Research progress of severe acute respiratory syndrome coronavirus 2 on aerosol collection and detection
Xinyu Zhng ,Yuting Chen ,Yueying Pn ,Xinye M ,Gui Hu ,Song Li ,Yn Deng,Zhu Chen,Hui Chen,∗,Ynqi Wu,Zhihong Jing,Zhiyng Li
a Hunan Key Laboratory of Biomedical Nanomaterials and Devices,Hunan University of Technology,Zhuzhou 412007,China
b State Key Laboratory of Quality Research in Chinese Medicine,Macau University of Science and Technology,Macau 999078,China
c Shenzhen Lemniscare Med Technol Co.Ltd.,Shenzhen 518000,China
d Department of Clinical Laboratory,the Affiliated Drum Tower Hospital of Nanjing University Medical School,Nanjing 210008,China
Keywords: Virus aerosol Aerosol transmission Aerosol collection Aerosol detection Protective measure
ABSTRACT The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in late 2019 has negatively affected people’s lives and productivity.Because the mode of transmission of SARS-CoV-2 is of great concern,this review discusses the sources of virus aerosols and possible transmission routes.First,we discuss virus aerosol collection methods,including natural sedimentation,solid impact,liquid impact,centrifugal,cyclone and electrostatic adsorption methods.Then,we review common virus aerosol detection methods,including virus culture,metabolic detection,nucleic acid-based detection and immunologybased detection methods.Finally,possible solutions for the detection of SARS-CoV-2 aerosols are introduced.Point-of-care testing has long been a focus of attention.In the near future,the development of an instrument that integrates sampling and output results will enable the real-time,automatic monitoring of patients.
1.Introduction
Since the outbreak of the novel coronavirus epidemic in late 2019,it has rapidly become a global pandemic and has become the biggest challenge for people in recent times [1].Transmission has gradually increased due to the lack of effective immediate clinical tests to rapidly and accurately identify patients already infected with SARS-CoV-2 [2,3].Furthermore,as clinical studies have revealed that asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected patients are highly contagious,but due to the lack of appropriate testing results,many have already had some spread at the societal level and are spreading very rapidly,with the number of cases increasing exponentially in many countries [4].After a period of common fight against the epidemic,the trend of increasing numbers of infections leveled off [5].Sequencing results have revealed that SARS-CoV-2 is similar to SARSCoV in sequence and that the illnesses caused by the virus are similar,with fever,cough and respiratory distress [6].As the novel coronavirus is an RNA virus with strong variability,the discovery of different variants of the novel coronavirus has been reported around the world [7].As variants emerge,they are more infectious and insidious [8].The contents of verification of the presence of SARS-CoV-2 aerosols were summarized in Supporting information,and verified by a series of experiments.
2.Aerosol deposition
The small particle size of aerosols confers them strong penetrating power and enables easy adsorption on the surface of objects.Thus,aerosols deposit in obscure places,leading to indirect infection,and in many cases,the source of infection cannot be identified [9,10].Conticiniet al.showed that air pollution not only causes rapid aerosol transmission of SARS-CoV-2 but also increases its lethality [11].Therefore,many scientists have conducted investigations to determine where aerosols are deposited.The contents of virus aerosols exist on surfaces,wastewater transmission of virus aerosols,and transmission of virus aerosols in public places were summarized in Supporting information,which are common but often overlooked.
3.Sampling of virus aerosols
The rapid spread of SARS-CoV-2 and the high mortality rate of its infection have decreased productivity.Therefore,it is paramount that the source of infection is identified and transmission contained at the earlist.The premise of SARS-CoV-2 detection is effective sampling.Microbes were first collected from the air by French microbiologist Bardes in 1861 [12].Since then,interest in the field of microbial collection has increased.To improve our understanding of the mechanisms of airborne transmission of viruses,air sampling techniques to detect the presence of aerosol viruses,efficiently collect and maintain their activity,and determine their distribution in aerosol particles are necessary [13].Several methods have been developed for sampling microbial aerosols,such as natural sedimentation [14],solid impact [15],liquid impact [16],centrifugal,cyclone [17] and electrostatic adsorption [18] methods.These methods are classified as either active or passive sampling[19,20].As summarized in Table S1 (Supporting information),the sampling performance and advantages and disadvantages of common aerosol samplers are clearly compared.
3.1.Passive sampling
The most conventional and commonly used sampling method is the natural sedimentation method.It was proposed by the German bacteriologist Robert Koch in 1881.This method collects microbial particles that fall from the air due to the effect of gravity into a Petri dish containing a culture medium within a certain period of time,and the results are observed microscopically.The particle concentration of microorganisms collected from the air is calculated using the Omeryanski formula [21].However,the effect of environmental factors,such as temperature,humidity and wind speed,on aerosols is not considered in this formula,so the calculation results vary markedly.Researchers generally use the natural sedimentation method to determine the community and structure of microorganisms,and because this method is simple and inexpensive,it can be performed multiple times.However,because this is a passive sampling method,the sampling conditions are difficult to control,and the sampling efficiency is low.Furthermore,this method is unsuitable for microbial aerosols with a molecular size<5 μm or for microbes that do not settle easily.
3.2.Active sampling
3.2.1.Solid impact method
The solid impact (or solid impingement) method involves the active sampling of microbial aerosols by striking on a solid medium in a Petri dish after gaining inertia.Cascade impactors are commonly used to obtain data on particle size and concentration to characterize ambient aerosols.The solid impact sampler uses the principle of impact,where air is moved over a solid sampling surface at a certain speed and directly collected in a Petri dish.The most common solid impact sampler is the Andersen sampler,proposed by Ariel Andersen in 1958 [22].The sampled air is injected into a gas nozzle using an air pump and flows through layers of different pore sizes (decreasing in size from top to bottom).It is mainly used to research the type and size distribution of particles in microbial aerosols.The inertia of the particles deflects them from the airflow and knocks them against a surface,usually a Petri dish containing a culture medium [23].The Andersen Class 6 sieve sampler is considered the standard for aerosol sampling by the International Society of Aerobiology.It is often used by researchers because of its high sensitivity,ability to separate particles by size,wide range of applications,and high collection efficiency [24].Because the Andersen 6-stage sieve sampler allows particulate collection according to particle diameter,some researchers have used the improve structure of Andersen samplers to collect the particles discharged from patients.Graltonet al.investigated whether respiratory viral infections are transmittedviadroplets or air and found that both large and small particles carrying viral RNA were produced when coughing and breathing,making both modes of transmission possible [25].Additionally,viability testing of airborne viruses and the sampling performance of Andersen samplers in indoor environments at low concentrations of virus aerosols revealed that adenovirus activity was not affected by prolonged nebulization conditions [26].
However,because the Anderson sampler requires many Petri dishes for collection,the operation is complicated,with plates needing to be cultured and the resulting colonies counted.If the colonies overlap,counting is difficult,and because the efficiency of virus collection is not ideal,the results are prone to errors.Furthermore,the small size of the virus particles means that they easily fall off the collection surface [27].Although the Andersen sampler can capture and classify particles according to their size and the sampling efficiency is highly stable,it is very inconvenient to carry the sampler outdoors.Collectively,these disadvantages mean that this sampling method cannot meet the requirements of rapid collection and detection.
3.2.2.Liquid impact method
Since Winslow designed the first liquid impingement sampler in 1908,more and more liquid impingement samplers have been developed [28].The liquid impact method is similar to the solid impact method,as shown in Fig.S4 (Supporting information),both use the principle of inertia.But the microbial aerosol in the airflow is deposited into a liquid medium instead of a solid medium[29].The use of a liquid medium at the time of sampling improves buffering,reduces microbial damage,and uses nonevaporative liquids with less particle re-aerosolization.Some studies on SARSCoV-2 used liquid impact samplers to collect virus aerosols with a particle size of 1–3 μm and detected the presence of virus particles using digital PCR,which has a higher level of sensitivity than RTqPCR [30].Furthermore,experiments investigating different sampling times revealed that long-term sampling with the liquid impact method led to the degradation of viral nucleic acids [30].
The collection efficiency of low-concentration microbial aerosols using the liquid impact method is inadequate,and it is unsuitable for use at low temperatures and for long-term collection.The virus is captured by jet airflow through the collection solution at high or low speed,without the need for culture [31].If a nutrient solution is added,the sample can be stored for analysis at a later stage.The first liquid impingement sampler was the AGI sampler,which had a significantly lower flow rate than the Andersen sampler [32].The more common liquid impingement samplers are the BioSampler and AGI-30,which have the advantages of high collection efficiency and good stability [33].As shown in Fig.S1(Supporting information),Zheng and Yao measured the physical and bioaerosol collection efficiency of the BioSampler sampler usingBacillus subtilisvegetative cells and achieved the highest sampling efficiency at a flow rate of 20 L/min [34].By improving the sampling membrane and sampling efficiency,researchers have also used liquid impact sampling of bacteria and viruses in animal husbandry for the detection of foot-and-mouth disease virus (FMDV) [35],avian influenza virus [36] and monkeypox virus[37] in an effort to reduce the loss of animals in the farming industry due to virus transmission in aerosols.
3.2.3.Centrifugal and cyclone methods
Both the centrifugal and cyclone methods use the impact principle to rotate an impeller in the volute at high speed so that the microbial aerosol deviates from its own gravity and is collected into a sampling medium.Centrifugal and cyclone samplers are small in size and easy to operate,carry and control.Laneet al.used a BC 251 two-stage cyclone sampler to collect virus-containing aerosols in nursing stations,ward corridors,and throughout the corridors of referral centers for critically ill patients with COVID-19.Although RT-qPCR results showed that SARS-CoV-2 was not detected in areas outside the patients’wards,the avoidance of direct contact was recommended [38].To verify that personal protective equipment protects against the spread of SARS-CoV-2 in public areas,another study used a sampling box containing a Teflon filter for collection,and the results showed that the concentration of SARS-CoV-2 RNA in a well-ventilated space was significantly decreased [39].
It must be noted that the efficiency of collecting small microbial aerosols is low,stability is poor,and microorganisms may be lost when the liquid evaporates [40].Investigation of the efficiency of recovery and quantification of FMDV from environmental surfaces and aerosols revealed that the higher the initial virus concentration,the higher the efficiency of recovery on a surface [35].During the prevention and control of the SARS-CoV-2 epidemic,the cyclone sampler has been identified as extremely suitable for collecting aerosols in real time in areas with dense traffic and obtaining the highest collection efficiency in the shortest time,thereby preventing problems before they occur.Thus,its usefulness during the SARS-CoV-2 epidemic cannot be overstated,and the large-scale outbreak of the epidemic has resulted in the continuous optimization of the cyclone sampler to achieve rapid sampling [40].Karagozet al.designed a new type of cyclonic sampler with a longer vortex length to increase its efficiency for aerosol capture [41].As shown in Fig.S5 (Supporting information),the cyclone sampler is suitable for portable detection of viral aerosols due to its simple internal construction.
3.2.4.Electrostatic deposition method
All microorganisms carry a charge,and this deviates under the action of an electric field.Thus,the application of an electric field during the sampling of microorganisms causes them to fall into the sampling medium.The use of a relatively mild electrostatic precipitation method for collection maintains the biological activity of bacteria and viruses to a greater extent [42].As early as 1997,Mainelis used an improved electrostatic aerosol sampler to collect three types of bacteria.Furthermore,a large number of experiments have confirmed that when the flow rate is 1 L/min,the collection efficiency of bacteria can reach 80% [43].Compared with solid impact samplers,electrostatic precipitation samplers can increase air concentration and maintain biological stability and activity.Additionally,they have the high collection efficiency for small microbial particles.If higher voltages are applied,more virus particles can be captured from the air.Electrostatic air samplers enable the sampling of low-concentration microbial aerosols and have lower power requirements and a lower drop in pressure than liquid impingement samplers [44].Kimet al.used an electrostatic air sampler to directly sample airborne viruses in a continuously flowing liquid,facilitating the enrichment process of aerosols for hydrosols [45].
However,the collection range of electrostatic aerosol samplers is limited,and high relative humidity of the surrounding air during the collection can lead to electricity leakage,resulting in an unsatisfactory enrichment effect.Maet al.designed and evaluated an integrated microfluidic electrostatic sampler with a maximum effective collection efficiency of approximately 40% at the corresponding voltage.Clinical sample experiments on bioparticle aerosols ofB.subtilisrevealed that the collection efficiency was approximately 16% due to the loss of particles from the hydrophobic mesh [46].However,when matched with an integrated detection machine,this type of automatic sampling device can form an integrated and portable experimental piece of equipment that can perform sampling and detection.As shown in Fig.S6 (Supporting information),Hanet al.proposed the concept of a personal electrostatic bioaerosol sampler,which has a sampling efficiency of up to 70% and can operate stably for a prolonged period,thereby making it convenient for laboratory and field research on various biological agents in the air [47].
3.2.5.Filtration method
The filtration method is convenient and often used to collect microbial aerosols.Following collection in the sampler,they can be directly analyzed and observed using electron microscopy.The most important component of the filtration method is the filter membrane on the sampler [48],and materials such as polytetrafluoroethylene,nylon and gelatin have long been used to collect aerosols,while samplers with gelatin filters have also been used for virus collection [49,50].Environmental friendly cellulose membranes are expected to eliminate water and airborne viruses,but many variables must be considered,such as pore size distribution,pH durability,biofouling,wet strength,flow rate,specified adsorption and flammability.
Liet al.compared BioSampler,gelatin filter and glass fiber filter samplers (all from SKC Ltd.,Dorset,UK).All samplers were used in the same environment,and they all only collected a small part of the total virus aerosol;therefore,the collection of samplers should be improved [51].In another study,polytetrafluoroethylene (PTFE)filters were installed in the rooms of inpatients with SARS-CoV-2 infection to study the aerosolization of the droplet transmission of SARS-CoV-2 as well as infection of respiratory viruses and appropriate protective measures in a hospital setting [52].Furthermore,a laboratory study evaluating the sampling efficiency of filters used to collect influenza virus and the effect of storage on the collected viral RNA found that optimal results were achieved if the viral RNA was extracted and amplified within 5 h of collection [53].
A filter sampler collects the microbial aerosol particles suspended in the air on the filter membrane by means of collision,sedimentation,etc.,through the filter device.Compared with other samplers,filter samplers have a higher efficiency,are convenient to carry,and are inexpensive.Furthermore,compared with liquid impingement samplers,the efficiency of filter samplers is better at low temperatures.However,because microorganisms are easily damaged when they accumulate on the filter material,longterm collection can reduce the filter permeability.Environmental factors also greatly interfere with collection using filter samplers.If the humidity is too high,a water film forms on the filter material,making it difficult to collect a sample [54].Additionally,secondary processing is required after collection,the operation is complicated,and the enrichment effect is quite different.Zhenet al.compared the overall performance of a gelatin sampler and the BioStage impactor for fungal collection and found that while the efficiency of the gelatin sampler was comparable to that of the impactor in indoor sampling,the efficiency decreased remarkably in outdoor sampling [55].In another study,the use of quartz fiber filters for outdoor sampling and collection of particulate matter during the SARS-CoV-2 epidemic lockdown in Madrid revealed high concentrations of SARS-CoV-2 in aerosols [56].
3.2.6.Other virus aerosol collection methods
The abovementioned methods for aerosol collection are more common in a laboratory environment or outdoors.However,there are several additional sampling methods that are used by researchers in experiments.The thermophoresis method of collection directly cultures samples on agar sheets.The collection process is quick and does not damage low-concentration aerosols.However,it is not widely used owing to the small sampling volume and short acquisition duration.Lednickyet al.collected air samples in the room of two patients with SARS-CoV-2 using the VIVAS air sampler based on the principle of gentle water vapor condensation and performed RT-qPCR and virus culture experiments.They found virus aerosols in the air circulating in the room and discovered that the aerosols could pass through air conditioning exhaust systems,leading to virus propagation [57].
Since the first method of microbial collection was introduced in 1881,several scientists have invented microbial samplers,and they are being constantly optimized to be more applicable and conducive to modern research.Different sampling methods have their own advantages and disadvantages,and the emergence of various samplers has greatly promoted research in microbial aerosols.As shown in Table S2 (Supporting information),it summarizes the main performance and sampling methods of common samplers in the market,which can be used in multiple places.Although the natural sedimentation method can maintain good biological activity,it leaks the outside air during the collection process;thus,it cannot be used for virus sampling.Compared with the natural sedimentation method,which is passive,active sampling methods are more practical,and the collection volume is higher.Compared with the solid impact sampler,the liquid impact sampler has a larger flow rate and also causes less damage to the microbial morphology,does not require complicated secondary culture,and can be directly added to a liquid medium for culture.Compared with centrifugal and cyclone samplers,the above samplers are bulky and inconvenient to carry.Therefore,when sampling outdoors,researchers tend to prefer centrifugal and cyclone samplers.Furthermore,the air flow is large,and the time is greatly shortened,making these instruments particularly suitable for collecting SARSCoV-2.Filter and cyclone aerosol samplers are commonly used to collect viral bioaerosols for culture-independent analysis because of their simplicity and ability to efficiently collect aerosol particles of various sizes.Many variables that affect the collection efficiency of aerosol samplers,such as the range of collection,size of the particles being collected,and environmental factors,affect the characterization and activity of microorganisms after collection;therefore,selecting a suitable sampler is crucial.To date,a perfect aerosol sampler suitable for any environment and the sampling of any microorganism has yet to be designed;thus,the sampling method can only be selected according to local conditions and consistent with the sample characteristics.
4.Methods to detect virus aerosols
Because the global pandemic caused by SARS-CoV-2 has wreaked havoc on society and caused worldwide panic,there is an urgent need for convenient,timely and accurate methods to detect SARS-CoV-2 [58].Sampling of microbial aerosols is thus essential;however,their detection more important,and efficient diagnostic testing is a critical intervention for pandemic management and control [59,60].As shown in Table S3 (Supporting information),it summarizes the common molecular detection methods and compares their advantages and disadvantages.The current rapid development of different technologies and molecular biology detection means that microscopic observation of the morphology of microorganisms is no longer the only option for microbial identification,and microbes can be identified according to smaller molecular structures and using molecular biology techniques [61,62].In everyday testing,researchers use various methods to identify microbes,including culture counting methods,nucleic acid detection,and immunological detection techniques [63–65].In clinical testing,nucleic acid-based methods are prone to false positives,and the use of antibodies has improved the accuracy of SARS-CoV-2 detection [66,67].New technologies integrating collection and detection are also undergoing rapid development,and efficient,accurate,and fast instant detection is favored [68,69].
4.1.In situ-based detection methods
The virus culture method and metabolism-based assays including electrical impedance tomography (EIT) and ATP bioluminescence are summarized in Supporting information.
4.2.Detection based on nucleic acid amplification
4.2.1.Detection based on non-isothermal amplification
PCR is a common technology used to detect viral nucleic acids.Through high-temperature denaturation,low-temperature annealing,and template extension at a suitable temperature,PCR exponentially amplifies trace amounts of DNA,enabling early,sensitive,and specific virus detection.The American scientist Kary Mullis improved DNA amplification by inventing the PCR.Then,the researchers improved the effciency of the PCR reaction by changing the heat-resistant polymerase and increasing the extension temperature [70,71].qPCR detection collects data using DNAbinding fluorescent dyes and fluorescent signals generated using DNA probes and photosensors that collect light signals of fixed wavelengths [72–74].As early as 2014,Silvaet al.collected and detected SARS-CoV-1 aerosols using RT-qPCR.Compared with conventional PCR,RT-qPCR showed higher sensitivity and more accurate detection (17.5%vs.39.5%) [75].PCR is the primary test for SARS-CoV-2.It is based on the detection of viral RNA and is one of the most widely used tests recommended by the World Health Organization (WHO) and Centers of Disease Control and Prevention for screening individuals in many countries [76].RT-qPCR is the gold standard for the detection of SARS-CoV-2 owing to its high amplification efficiency and strong specificity,and large-scale detection using this technique has been performed in many countries[77–79].As shown in Fig.1,RT-qPCR is used to determine the viral load of SARS-CoV-2 in samples collected from positive patients by detecting SARS-CoV-2-specific RNA sequences.Owing to the high viral load in the nose and throat,samples are primarily obtained from oropharyngeal and nasopharyngeal swabs of the respiratory tract,following which viral RNA is extracted and amplified [80,81].
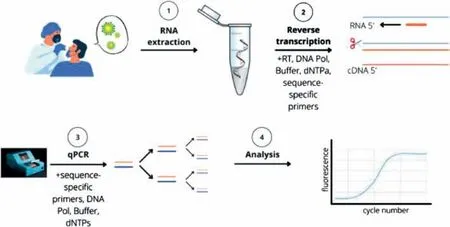
Fig.1.Example of PCR testing workflow during COVID-19 outbreak.Reproduced with permission from [58].Copyright 2022,Talanta.
A PCR-based study of presymptomatic and asymptomatic infection identified 43 positive patients from 61 samples acquired from patients who did not show symptoms of infection [82].However,RT-qPCR requires manual manipulation [83];it requires a highly secure laboratory,and the process from extraction to testing is time consuming,thereby indicating that it does not meet the expectations of point-of-care testing (POCT) [84].
4.2.2.Detection based on isothermal amplification
(1) Loop-mediated isothermal amplification (LAMP):
In 2000,Notomi invented the LAMP technique,a new isothermal nucleic acid amplification method suitable for genetic diagnosis [85].As shown in Fig.S7 (Supporting information),four specific primers (F3,B3,FIP and BIP) and two loop primers (LF and LB)were designed to target six regions of the gene of interest.Under the action of DNA polymerase-based strand displacement,the amplification leads to substantial amplification at a constant temperature of 60–65 °C for 45 min [86].
Since the development of this technique,LAMP has made great strides in its application as a molecular diagnostic technique[87,88].It has been used to successfully detect human respiratory pathogens [89,90],viruses [91] and bacteria [92].Huanget al.used a visual LAMP detection method to detect SARS-CoV-2 RNA and reported the experimental results through color changes,enabling the results of viral RNA amplification to be read with the naked eye without the need for expensive equipment [93,94].Kitagawaet al.improved the RT-LAMP system using a fluorescence quantitative method to display the amplification value more intuitively and were able to detect 1.0× 101cp/μL within 35 min [95].The advantage of LAMP is that it eliminates the time-consuming process of the thermal cycling step in PCR,achieving 109–1010copies in the shortest time [96,97].However,LAMP also has disadvantages compared with PCR.For example,LAMP is several times costlier than PCR,making it unsuitable for large-scale nucleic acid sampling.Yuanet al.also developed a detection method with high precision,specificity and quantification of nucleic acids by improving the digital droplet LAMP system to quantify the target DNA concentration by the number of droplets and significantly reduced the limit of pathogen detection [98].
(2) Recombinase-mediated isothermal nucleic acid amplification(RAA) and recombinase polymerase amplification (RPA):
Compared with the abovementioned amplification methods,RPA and RAA can provide simple,convenient,and portable POCT diagnosis [99].As shown in Fig.S8 (Supporting information),it the amplification principle of RPA,which is simpler than LAMP and does not require high temperature.Piepenburget al.designed a new amplification method that drives primer targeting by isothermal recombinase coupled with strand-displacement DNA synthesis.The amplification reaction has high sensitivity and specificity and is faster.Compared with LAMP,it can be used at lower reaction temperatures [100,101].Fanet al.detected African swine fever using two isothermal amplification methods,RAA and RPA,resulting in a detection rate of 96.59% and 97.73%,respectively,which were similar to the detection rates of the commonly used gold standard PCR,and omitted the tedious amplification step.Simple amplification procedure also shortens the amplification time,and results can be obtained within 16 min [102].Such isothermal amplification techniques are particularly well suited to resource-constrained settings that lack the infrastructure,equipment,and skills to support the use of PCR as a diagnostic tool [103].However,because the reaction temperature is generally approximately 37 °C and the reagents cannot be stored in an environment with a room temperature higher that 37 °C,it is easy to elicit an enzyme reaction.
4.3.Immunology-based assays
The properties of coronaviruses greatly affect the diagnostic performance of molecular and serological assays,as well as the speed of infection and the immune responses that occur in the body of the infected person [104].Anti-SARS-CoV-2 IgM is not detected in individuals who have not been infected with SARS-CoV-2 and is also absent in the early stage of infection.After exposure to SARS-CoV-2 infection,IgM values increase,peaking in the third week [105].The detection sensitivity is significantly higher than that of RT-qPCR 1 week after the disease onset.These findings indicate that the combination of molecular and serological tests is crucial for the diagnosis of patients with newly diagnosed COVID-19 at different stages [106].
4.3.1.Enzyme-linked immunosorbent assay (ELISA)
ELISA is both a qualitative and quantitative method of detection where an antigen or antibody specifically binds to a solid phase carrier [107].ELISA has the advantages of good specificity,high sensitivity,and stable results.The test is quick and simple to perform,making it suitable for large-scale routine sampling[108].Harritshøjet al.evaluated 15 commercial and 1 in-housedeveloped anti-SARS-CoV-2 kits in 16 laboratories using clinical samples from asymptomatic,mild,or moderate COVID-19 patients and confirmed the results using nucleic acid amplification tests.Nearly 95% of the kits could detect SARS-CoV-2 IgG with a specificity of ≥99% [109].Another study compared ELISA test kits from four different manufacturers and found that all of them achieved a sensitivity of at least 98% and a specificity of up to 98% [110].The detection of SARS-CoV-2 antibodies using ELISA is relatively reliable,and RT-qPCR detection plays a role in auxiliary diagnosis.A meta-analysis of studies on ELISA-based tests found that the N gene-based antigen was better than the S gene-based antigen,which was different from the RT-qPCR with the N gene as the target [111].Liuet al.developed a simple nanozyme chemiluminescence paper test paper using the ELISA method and test strips as the carrier,which showed high sensitivity and facilitated early screening [112].However,in the early stage of infection with SARSCoV-2,ELISA testing cannot detect antibodies,making it easier for the virus to spread in the community [113].Therefore,it should be used in conjunction with RT-qPCR as an auxiliary test.
4.3.2.Colloidal gold immunochromatography assay (GICA)
GICA is a new method of immunolabeling using colloidal gold as a tracer marker that is applied to antigens and antibodies [114].The introduction of colloidal gold in immunochemistry by Faulk and Taylor in 1971 opened new avenues for immunological detection methods [115].Owing to its advantages of rapidity,short turnaround time and accurate results,GICA is widely used in medical testing,compensating for the limitations of RT-qPCR,which requires a clean laboratory to avoid PCR contamination and specially trained nucleic acid testing technicians [116].Liaoet al.developed a SARS-CoV-2 IgG/IgM combined antibody test strip based on GICA.Compared with RT-qPCR,there was no significant difference in sensitivity,and the goal of POCT detection was achieved[117].Rashidet al.tested nine one-step kits based on the principle of colloidal gold-labeled immunochromatography and obtained results within 15 min,with specificities ranging 98.7%–100%,but with reduced sensitivity [118].
4.4.Other detection methods
High-throughput sequencing technology can be used to obtain the complete information on unknown sequences from complex samples in a single test and is highly accurate with good sensitivity.During the spread of SARS-CoV-2,this technology can be used to accurately identify virus mutations,thus helping to identify the source of new COVID-19 outbreaks [119].However,owing to its high cost and the need for specially trained professionals,high-throughput sequencing is unsuitable for comprehensive nucleic acid detection applications;thus,it is only performed when determining sequence mutations [120].
Because of the frequent outbreaks of SARS-CoV-2,monitoring the epidemic is critical.Lara-Jacoboet al.proposed a method based on mass spectrometry to monitor SARS-CoV-2 proteins in urban wastewater.They detected specific SARS-CoV-2 proteins 5–6 days ahead of clinical cases,and this method could be used as an early warning system for epidemic prevention and control [121].In addition,quantitative proteomic analysis of SARS-CoV-2-positive urine samples can also be performed using mass spectrometry,and the host can be identified after infection [122].
De Puiget al.developed minimally instrumented SHERLOCK,a clustered regularly interspaced short palindromic repeats (CRISPR)-based diagnostic test that allows users to self-test SARS-CoV-2 and its multiple viral variants at home using a saliva sample without the need for additional equipment.Only three steps are performed: spit,wait,and scan.The results are either positive or negative and are obtained within 20 min [123].If this technology can be widely used in the detection of SARS-CoV-2,it will not only reduce costs and greatly decrease the detection time but also make a great contribution to the development of rapid detection tests[124].Nouriet al.designed a method for detecting SARS-CoV-2 using solid-state CRISPR-Cas12a-assisted nanopores and introduced a nanopore-sized counting method to measure particle size distribution and relative abundance,which can be quantitatively measured as a negative and positive standard.This method has strong specificity and good sensitivity for the fast and specific detection of SARS-CoV-2 [125].
With the continuous development of molecular biology,an increasing number of detection technologies have been invented,which are more suitable for modern technologies in daily life.Although the virus culture method needs time to cultivate microorganisms,the morphology and number of cells can be observed,which lays the foundation for the detection of microbial aerosols.After the outbreak of SARS-CoV-2,RT-qPCR became the gold standard for SARS-CoV-2 detection owing to its low cost,strong specificity,and high sensitivity,but the process is time consuming.There is also an increasing body of research on other methods,such as RT-LAMP and CRISPR-based detection technology [126].Some researchers have designed a CRISPR-based rapid detection method,but there are few experimental samples and thus it cannot be used for clinical detection.Although IgG and IgM antibodies do not appear until 1–3 weeks after viral infection,antibody testing has also been added to the standard for diagnosing SARSCoV-2 in the seventh edition of the New Coronavirus Diagnosis and Treatment Protocol,issued in 2020 [127,128].The production of antibodies provides great protection,and epidemiological investigations using antibodies can be rapidly performed at a low cost.Many research teams have designed a large number of kits for immunological detection based on enzyme-linked immunosorbent chromatography,colloidal gold chromatography and fluorescence immunochromatography [129–134],which have greatly reduced the detection time,do not require specialized personnel,and have reduced aerosol production.
Currently,POCT diagnosis,as a rapid detection tool,also has great applications during the prevention and control of the new crown epidemic.Because of the rapid spread of the epidemic,researchers have proposed the use of an integrated extraction and detection test.The common integrated detection technologies include lateral flow fixation technology [135],cartridge-based detection technology,and microfluidic-based detection technology [136].The contents of integrated detection technology are summarized in Supporting information.
Nowadays,hospital-acquired infections are noteworthy,and the contents of response to SARS-CoV-2 prevention and control deployment are summarized in Supporting information.
5.Discussion and outlook
The ongoing COVID-19 pandemic has affected the entire world and caused millions of deaths.Medical personnel are also becoming increasingly aware of the symptoms of SARS-CoV-2 infection.However,the RNA virus has a high rate of mutation and undergoes rapid transmission.Thus,effective suppression of SARS-CoV-2 has become a difficult problem,and it is challenging to prevent and control its spread.Therefore,protective vaccination against SARSCoV-2 is essential to halt the spread of the COVID-19 pandemic.Because early clinical intervention is particularly important for new COVID-19 patients,aerosol detection methods need to be more rapid.
The results of the study confirm that SARS-COV-2 aerosol are infectious and can survive for several hours even when exposed to air.It is therefore important to pay attention to the characteristics of aerosol transmission and its presence,and to eliminate the spread of viral aerosols at the source.For example,in public places and confined spaces with high viral loads,it is important to take personal disinfection precautions.With the spread of several variants of the new coronavirus epidemic around the world and the high level of population movement,which is undoubtedly the greatest means of transmission,we need higher levels of protection in the form of masks and protective clothing,and we need to ensure that we have sufficient protective gear to keep us safe during an epidemic.
During the rapid spread of the epidemic,digital media are used as much as possible to promote proper protective measures.The symptoms caused by the current epidemic strains are also published in a timely manner to guide the public to seek medical attention without delaying the best diagnosis period.When new mutant strains are detected,the epidemiological situation is also announced to avoid panic and adverse effects on the control of the epidemic.
Microbial aerosol detection has attracted increasing attention after the outbreak of SARS-CoV-2.The emphasis has shifted from RT-PCR to POCT,focusing on rapid detection,and researchers must pay more attention to practical aspects,such as simple operation and microbial aerosol generation.Because aerosols lead to contamination,the current demand for methods of detection of microbial aerosols is high,and an increasing number of researchers are pursuing the design of an instrument that integrates sampling and output to realize automatic real-time monitoring of patients with SARS-CoV-2 infection [137–139].Although there are some emerging technologies,they are unsuitable for clinical use.It is expected that there will be many false negatives and false positives in the test process,which will greatly impact the test results.Therefore,new technologies must be highly sensitive and specific as well as cheap and easy to operate.Currently,protective measures are the best defense against SARS-CoV-2.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported by the NSFC (Nos.61701176 and 62071119),Macao FDCT (No.0065/2020/A2),Natural Science Foundation of Hunan Province of China (Nos.2022JJ50052,2018JJ3130 and 2020JJ5145),Hunan Key R &D Projects (No.2021SK2003),Nanjing Important Science &Technology Specific Projects (No.2021-11005),2022 Special Project for the Construction of Innovative Provinces to Fight the COVID-19 Outbreak (No.2022SK2115),Open Funding of State Key Laboratory of Oral Diseases (No.SKLOD2022OF05) and Shenzhen Innovation and Entrepreneurship Program Innovation and Entrepreneurship Special Project (No.20220624181237005).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108378.
 Chinese Chemical Letters2024年1期
Chinese Chemical Letters2024年1期
- Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
