenching reaction occurred between the free radical and the excess oxidant,resulting in a decrease in the rate constant (Eqs.(9)–(10))[32,42].Nevertheless,the results of this experiment presented the fact that thekobsremoved by CMI-MI showed a linear trend with the amount of H2O2:kobs=0.234×[H2O2]+0.062.The results are consistent with the previously reported studies on removing betrixaban by UV/H2O2processes [52].This may be because the amount of oxidant is not sufficient for the quenching reaction to occur.In practice,excessive use of H2O2would increase the actual cost,so it is necessary to control the amount of H2O2according to the real situation.To observe significant inhibition and promotion in subsequent experiments,the optimal concentration of H2O2was set to 0.3 mmol∙L–1.
3.4.Effect of initial pH and different anions
It is well known that the contents of acid and alkaline and inorganic ions in different wastewater are different due to the influence of the wastewater source [53].An excellent water treatment process can withstand pH and some common ion parameter tests.Considering the actual water quality of circulating cooling wastewater,the effects of pH and common anions on the treatment efficiency of CMI-MI were studied.As can be seen from Fig.S4,kobsdid not change in the range of pH from 4 to 10,suggesting that pH had no significant effect on the removal of CMI-MI in the UV/H2O2process.Similar results were obtained in the degradation of HPTS (Solvent Green 7) and PTSA by UV/H2O2advanced oxidation process [43,50].Therefore,it is concluded that the UV/H2O2process has a wide range of pH adaptability in the degradation of organic pollutants in wastewater,which is a vital advantage in engineering applications.
Common ions in circulating water cooling wastewater include.Their presence may affect the generation or consumption of free radicals,thereby promoting or inhibiting the removal of target pollutants[54].Hence,in addition to studying the influence of pH,it is also essential to explore the influence of ions.As shown in Fig.3,kobsbarely changed when the concentrations ofand Cl-were increased from 0 to 10 mmol∙L–1,indicating that the presence ofand Cl-in the solution did not interfere with the removal rate of CMI-MI.This was attributed to the following reasons: (i)rarely reacted with ∙OH,(ii) the reaction between Cl–and ∙OH and a rapid backward reaction occured simultaneously,which maintained the stability of the ∙OH concentration (Eq.(11)) [55,56].

Fig.3.Effects of different anions on CMI-MI degradation under UV/H2O2 system.
Experimental conditions: [CMI-MI]0=30.0 mg∙L–1;[H2O2]0=0.3 mmol∙L–1;pH=7 (4 mmol∙L–1phosphate buffer),Iavg=7.26 mW∙cm-2.
3.5.Evaluation of EEO
Among the essential factors (operating cost,treatment efficiency,operability,feasibility)in choosing a waste treatment technology,operating cost is the most critical factor to be considered[57].The degradation process with UV-based advanced oxidation technologies consumes electricity,which accounts for a significant portion of operating costs.TheEEOindex is an important index to evaluate power efficiency in the water treatment process.Fig.4 showed theEEOchanges in UV/H2O2and UV/PS treatment systems with the dosage of oxidant increasing.It can be seen thatEEOdecreased with increasing concentration of oxidant.TheEEOvalue of the oxidizer was more than 30%lower than that of the UV degradationEEOalone when the dosage of the oxidizer was increased to 0.3 mmol∙L–1.TheEEOvalue was more than 60%lower than that of UV degradation alone when the dosage of oxidizer was increased to 0.5 mmol∙L–1.These phenomena indicated that the increase of oxidizer dosage could reduce the power consumption of operating costs.However,this means that oxidant costs would increase,so it needs to be taken into consideration.Compared with UV/PS treatment system,theEEOvalue of UV/H2O2treatment system was lower under the same conditions,which indicateed that UV/H2O2treatment system had lower energy consumption in the degradation of CMI-MI,and had more economical advantages in practical application.

Fig.4.EEO changes in UV/H2O2 and UV/PS treatment systems with the dosage of oxidant increasing.Experimental conditions: [CMI-MI]0=30.0 mg∙L–1; Iavg=7.26 mW∙cm-2.
3.6.DOC removal
Photodegradation of organic pollutants usually disrupts the structure of organic molecules,with some eventually converted to CO2and H2O,and others creating intermediates that are difficult to mineralize.The DOC mineralization rate is an important index to observe whether the reaction is thorough and whether the pollutant is completely degraded.
The change of concentration of DC/DOC/IC with the extension of the UV irradiation time in UV/H2O2treatment system was shown in Fig.5(a).It was evident that the DOC of the solution showed a noticeable decline with the increase of UV illumination time,while the IC showed an increasing trend.After 40 min ultraviolet exposure,concentration of DOC decreased from 10.2 mg∙L–1to 6.6 mg∙L–1,and the removal rate of DOC was 35%.This indicates that a small part of the degraded pollutants in the solution was converted into small inorganic molecules,and a large part was converted into intermediate organic matter.CMI-MI could be effectively mineralized by UV/H2O2technology,and the extension of ultraviolet illumination time could promote the increase of mineralization rate.It can be clearly seen from Fig.5(b) that the DOC removal rate of the pollutant solution treated with UV/H2O2was lower than that after UV degradation alone.

Fig.5.(a)The change of concentration of DC/DOC/IC with the extension of UV irradiation time in the UV/H2O2 treatment system,and(b)the DOC mineralization rates in the UV and UV/H2O2 systems.
3.7.Identification of degradation products
In order to investigate the organic matter produced by CMI-MI during UV/H2O2degradation,the carbon (13C NMR) and hydrogen(1H NMR) spectra of CMI-MI stock solution and its degraded solution were characterized by NMR spectroscopy.It could be clearly seen from Fig.S5(a)and(b)that the position of signal peak corresponding to1H and13C of CMI-MI.By comparing the nuclear magnetic hydrogen spectra (Fig.S5(a) and (c)) before and after degradation,it was observed that the original signal peak intensity of CMI-MI was weakened,indicating that most of the initial CMIMI was oxidized and degraded during the degradation process.In addition,new signal peaks near 2.5 and 8.3 were observed in Fig.S5(c).Through the comparison of the results of nuclear magnetic carbon spectrum (Fig.S5(b) and (d)),it was observed that the signal intensity of CMI-MI at 110 and 140–150 after degradation was significantly reduced,but the change of signal peak was not consistent with that of 25 and 170.It was inferred that these two signal peaks were the signal peaks of CH3NH2.HCl and HCOOH.This was consistent with the new signal peak in the nuclear magnetic hydrogen spectrum.CH3NH2existed in the form of CH3NH2.-HCl because the degraded solution was acidic and Cl-was produced.Therefore,the main organic compounds degraded by UV/H2O2were CH3NH2and HCOOH.
The inorganic products produced in the UV/H2O2process were detected by ion chromatography,as shown in Fig.S6.No nitrogen-containing inorganic ions were produced in the whole process,indicating that organic nitrogen in CMI and MI had not been mineralized into,but still existed in organic form.This is consistent with the results of NMR identification.In the reaction process,Cl-andwere detected,and the ion concentration gradually increased with the extension of degradation time.This indicates that chlorine atoms on CMI heterocyclic ring were gradually replaced by hydroxyl groups to form chloride ions during degradation.Both CMI and MI molecules contained an electron-rich group containing sulfur atoms,so they were easily oxidized by ∙OH to form a sulfone structure,which was then hydrolyzed into[6].In summary,the proposed degradation pathway were shown in Fig.6.
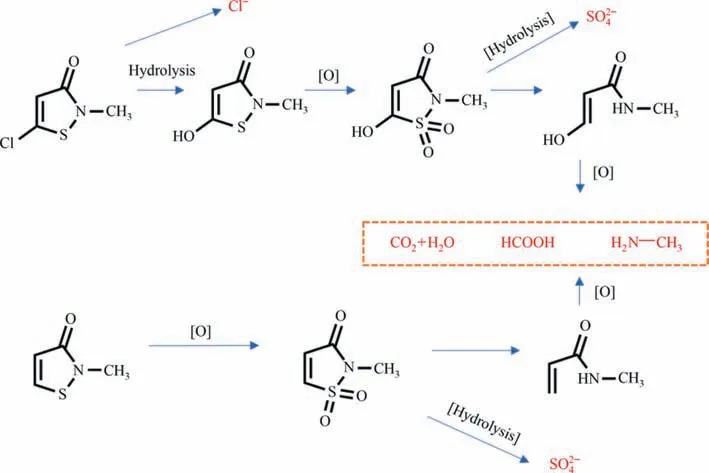
Fig.6.Possible degradation pathways of CMI-MI in the UV/H2O2 system.
3.8.Biological degradation
In order to explore the biodegradation performance of the inoculum,25 mg∙L–1sodium acetate was used as a reference to set up a reference group.As can be seen from Fig.S7,the removal rate of sodium acetate reached more than 90% after 28 days of biodegradation,indicating that the inoculum had good activity and could be used for further experiments.
The changes of DOC after the degradation of high-concentration CMI-MI in UV and UV/H2O2systems at different times were shown in Fig.7(a).As can be seen from Fig.7(a),after 3 h and 4 h of direct UV photolysis,the residual rates of DOC in the solution were 44.5% and 40%,respectively.After 1 h and 2 h of UV/H2O2photolysis,the residual rates of DOC in the solution were 59% and 44.5%,respectively.Although the degradation time reached 3 h under UV conditions,the removal rate of DOC was the same as that after 2 h under UV/H2O2condition,reflecting that oxidant played an essential role in promoting the mineralization of pollutants.The pollutants were not completely mineralized in UV and UV/H2O2systems,indicating that organic intermediates were generated during the reaction,which was consistent with the inferred results of the above products.
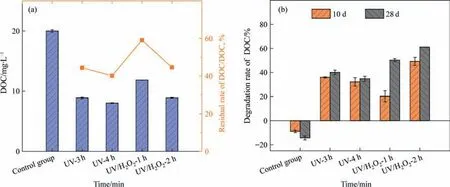
Fig.7.(a)Changes of solution DOC after degradation in UV and UV/H2O2 systems for different time.[CMI-MI]0=60 mg∙L–1,[H2O2]0=1.2 mmol∙L–1,Iavg=7.26 mW∙cm-2;(b)The changes of DOC removal rate in rebiodegradation before and after advanced oxidative degradation.
The comparison of DOC removal rates of pollutants directly biodegraded and biodegraded after advanced oxidative degradation was shown in Fig.7(b).Surprisingly,in the highconcentration CMI-MI solution group,DOC did not decrease but increased during 28 d of biodegradation.This is mainly because CMI-MI itself was a biocide,which could react with DNA and other structures in microbial cells,resulting in microbial inactivation.Then the dead microorganisms release some soluble organic matter inside the cell [23–25].In addition,after UV/H2O2photolysis for 2 h,the DOC removal rate reached 60% after biodegradation for 28 d,which indicated that the UV/H2O2process improved the biodegradability of CMI-MI wastewater.It was attributed to the fact that sulfur atoms in theheterocyclic ring of CMI-MI molecules were attacked and destroyed to form sulfate ions in UV/H2O2system,which reduced the toxicity of the solution.The increase of photolysis time had no significant effect on the biodegradation rate in the UV degradation system.The increase of photolysis time significantly promoted the biodegradation rate in the UV/H2O2degradation system.In conclusion,the UV/H2O2treatment process could reduce the toxicity of CMI-MI and improve the biochemical properties of CMI-MI.
4.Conclusions
In this study,the removal performance,parameter influence,degradation products and enhancement of subsequent biodegradation of CMI-MI in UV/H2O2system were systematically studied.The degradation rate of CMI-MI could reach 90% under UV irradiation for 20 min when the dosage of H2O2was 0.3 mmol∙L–1.The DOC mineralization rate of CMI-MI could reach 35% under certain conditions ([H2O2]=0.3 mmol∙L–1,UV irradiation for 40 min).The degradation rate of CMI-MI decreased with the increase of substrate concentration and increased with the increase of H2O2dosage.Andkobswas inversely proportional to the concentration of CMI-MI and proportional to the concentration of H2O2.The degradation rate of CMI-MI was almost unaffected by pH.The presence ofinhibited the removal rate of CMI-MI because of the complex reactions between them and free radicals.Compared with UV/PS treatment system,theEEOvalue of UV/H2O2treatment system was lower under the same conditions,which indicated that UV/H2O2treatment system had lower energy consumption in the degradation of CMI-MI.In the UV/H2O2system,CMI-MI was degraded to produce two products,namely HCOOH and CH3NH2.There was also the formation of Cl-and.After UV and UV/H2O2photolysis,the CMI-MI solution was biodegraded,and the biochemical properties were obviously improved,especially the treatment effect of UV/H2O2was better.
CRediT Authorship Contribution Statement
Jinzhi Cui:Conceptualization,Data curation,Methodology,Visualization,Writing–original draft.Guiqiao Wang:Supervision.Xing Rong:Visualization,Software.Wensu Gao:Supervision,Validation.Yaxin Lu:Supervision.Yawen Luo:Supervision.Lichao Zhang:Supervision.Zhongfa Cheng:Resources.Canzhu Gao:Supervision,Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thanks for the support of experimental Instrument Platform of Shandong Taihe Water Treatment Technology Co.,LTD.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.06.023.
 Chinese Journal of Chemical Engineering2024年1期
Chinese Journal of Chemical Engineering2024年1期
- Chinese Journal of Chemical Engineering的其它文章
- Flower-like tin oxide membranes with robust three-dimensional channels for efficient removal of iron ions from hydrogen peroxide
- Experimental study on the activation of coal gasification fly ash from industrial CFB gasifiers
- Enhanced stability of nitrogen-doped carbon-supported palladium catalyst for oxidative carbonylation of phenol
- Solubility of iron(III) and nickel(II) acetylacetonates in supercritical carbon dioxide
- Filtration performance and modeling of granular bed for dust removal from coal pyrolytic vapors
- Copper slag assisted coke reduction of phosphogypsum for sulphur dioxide preparation
