Recognition and chirality sensing of guanosine-containing nucleotides by an achiral tetraphenylethene-based octacationic cage in water✰
Honghong Duan ,Ting Yang ,Qingfang Li,Fan Cao,Pingxia Wang,Liping Cao
College of Chemistry and Materials Science,Northwest University,Xi’an 710069,China
Keywords: Host-guest recognition Chirality sensing Tetraphenylethene Achiral cage Nucleotide
ABSTRACT The development of molecular probes or systems with the ability of multiple orthogonal responses is an effective approach to precisely detect biomolecules with similar chemical structures.Herein,we report the synthesis of a water-soluble TPE-based octacationic cage (1) with the compressed TPE-containing bilayer,which endows it with good fluorescence properties and potential conformation chirality.As a result, 1 exhibits molecular recognition for anionic nucleotides within its two “claw”-like cavities to form 1:2 host-guest complexes in water,companying with selective turn-off fluorescence and turn-on CD responses to G/GTP over other nucleotides.
Nucleotides are essential for life because they involved in multiple cellular functions [1–3].Especially,ATP as energy source is pivotal in living systems [4],and GTP is involved in RNA synthesis and the citric acid cycle and acts as an energy source for protein synthesis [5].Therefore,detection of nucleotides has been developed and mainly focused on fluorescent sensors [6–10],involving direct sensing [11,12],cation-based recognition [13–19],and indicator displacement assays [20–27].Supramolecular chemists also have designed molecular clamps [14],bowls [28],tubes [29],and others [30–37] to act as host molecules for recognizing nucleotides as guests.However,most of these molecular probes,hosts,or systems only can exhibit a fluorescence signal,which could be induced by other irrelevant factors to result in a false diagnosis.On the other hand,all nucleotides with a glucose unit are chiral molecules [38].However,the chiral factor of nucleotides was usually ignored or did not express in their optical detection.In host-guest recognition,the chiral conformation of the host can be induced by chiral guests to exhibit chiroptical responses [39,40].Until now,there have been few reports of the simultaneous incorporation of fluorescence and chiral units in the structural design of supramolecular hosts [29,33,34,39].Therefore,developing multiple-responsive molecular probes or systems with fluorescence and chiroptical properties for detecting or imaging these chiral biomolecules is desired and challenging in aqueous solution.
Tetraphenylethylene (TPE) and its derivatives possess aggregation-induced emission (AIE) properties and dynamic right-handed (P) and left-handed (M) rotational conformations.Therefore,TPE will be an ideal building block for the design of molecular probes or hosts with the ability of dual responses including fluorescence and chiroptical signals [41–48].Based on this strategy,we have developed a TPE-based cage with a proper cavity (height: ∼7 ˚A),which can encapsulate aromatic groups to achieve the recognition and stabilization of natural base pairs in aqueous media [34,49].Herein,we report the synthesis of a TPE-based octacationic cage (1) with a compressed cavity (height:∼5 ˚A),which consists of two tetrapyridinium TPE moieties as central faces and fourm-xylylene moieties as surrounding pillars.In the cage structure,two TPE faces were tightly locked in a shorter distance to form the bilayer structure,resulting in the high absolute quantum yields (φ Fup to 40.2%) in the solution state.Taken advantage of the fluorescence and chiral rotational conformation of the TPE units,1can form 1:2 host-guest complexes with various nucleotides and exhibit selective fluorescence and circular dichroism (CD) responses to 2′-deoxyguanosine-5′-diphosphate(G) and guanosine-5′-triphosphate (GTP) over other nucleotides in water.
As shown in Scheme 1,1·8PF6-was synthesized by two-step SN2 reactions.Firstly,the tetrapyridyl TPE compound (2) was reacted with 1,3-bis(bromomethyl)benzene (3) in a molar ratio of 1:10 to obtain an acyclic tetrapyridinium compound (4) at 90 °C for 3 days (Figs.S1–S3 in Supporting information).Next,2and4were mixed at 110 °C for 3 days to obtain a yellow powder of crude1·8PF6-.The crude product was purifiedviacolumn chromatography to give pure1·8PF6-in ∼16% yield.Furthermore,1·8PF6-was transferred to water-soluble1·8Cl-by adding an excess amount of tetrabutylammonium chloride (TBACl) in ∼80% yield.1H,13C,COSY NMR spectroscopy and electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) supported the formation of1(Figs.S4-S10 in Supporting information).
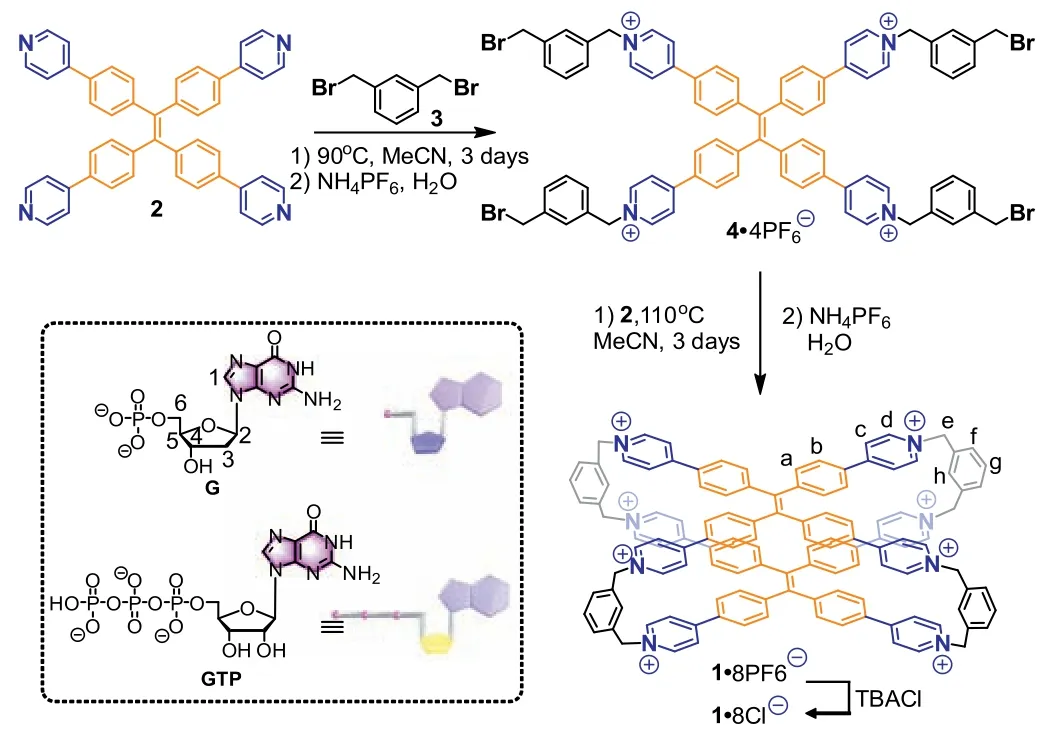
Scheme 1.Synthesis of 1 and chemical structures of G/GTP.
Fortunately,X-ray single crystals of1were obtained by slow vapor diffusion of ethyl ether from a solution of1·8PF6-in MeCN or acetone from a solution1·8Cl-in H2O,respectively (Tables S1 and S2 in Supporting information).In the X-ray crystal structure,1·8PF6-possesses a cuboid internal cavity with a size of approximately 17.1 ˚A (length) × 11.5 ˚A (width) × 5.1 ˚A (height),and two TPE units adoptPandMrotational conformation in a mesomeric pattern (Fig.1a).Compared with the previous cage [49],the distance of ∼5.1 ˚A between two TPE units is shorter,which makes it insufficient to accommodate the aromatic guests in the central cavity.In addition,the rotational directions of the four-surroundingm-phenyl pillars are inconsistent (Fig.1b),and the adjacent cages are stacked to form a porous network structure throughπ···πinteraction between twom-phenyl pillars (Fig.1c).The crystal structure shows that1·8Cl-has the similar size with1·8PF6-(Fig.1d).However,fourm-phenyl pillars show “claw”-like conformation,which make two potential recognition sites (triangle area)–“claw”–like cavities–with the size of ∼9 ˚A–∼12 ˚A when combined with the 60°-angle concave wall of TPE units (Fig.1e).These cavities could bind with guest molecules to form a host-guest complex in a ratio of 1:2.Furthermore,cages accumulate with each other to form a network structure (Fig.1f and Fig.S11 in Supporting information).
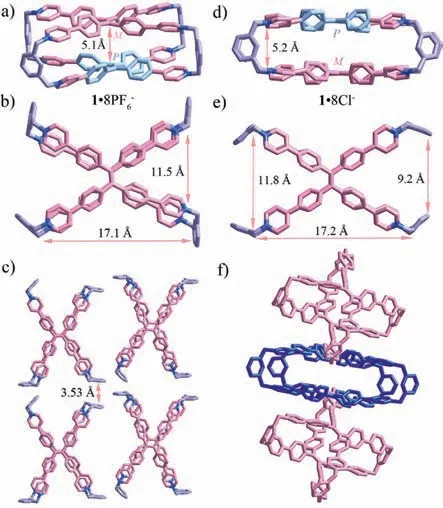
Fig.1.X-ray crystal structures of 1·8PF6-: (a) side view and (b) top view,(c) stack packing;and 1·8Cl-: (d) side view and (e) top view,(f) stack packing.Counter ions and hydrogen atoms are omitted for clarity.
Utilizing restriction of intramolecular rotations (RIR) mechanism is a good strategy for designing the aggregation-state luminescent materials [39,50].The compressed TPE-containing bilayer in cage molecules can efficiently restrict the rotation of the TPE units,making it with good fluorescence property in different solvents.The solution of1·8PF6-in both good solvents (e.g.,MeCN and DMSO) or poor solvents (e.g.,H2O,MeOH,THF,CHCl3and CH2Cl2) showed a bright yellow-green emission centered approximately at 530 nm (Figs.2a and b) with stable optical properties and high absolute quantum yields (13.6%–40.2%,Table S3 in Supporting information).Fluorescence enhancement was not observed when different amounts of water (poor solvent) were added to acetonitrile (good solvent) solution (Fig.2c and Fig.S12a in Supporting information),indicating that the rotation of TPE units in the cage could be effectively restricted by shortening the distance between two TPE units,which cause that the AIE effect is negligible.Similarly,water-soluble1·8Cl-showed similar emission centered at ∼530 nm in different solvents to give highφ Fof 13.6%∼32.7% (Figs.2d and e,Table S3 in Supporting information).When the poor solvent MeCN is gradually added to the solution of1·8Cl-in H2O,fluorescence quenching occurs owing to the aggregation-caused quenching (ACQ) effect (Fig.2f and Fig.S12b in Supporting information).In this case,therefore,both AIE and ACQ phenomena occurred on the same molecules.Due to the compressed TPE-containing bilayer structure of the cage effectively limits the rotation of TPE units,however,1·8Cl-still exhibits high quantum yields and long lifetimes (τ=5.15 ns) in both poor and good solvents (Table S4 and Fig.S13 in Supporting information).Therefore,the design of cage-like molecule featuring the compressed TPE-containing bilayer can contribute to promote fluorescence properties with high quantum yield and long lifetime.

Fig.2.Fluorescence spectra (a) and (b) images of 1·8PF6- in different solvents.(c)Fluorescence spectra 1·8PF6- versus H2O fraction in MeCN–H2O mixture.Fluorescence spectra (d) and (e) images of 1·8Cl- in different solvents.(f) Fluorescence spectra 1·8Cl- versus MeCN fraction in MeCN–H2O mixture.
Given hydrophobic effect and electron-deficient features,this pyridinium-based cage with two “claw”-like hydrophobic cavities can be an ideal receptor for anionic nucleotide molecules in aqueous media.Therefore,cage could utilize these “claw” cavities to capture two guests,forming the “crab” complex (Fig.3a).The hostguest recognition between1·8Cl-and G in D2O was observed by1H NMR titration experiments (Fig.3b): (1) when 0–6.0 equiv.of G was added into the solution of1·8Cl-in D2O,the TPE proton signals (Haand Hb) of1·8Cl-showed obvious upfield shifts (Δδ>0.1 ppm) due to the shielding effect from the aromatic guanine unit of G.At the same time,Haand Hbwere split into multiple peaks,because the chiral ribose moiety of G decreases the symmetry of the cage.(2) The other proton resonances of1·8Cl-almost no significant shifts had occurred.This result shows that G dose not enter the central cavity but the “claw”-like cavities at two sides.(3) the proton resonances of guanine (H1) and the ribose moiety (H2–6) of G were obviously shifted upfield,indicating that whole G molecule is shielded by the “claw”-like cavities of1·8Cl-.ESI-TOF-MS provided evidence for the 1:2 stoichiometry of1·8Cl-⊃G2(Fig.3c).DFT theoretical simulation shows that model I is stable,in which two G are located“claw”-like cavities of1·8Cl-(Fig.3d,left).In addition,1·8Cl-exhibited similar recognition behavior for other nucleotides by NMR experiments (Figs.S14-S22 in Supporting information).Meanwhile,ESI-TOF-MS,UV–vis titration,and Job’s plots also strongly supported the formation of these 1:2 host-guest complexes (Figs.S23-S43 in Supporting information).Due to the number of phosphate groups and the different base units,1·8Cl-and guests exhibit different first-step binding constants (Ka) of ∼102–∼103L/mol with the selectivity for G/GTP(Table S5 and Fig.S44 in Supporting information),indicating that the electrostatic interaction between positively-charged pyridinium units of host and negatively-charged phosphate groups of guests and hydrophobic effects from the “claw”-like cavity are main driving forces in these aqueous host-guest complexation.
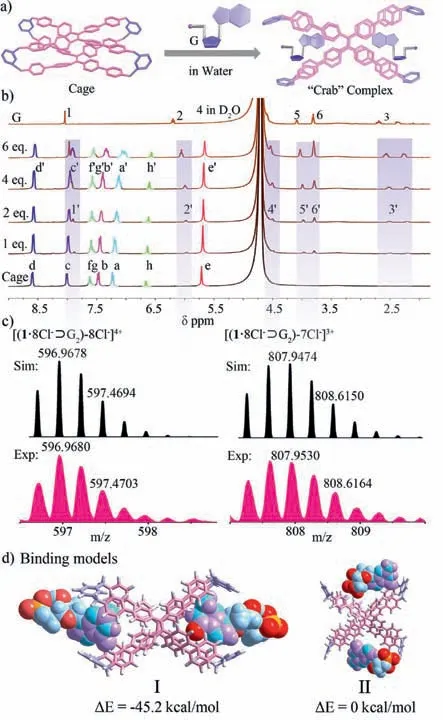
Fig.3.(a) Schematic representation for the formation of 1·8Cl-⊃G2.(b) 1H NMR spectra (400 MHz,D2O,298 K) of 1·8Cl-(0.40 mmol/L) titrated with G (0–6.0 equiv.).Primes (’) denote the resonances within the host-guest complex.(c) ESITOF-MS of 1·8Cl-⊃G2.(d) DFT calculations for three theoretically possible binding models of 1·8Cl-⊃G2.
Based on the host-guest recognition,the multiple optical responses of1·8Cl-to nucleotides were further studied by fluorescence and CD titration experiments (Fig.4).Fluorescence spectra of1·8Cl-titrated with ATP did not show any significant change in the intensity of the emission although they have strong binding when compared with other guests (Fig.4a).However,a dramatic fluorescence quenching of1·8Cl-at 530 nm when GTP was added,which could be attributed to the strong photoinduced electron transfer(PET) between electron-rich guanine units of GTP and electrondeficient pyridinium units of1·8Cl-(Fig.4b).The detection limits of1·8Cl-was calculated to be ∼500 nmol/L and ∼60 nmol/L for G and GTP,respectively (Fig.S45 in Supporting information).In all,1·8Cl-showed fluorescence quenching responses to guaninecontaining nucleotides including G and GTP,and no fluorescence response to other nucleotides (Fig.4c,Figs.S46 and S47 in Supporting information),indicating that cages exhibit excellent selectivity through fluorescence response to guanine-containing nucleotides over others.
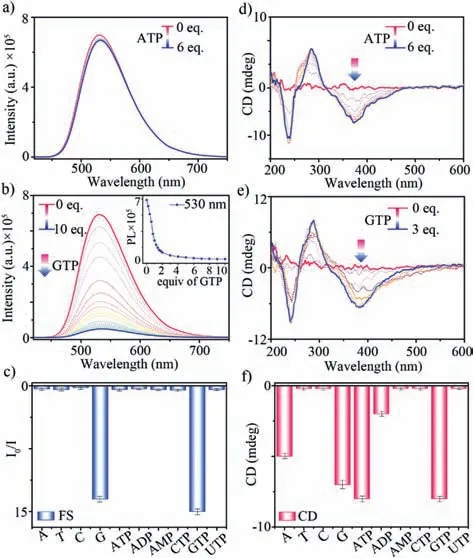
Fig.4.Fluorescence spectra of 1·8Cl- (10 μmol/L) titrated with (a) ATP and (b) GTP.(c) I/I0 (530 nm) of 1·8Cl- (10 μmol/L) with nucleotides (6.0 equiv.).CD spectra of 1·8Cl- (20 μmol/L) titrated with (d) ATP and (e) GTP.(f) CD intensity (380 nm) of 1·8Cl- (20 μmol/L) with nucleotides (3.0 equiv.).
Given theP/Mrotational conformation of TPE units,CD experiment was employed to evaluate the chiral response of1·8Clto nucleotides.As expected from the mesomeric conformation of cage,1·8Cl-did not exhibit any obvious Cotton effects in water,because two TPE units in cage adopt a mesomericP/Mrotational conformation in the solution state.Interestingly,when adenine-/guanine-containing nucleotides including A/ATP and G/GTP were titrated into the solution of1·8Cl-in water,CD signals from new negative Cotton effects at 310–450 nm,strongly confirming that theProtational conformation of TPE units was induced by guests(Figs.4d and e,Fig.S48 in Supporting information).At the same time,other adenine-containing nucleoside (ADP and AMP) with less phosphate groups could induce weaker or no Cotton effects of1·8Cl-in the host-guest complexation through the supramolecular chirality transfer,due to weaker binding between host and guest.The chirality induction from other chiral nucleosides to cage was ineffective,which only showed no or very weak CD signals (Fig.4f,Figs.S49 and S50 in Supporting information).These results support that the purine bases with large aromatic units could be bound tightly within the “claw”-like cavity to limit and induce the chiral rotation of TPE units.However,the binding of cage and pyrimidine bases were not enough strong to effectively limit the rotation of TPE units,resulting no CD response.As a result,1·8Cl-can exhibit multiple orthogonal responses of fluorescence and CD to different types of nucleotide molecules: (1) Dual responses of fluorescence and CD to guanine nucleotides;(2) Only CD response to adenine ones;(3) No response to all pyrimidine compounds (Figs.4c and f).
In conclusion,we have designed and synthesized a compressed TPE-based cage (1) with two “claw”-like cavities.1can encapsulate two nucleotide molecules inside two “claw”-like cavities to form 1:2 host-guest complexesviahydrophobic effects and electrostatic interactions in water.Given the fluorescence andP/Mrotational conformation of TPE units,1as a chiroptical and fluorescent sensor can exhibit multiple orthogonal responses of fluorescence and CD to different types of nucleotide molecules in aqueous solution.Therefore,this water-soluble cationic cage with the ability of multiple orthogonal responses can promote the accuracy for the detection of biomolecules with similar chemical structures in the biocompatible media.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.22122108 and 21971208),the Natural Science Basic Research Plan for Distinguished Young Scholars in Shaanxi Province of China (No.2021JC-37),and the Fok Ying Tong Education Foundation (No.171010).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108878.
 Chinese Chemical Letters2024年1期
Chinese Chemical Letters2024年1期
- Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
