Phosphorous and selenium tuning Co-based non-precious catalysts for electrosynthesis of H2O2 in acidic media
Jingxin Xie ,Lijie Zhong,∗ ,Xin Yng ,Dequn He ,Knglong Lin ,Xioxi Chen ,Hun Wng,Shiyu Gn,Li Niu,b,∗
a Guangdong Engineering Technology Research Center for Photoelectric Sensing Materials &Devices,Guangzhou Key Laboratory of Sensing Materials &Devices,Center for Advanced Analytical Science,School of Chemistry and Chemical Engineering,Guangzhou University,Guangzhou 510006,China
b Department of Chemistry and Environment Science,Fujian Provincial Key Laboratory of Modern Analytical Science and Separation Technology,Minnan Normal University,Zhangzhou 363000,China
Keywords: Oxygen reduction reaction Electrochemical H2O2 production Electrocatalysis Non-precious electrocatalyst Acidic media
ABSTRACT Electrosynthesis of hydrogen peroxide (H2O2) is an on-site method that enables independent distribution applications in many fields due to its small-scale and sustainable features.The crucial point remains developing highly active,selective and cost-effective electrocatalysts.The electrosynthesis of H2O2 in acidic media is more practical owing to its stability and no need for further purification.We herein report a phosphorus and selenium tuning Co-based non-precious catalyst (CoPSe) toward two-electron oxygen reduction reaction (2e– ORR) to produce H2O2 in acidic media.The starting point of using both P and Se is finding a balance between strong ORR activity of CoSe and weak activity of CoP.The results demonstrated that the CoPSe catalyst exhibited the optimized 2e– ORR activity compared with CoP and CoSe.It disclosed an onset potential of 0.68 V and the H2O2 selectivity 76%-85% in a wide potential range (0–0.5 V).Notably,the CoPSe catalyst overcomes a significant challenge of a narrow-range selectivity for transitionmetal based 2e– ORR catalysts.Finally,combining with electro-Fenton reaction,an on-site system was constructed for efficient degradation of organic pollutants.This work provides a promising non-precious Co-based electrocatalyst for the electrosynthesis of H2O2 in acidic media.
Hydrogen peroxide (H2O2) is a bulk chemical that has been applied in a variety of fields,such as paper and textile bleaching [1],disinfection [2,3],environmental wastewater treatment [4,5] and chemical synthesis engineering [6].Traditional anthraquinone process represents state-of-the-art industrial-scale H2O2production[7].This process involves hydrogenation of an anthraquinone and O2oxidation in organic solvent.The resource of H2remains facing sustainable challenging and the produced concentrated H2O2either faces the safety issue.Recently,electrosynthesis of H2O2through a two-electron oxygen reduction reaction (2e–ORR) process [8–11] has drawn intensive attention due to its on-site and decentralized characteristics that enable both large-and small-scale use,particularly toward distribution applications by rational design of electrochemical reactors [12–14].Combining electro-Fenton process,it can produce highly reactive hydroxyl radicals to oxidize organic pollutants in sewage [15].Hitherto,electrosynthesis of H2O2in alkaline media has achieved significant progress [16–19].How- ever,it should gain more attention in acidic media [20] since H2O2is not stable and requires post purification in alkaline media.In addition,carbon materials,for example,even heated bare glass carbon [9] have been demonstrated nearly without overpotential in alkaline electrolyte [21–24].
A few representative 2e–ORR electrocatalysts have been reported in acidic media.Typically,noble metal alloys,such as Au-Pd [25],Pt-Hg [26],Pd-Hg [27] and Au-Pt-Ni [28] have achieved both high acitivty and selectivity.To lower its mass loading,single atomic catalysts (SACs) have made significant progress but the stability should be further examined and improved [29–33].Recently Pt and Pd doped with non-metal elements,for example PtP2[34],PtSCNx[35,36],Pd4Se [37] and PtSe2[38] could be an effi-cient strategy to gain both activity and stability.Another type of 2e–ORR electrocatalysts is non-precious metal catalysts,in which Co-based materials are the most promising due to its high H2O2selectivity compared other transition metals [39–42].For example,the atomic level Co-N-C material exhibits a certain H2O2selectivity (∼40%-80%) [43] and further oxygen functionalization enhanced the selectivity [44].However,the stability remains an issue due to the degradation of active center by H2O2,which has either been encountered in the early studies of proton-exchange membrane fuel cell [45].Another representative Co-based electrocatalysts are the metal sulfides and selenides.The first reported example of cobalt disulfide (CoS2) disclosed a selective 2e–ORR with a yield of H2O2up to ∼70% at 0.5 V in acidic media [46].The lattice anion of S element separates adjacent Co active sites,which suppresses the association of O–O bond,leading to a selective 2e–ORR.Lately,the CoSe2further increased the H2O2selectivity to>80% at high potential region based on the enhanced isolation effect by larger anion of Se element [47].The progress of CoS2and CoSe2has promoted the development of non-precious metal-based 2e–ORR catalysts in acidic media.However,a significant challenge for the Co-based electrocatalyst is sharply decreased selectivity at low potentials.Very recently,NiSe2catalysts [48] or NiS2nanosheets[49] and mixed transition metal sulfide [50] have been proposed to improve this issue,but the ORR activity decreased partially due to the difference of∗OOH binding affinity on Co and Ni active metal centers.Therefore,tuning non-precious metal-based catalysts to gain both activity and selectivity remains to be addressed toward electrosynthesis of H2O2in acidic media.
In this work,we reported a phosphorous and selenium tuning cobalt-based nonprecious electrocatalyst (CoPSe) to produce H2O2in acidic media.The idea is using P and Se elements to realize an optimal 2e–ORR process on Co active center compared individual P and Se.The results demonstrated that CoSe/C preferred a 4e–ORR activity while the CoP/C exhibited weak 2e–ORR activity.However,the CoPSe catalyst achieved a high 2e–ORR activity with an onset potential of 0.68 V and the selectivity is up to 76%-85% in a wide potential range (0–0.5 V).It also exhibits improved stability in acid media compared with present Co-based 2e–ORR catalysts.An electro-Fenton setup based on CoPSe catalyst has been applied for the degradation of organic pollutants,which provides a promising application prospect for on-site water treatment.
The CoPSe/C catalyst was prepared by a simple two-step process (Fig.1a).The precursor of Co(MeIm)2was firstly pyrolyzed to form Co/C under 650 °C.The dia-Co(MeIm)2is a precursor of Co organic complex,which was prepared by mixing cobalt acetate and 2-methylimidazole (mole ratio 1:2).And then,the Co/C was further treated by phosphorus and selenium (see experimental section in Supporting information for details).The Co/C exhibits a sheet-like morphology by scanning electronic microscopy (SEM) (Fig.1b and Fig.S1 in Supporting information).After P and Se treatment,the CoPSe/C shows a uniform particle structure (Fig.1c) and the highresolution SEM discloses a maintained sheet structure,but more wrinkles were observed (Fig.1d).Further transmission electronic microscopy (TEM) characterization revealed the CoPSe/C nanoparticles supported in carbon layer matrix (Fig.1e).The particle size of CoPSe/C nanoparticles is about 50 nm (Fig.1f).The high-resolution TEM images demonstrate clear lattice spacing distances of 0.28 nm and 0.25 nm for CoPSe/C nanoparticles,corresponding to lattice(200) and (210) planes,respectively (Figs.1g and h).The corresponding energy-dispersive X-ray spectroscopic mapping reveals a uniform distribution of elements of Co,P,Se and C in the CoPSe/C composite (Fig.1i).The morphology and composition distribution of the CoSe/C and CoP/C nanocrystals are studied as depicted in Figs.S2-S5 (Supporting information),respectively,showing uniform Co,P,and Se distribution in the composites.
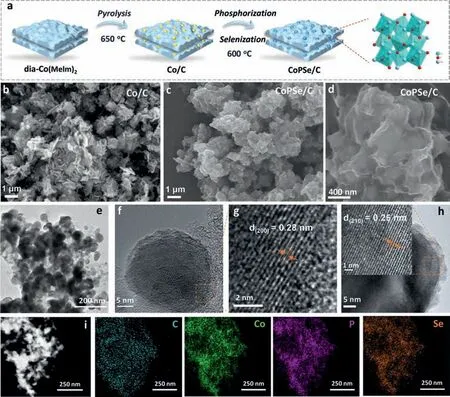
Fig.1.Synthesis and morphology of CoPSe/C catalyst.(a) Schematic diagram of CoPSe/C synthesis process.(b-d) Representative SEM images of Co/C and CoPSe/C.(e-h) TEM and HRTEM image of CoPSe/C.(i) Element mapping images of CoPSe/C.
The crystal structures of the catalysts were further examined by the X-ray diffraction (XRD).The CoPSe/C discloses a cubic structure with strong diffraction patterns of (200) at 2θ=32.0° and (210) at 2θ=35.8° (orange line,Fig.2a).This result is consistent with the TEM observation of the two crystal fingerprints (d(200)andd(210))(Figs.1g and h).The XRD patterns of CoSe/C agree with the structure of monoclinic Co6.8Se8,in which main crystal faces of (220),(221) and (040) are clearly distinguished.The CoP/C sample was identified to an orthorhombic structure but exhibits weak crystallinity with main crystal faces of (011) and (211).In addition to the structures of anchored metal catalysts,the carbon support was also examined by the Raman spectra (Fig.2b).Typical D and G bands for the carbon materials are observed.The D band represents the defect structure of carbon plane,for example sp3carbon and heterogeneous atom-doped carbon (e.g.,P/Se).The G band discloses the complete carbon plane structure (graphitic carbon).The intensity ratio between D and G bands (ID/IG) could reflect the degree of defects.It is found that the CoPSe shows the highestID/IG(1.08),which was originated from the both phosphorization and selenization.These defects may enhance the interaction between Co sites and carbon support,which is beneficial to the stability for the electrocatalysis of ORR.
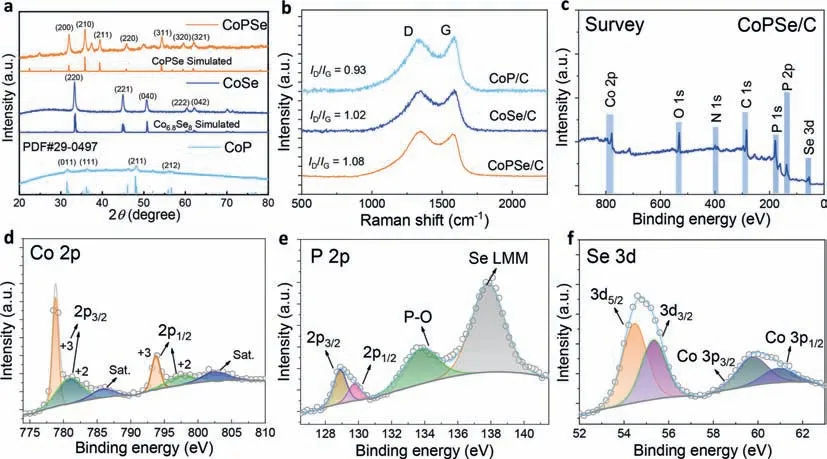
Fig.2.Structures and compositions of the catalysts.(a) XRD patterns of CoPSe/C,CoSe/C and CoP/C.(b) Raman spectra of the three catalysts under 514 nm laser excitation.(c) XPS survey spectrum of CoPSe/C.(d-f) High-resolution XPS spectra of (d) Co,(e) P and (f) Se for CoPSe/C.
To further understand the compositions of CoPSe/C,the X-ray photoelectron spectroscopy (XPS) was used to examine the elements.Fig.2c shows its XPS survey spectra.The XPS spectrum of CoSe/C and CoP/C show the predominant signals of Co,P and Se(Fig.S6 in Supporting information).High-resolution Co 2p spectrum of CoPSe/C includes peaks at 793.6 eV and 778.9 eV correspond to Co 2p1/2and 2p3/2of CoPSe/C,respectively,as well as peaks with two shakeup satellite peaks assigned to oxidized cobalt species,indicating the coexistence of both Co3+and Co2+(Fig.2d).In the fitted P 2p spectrum (Fig.2e),two splitting peak regions centered on 128.9 eV and 129.8 eV are located in 2p3/2and 2p1/2orbitals respectively,indicating that P is bonded with Co.The peak region centered on the binding energy of 133.8 eV belongs to the oxidation state P,which may be formed when the catalyst is exposed to air.In addition,the additional peak at 138.1 eV can be attributed to the overlap of the Se Auger emission line with the P 2p core level.The high-resolution Se 3d spectra (Fig.2f) show two peaks centered on 54.5 and 55.3 eV (Se 3d5/2and Se 3d3/2),which also indicates the bonding of Co with Se.
The above structural characterizations demonstrated welldefined morphology and composition of CoPSe/C catalyst.In this section,its 2e-ORR performances in acidic electrolyte were examined in detail.To elucidate the effects of P and Se on the catalysts,the controlled experiments of CoP/C and CoSe/C were compared.Fig.3a shows rotating ring-disk electrode (RRDE) voltammograms for the three Co-based catalysts in O2-saturated 0.1 mol/L HClO4with a scanning potential window between 0.0 and 0.8 V(versusRHE).It is found that the CoSe/C has an onset potential of 0.74 V (higher than theoretical value of 0.7 V),high limiting current density (jL=4.8 mA/cm2) and relatively low H2O2selectivity(∼20%-40%),which indicates a dominated 4e-ORR process.The CoP/C catalyst exhibits an onset potential of 0.61 V,much lowerjL(∼3.5 mA/cm2) and H2O2selectivity of ∼40%-60%.Although the selectivity is enhanced,the activity for 2e-ORR is low (overpotential up to 90 mV).The CoPSe/C showed the best activity with an onset potential of 0.68 V (20 mV overpotential) and ∼80% selectivity was resulted over a wide potential window and the maximum selectivity is up to ∼85% (Fig.3b).
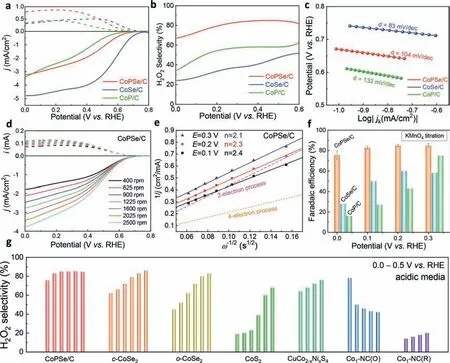
Fig.3.Electrochemical ORR in 0.1 mol/L HClO4.(a) Rotating-ring-disk-electrode (RRDE) voltammograms of CoPSe/C,CoSe/C and CoP/C in O2-saturated 0.1 mol/L HClO4 at a scan rate of 10 mV/s.(b) The H2O2 selectivity over the potential window from 0.00 to 0.60 V (vs. RHE).(c) Tafel plots for CoPSe/C,CoSe/C and CoP/C.(d) RRDE for the CoPSe/C at different rotating speeds.(e) The determined electron transfer numbers of CoPSe/C at different potentials from RRDE.(f) FE% of CoPSe/C at different electrolysis potentials determined by KMnO4 titration.(g) A comparison of H2O2 selectivity between CoPSe/C and other reported representative Co-based non-precious 2e– ORR catalysts in acidic media.For the CoPSe/C,the selectivity at 0–0.3 V was determined by bulk electrolysis and the data at 0.4 V and 0.5 V was obtained from RRDE test.The comparison data was cited from c-CoSe2 (0–0.5 V) [47], o-CoSe2 (0–0.5 V) [47],CoS2 (0–0.5 V) [46],CuCo2-xNixS4 (0.2–0.5 V) [50],Co1-NC(O) (0.1–0.5 V) [43] and Co1-NC(R) (0.2–0.5 V)[43].
The kinetic performances of three cobalt-based materials were further evaluated by Tafel plots (Fig.3c).The kinetic current density (jk) was obtained by using the K-L equation.The Tafel slope could suggest the differences in their rate-determining step [51].The slope of CoSe/C was determined to 83 mV/dec,which is close to generally observed Pt/C catalyst [52],indicating a 4e-ORR process.However,the CoPSe/C exhibits a Tafel slope of 104 mV/dec,much higher than CoSe/C,corresponding to the values for general 2e-ORR,in which the controlling step is determined by the formation of the∗OOH intermediate through the proton transfer step.The CoP/C of 132 mV/dec indicates a difficulty for the 2e-ORR leading to a high overpotential (Fig.3a).
The selectivity of ORR could be further examined by the number of electron transfer.Therefore,to demonstrate the ORR pathway of CoPSe/C catalyst and H2O2generation,the RRDE at different rotation speeds was examined from 400 rpm to 2500 rpm (Fig.3d).The cathodic limiting current density increases with the rotation speed owing to the controlled thickness of the diffusion layer(mass transfer).The relationship between current density and rotational speed can be described by Koutecky-Levich (K-L) equation.As shown in Fig.3e,the K–L plots display good linearity and the numbers of electron transfer are around 2.1–2.4,which confirms that CoPSe/C prefers the 2e-ORR pathway to generate H2O2.To compare the selectivity of the three catalysts,bulk electrolysis tests were further carried out.The concentration of H2O2was titrated with KMnO4solution.The Faradaic efficiency (FE%) was shown in Fig.3f.The CoPSe/C reaches FE% of 76%-85% at the potentials of 0.1–0.3 V.The comparison materials of CoSe/C and CoP/C were also tested.However,their FE% is below 50%,which further demonstrates the advantage of CoPSe/C catalyst.Although the H2O2is less stable in neutral and alkaline medias,we also tested its performances in 0.1 mol/L PBS (pH 7) and 0.1 mol/L KOH (pH 13) (Fig.S7 in Supporting information).All three catalysts exhibit decreased H2O2selectivity,particularly in alkaline electrolyte (<40%).The onset potentials are far than 0.7 V,indicating that the 4e–ORR as a dominant process occur in these electrolytes.These results are consistent with early studies of many reported transition metalbased ORR catalysts for alkaline fuel cells.Finally,the H2O2selectivity is compared between CoPSe/C and other reported representative Co-based non-precious 2e–ORR catalysts in acidic media (Fig.3g and Table S1 in Supporting information).The CoPSe/C discloses the selectivity of 76%-85% in a wide potential range (0–0.5 V),which overcomes the difficulty of a narrow-range selectivity for transition-based 2e–ORR electrocatalysts.
After examining its 2e–ORR electrochemical activity and selectivity,the stability was further evaluated in this section.Cyclic voltammetry (CV) tests and long-term electrolysis were both used.After 3000 CV cycles,the RRDE curves of CoPSe/C showed very slightly shifted half-wave potential (∼4 mV) and nearly unchanged H2O2selectivity (Fig.4a).Moreover,the long-term stability of CoPSe/C catalyst on an 8 mm glass carbon electrode was evaluatedviathe chronoamperometric test at a constant electrolysis potential of 0.1 V (vs.RHE) through an H-type electrolytic cell (Fig.4b).After 2 h,the current density basically maintains a stable value.The produced H2O2was sampling each hour and titrated with KMnO4solution.For the 10 h electrolysis,the total concentration of H2O2was determined to 0.45 mmol/L.The average productivity of H2O2is up to 87 mmol gcat-1h-1.And it is found that the production rate is relatively fast for the first 4 h while it gradually decreases with the extension of electrolysis time.The possible reason is due to the used non-flow H-type cell,which leads to accumulation of H2O2in the cell or the surface of electrode interface.The morphology and composition changes of CoPSe catalyst were further examined after electrolysis test.The CoPSe nanoparticles are still distributed on the carbon (Fig.4c) and the main lattice face(210) remains clearly distinguished (Fig.4d).The Co,P and Se elements were still uniformly distributed in the catalysts as shown in the elemental mapping images (Fig.4e).The high resolution XPS spectra of Co 2p,P 2p,and Se 3d also fit well with corresponding binding bands without apparent shifts of binding energy compared with initial CoPSe/C catalyst (Figs.4f-h).Although all these results disclose a certain stability for the CoPSe,it should be noted that the Co element has been detected in the electrolyte after longterm electrolysis by highly-sensitive ICP-MS test (ca.57 μg/L).The ratio of Co dissolution was determined ∼10% relative to initially loaded CoPSe/C on the surface of electrode.In addition,the XPS intensities of Co/P/Se elements decreased after long-term electrolysis(Figs.4f-h),which either indicates the dissolution of Co element.The repeat dissolution and electrodeposition resulted in the aggregation of CoPSe nanoparticles (Fig.4c).This is indeed a daunting challenge for the transition metal-based 2e–ORR catalysts in acidic media.More efforts should be further devoted to further improve its stability.
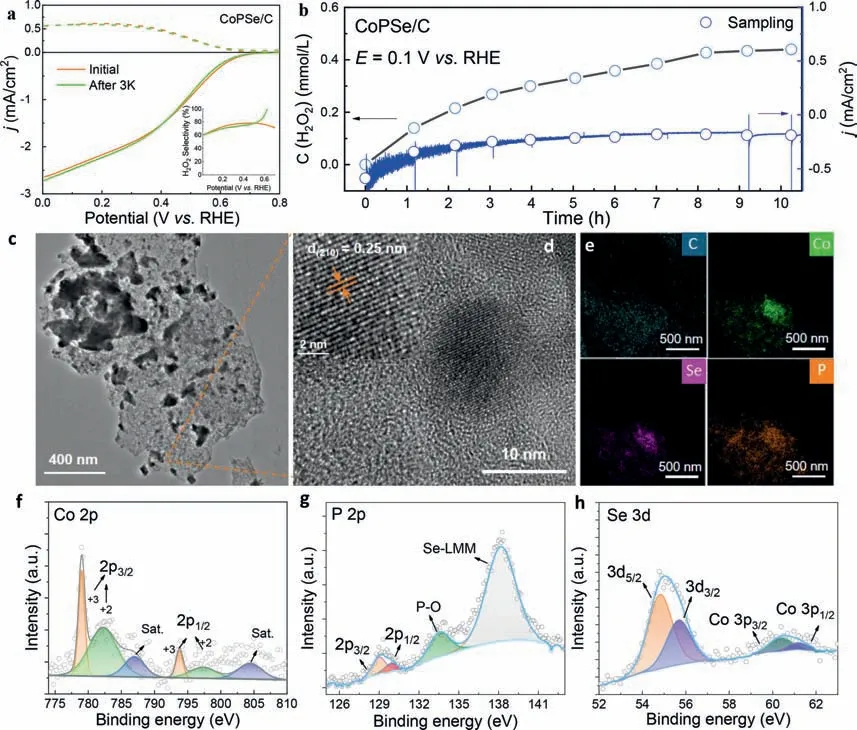
Fig.4.Stability examination of CoPSe/C catalyst.(a) RRDE voltammograms of CoPSe/C before and after 3000 cyclic tests in 0.1 mol/L HClO4.The inset shows the H2O2 selectivity before and after cyclic tests.(b) Continual long-term electrolysis of CoPSe/C at 0.1 V (vs. RHE) and H2O2 accumulation concertation during stability test.(c) TEM images,(d) HRTEM images and (e) element mapping of the catalyst after electrolysis.(f-h) High resolution XPS of Co,P and Se.
Overall,the above experimental results demonstrate an optimal 2e–ORR process for CoPSe/C catalyst compared with CoSe/C and CoP/C.The comprehensive performances are also compared with state-of-the-art transition metal based 2e–ORR electrocatalysts (Table S1).It is found that the CoPSe/C exhibited the advantages in relatively low overpotential,high H2O2selectivity in a wide potential range,and improved stability at a certain degree.
Advanced oxidation processes (AOPs) that generates highly active radicals (e.g.,•OH and Cl•) from soluble oxidants (H2O2,O3and HOCl,etc.),are state-of-the-art wastewater treatment technology for the removal of organic pollutants [15,53].Among them,H2O2could bein situgenerated at the cathode through electrochemical 2e–ORR (Eq.1) and decomposed into highly oxidizing hydroxyl radical (•OH) (2.7 V) by external addition of Fe2+(Eq.2).Furthermore,Fe2+reacts with H2O2to oxidize to Fe3+,and then directly reduces back to Fe2+at the same cathode (Eo=0.77 V,Eq.3).The produced strongly oxidant•OH could oxidize the organic pollutants(Eq.4).The whole electro-Fenton process remarkably increases the production rate of•OH and organic mineralization ability compared with traditional chemical Fenton process.The catalytic activity of iron ion mainly depends on the pH value of solution.This Electro-Fenton system is effective in acidic reaction media,like optimal pH 2.8 to 3.0,while the Fenton activity declines sharply in neutral or alkaline solution,due to precipitation of dissolved Fe2+.The above results have demonstrated that CoPSe/C was enabled as a promising electrocatalyst for electrosynthesis of H2O2in acidic media,which could be used for electro-Fenton degradation of organic pollutants.
To examine the activity of CoPSe/C as an electro-Fenton catalyst,a proof-of-concept application was conducted using the electro-Fenton degradation of phenol and rhodamine B (RhB) as model reactions (Fig.5a).An H-cell was used with a Nafion membrane as a separator (Fig.S8 in Supporting information).The electrolyte consisted of O2-saturated acidified 0.5 mol/L Na2SO4solution with 0.5 mmol/L Fe2+,followed by the addition of phenol or RhB (20 or 40 mg/L) as the degradation objects.The cathode was prepared by spraying CoPSe catalyst onto carbon paper (CoPSe/CP) and applying a constant voltage to achieve efficient H2O2production (Fig.5b).The change of phenol content and the aromatic and carboxylic acid intermediates produced during the degradation were identified by HPLC.Fig.5b presents the degradation degree diagrams of phenol solutions and their chromatograms at different degradation times (Fig.5c and Fig.S9 in Supporting information).The peak appeared when retention time was 6 min,which was related to phenol.With the increase of degradation time,phenol gradually attenuated and disappeared after ∼60 min (20 mg/L) and ∼130 min(40 mg/L).The phenol peak disappeared,indicating that all intermediates were decomposed.
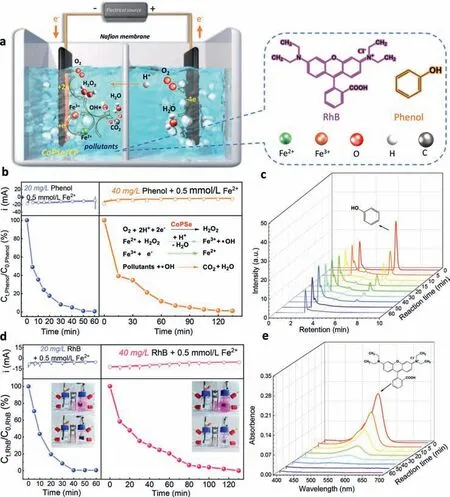
Fig.5.Electro-Fenton degradation of organic pollutants.(a) Schematic illustration of the electro-Fenton process.(b) Electro-Fenton degradation of Phenol.Top: current-time curves for degradation of phenol solutions with 20 mg/L and 40 mg/L at 0.1 V (vs. RHE).Bottom: decays of the concentration of phenol over time.(c) HPLC examines the degradation of phenol solution (20 mg/L).Corresponding HPLC curves for the degradation of 40 mg/L phenol solution are shown in Fig.S9 (Supporting information).(d) Electro-Fenton degradation of RhB.Top: current-time curves for degradation of RhB solutions with 20 mg/L and 40 mg/L at 0.1 V (vs. RHE).Bottom: decays of the concentration of RhB over time.(e) UV–vis absorption spectra of the RhB solution (20 mg/L) during the degradation.Corresponding UV–vis curves for the degradation of 40 mg/L RhB solution are shown in Fig.S10 (Supporting information).
Another organic pollutant of RhB was further degraded (Fig.5d).To determine the degree of degradation of RhB,UV–vis spectrophotometry was used to monitor the change of its absorbance (Fig.5e and Fig.S10 in Supporting information).The CoPSe/CP exhibited relatively stable galvanometric responses for the RhB degradation (Fig.5d,top).The characteristic absorption peak of 554 nm gradually decays along with degradation time.The CoPSe/C cathodic electrode completely degraded RhB solutions in ∼60 min (20 mg/L) and ∼135 min (40 mg/L).These results indicate that CoPSe can be used forin-situelectrochemical synthesis of H2O2and as a cathode in electro-Fenton reaction for real-time degradation of persistent and toxic organic pollutants in wastewater.
Finally,we use the density function theory (DFT) calculation in comparison with literatures to illustrate the structure-propertyactivity relationship (Fig.S11 in Supporting information).The CoPSe in this work andc-CoS2,c-CoSe2ando-CoSe2[47] in literatures were compared.The CoPSe constitutes two main crystal faces(210) and (200),which was proved by the TEM (Figs.1g and h) and XRD results (Fig.2a),so that the 2e–ORR process was calculated on both crystal faces.The crucial intermediate of∗OOH was calculated.According to their free energy curves (Fig.S11a),it is found that the CoPSe (210) and (200) exhibit downhill binding affinity of O2to∗OOH,which are–0.17 eV and–0.38 eV,respectively.This result indicates both crystal faces are active toward 2e–ORR.However,the CoPSe (210) is closer to the ideal state (0 eV).In addition,the bond length of Co-O (1.846 ˚A) on CoPSe (200) is shorter than Co-O (1.888 ˚A) on CoPSe (210),which indicates over binding of∗OOH on (200) (Fig.S11b).A longer of O–O bond length on (200)relative to (210) further suggests relatively easier dissociation of this bond,leading to unfavorable 2e–ORR.Furthermore,compared withc-CoS2(100),c-CoSe2(100) ando-CoSe2(101),the binding energy of∗OOH on CoPSe (210) is also closer to 0 eV,which demonstrates its relatively more active toward 2e–ORR.Overall,both free energy and bond length results identified the active crystal faces CoPSe (210).An optimal binding energy of∗OOH promotes its high activity of 2e–ORR.
In summary,we have developed a cobalt phosphate selenium ternary catalyst (CoPSe/C) for selective 2e–ORR electrosynthesis of H2O2in acidic media.The catalysts disclosed an onset potential of 0.68 V (vs.RHE) and H2O2selectivity of 76%-85% in a wide potential range,which outperforms state-of-the-art representative Cobased 2e–ORR electrocatalysts.This research emphasized the importance of 2e–ORR in acidic media and the selectivity in a wide potential range.Toward non-precious 2e–ORR electrocatalysts,we demonstrated that the co-tuning effects of P and Se elements could improve the performances of Co-based 2e–ORR electrocatalysts.An on-site electro-Fenton setup has exhibited an efficient degradation of organic model pollutants,which has the potential in applications of wastewater treatment.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work has received financial support from the National Natural Science Foundation of China (Nos.21805052,21974031,2278092),Science and Technology Research Project of Guangzhou(Nos.202102020787 and 202201000002),Department of Science&Technology of Guangdong Province (No.2022A156),Key Discipline of Materials Science and Engineering,Bureau of Education of Guangzhou (No.20225546) and the Innovation &Entrepreneurship for the College Students of Guangzhou University(No.XJ202111078175).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108472.
 Chinese Chemical Letters2024年1期
Chinese Chemical Letters2024年1期
- Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
