Human pluripotent stem cell-derived kidney organoids: Current progress and challenges
Hong-Yan Long,Zu-Ping Qian,Qin Lan,Yong-Jie Xu,Jing-Jing Da,Fu-Xun Yu,Yan Zha
Abstract Human pluripotent stem cell (hPSC)-derived kidney organoids share similarities with the fetal kidney.However,the current hPSC-derived kidney organoids have some limitations,including the inability to perform nephrogenesis and lack of a corticomedullary definition,uniform vascular system,and coordinated exit pathway for urinary filtrate.Therefore,further studies are required to produce hPSCderived kidney organoids that accurately mimic human kidneys to facilitate research on kidney development,regeneration,disease modeling,and drug screening.In this review,we discussed recent advances in the generation of hPSCderived kidney organoids,how these organoids contribute to the understanding of human kidney development and research in disease modeling.Additionally,the limitations,future research focus,and applications of hPSC-derived kidney organoids were highlighted.
Key Words: Kidney;Organoids;Human pluripotent stem cell;Development;Vascular system;Disease modeling
INTRODUCTION
The kidney is a complex organ consisting of over 20 cell types and is responsible for producing urine.Additionally,the kidney ensures normal body activity and metabolism through reabsorption and endocrine functions[1].According to the Global Burden of Disease Alliance,chronic kidney disease (CKD) is predicted to be one of the top five diseases causing loss of life expectancy by 2040[2].CKD is a major global health problem with increasing incidence and prevalence;it affects approximately 10% of the global population and can lead to end-stage renal failure[3].Currently,the primary treatments for kidney diseases include medications,dialysis,and kidney transplantation[4].By 2020,the number of individuals receiving renal replacement therapy exceeded 2.5 million and was expected to double to 5.4 million by 2030[5].Recently,there has been an increase in the number of people with end-stage renal disease who require dialysis or transplantation[6],presenting a serious and growing public health issue.Therefore,there is an urgent need to develop effective treatments for CKD,and pluripotent stem cells (PSCs) possess potential applications for CKD treatment due to their multi-directional differentiation potential.
PSCs are a class of cells with the potential for self-renewal and multi-directional differentiation.Organoids are a group of highly PSCs induced into a three-dimensional (3D) structure that closely mimics human organs structurally and functionally under specific differentiation conditions[7-9].Organoids are physiologically complicated systems for studying organ development,drug screening,and disease modeling because they frequently possess cell types from three germ layers and a structural organization comparable to that of human organs (Figure 1A)[7,10,11].
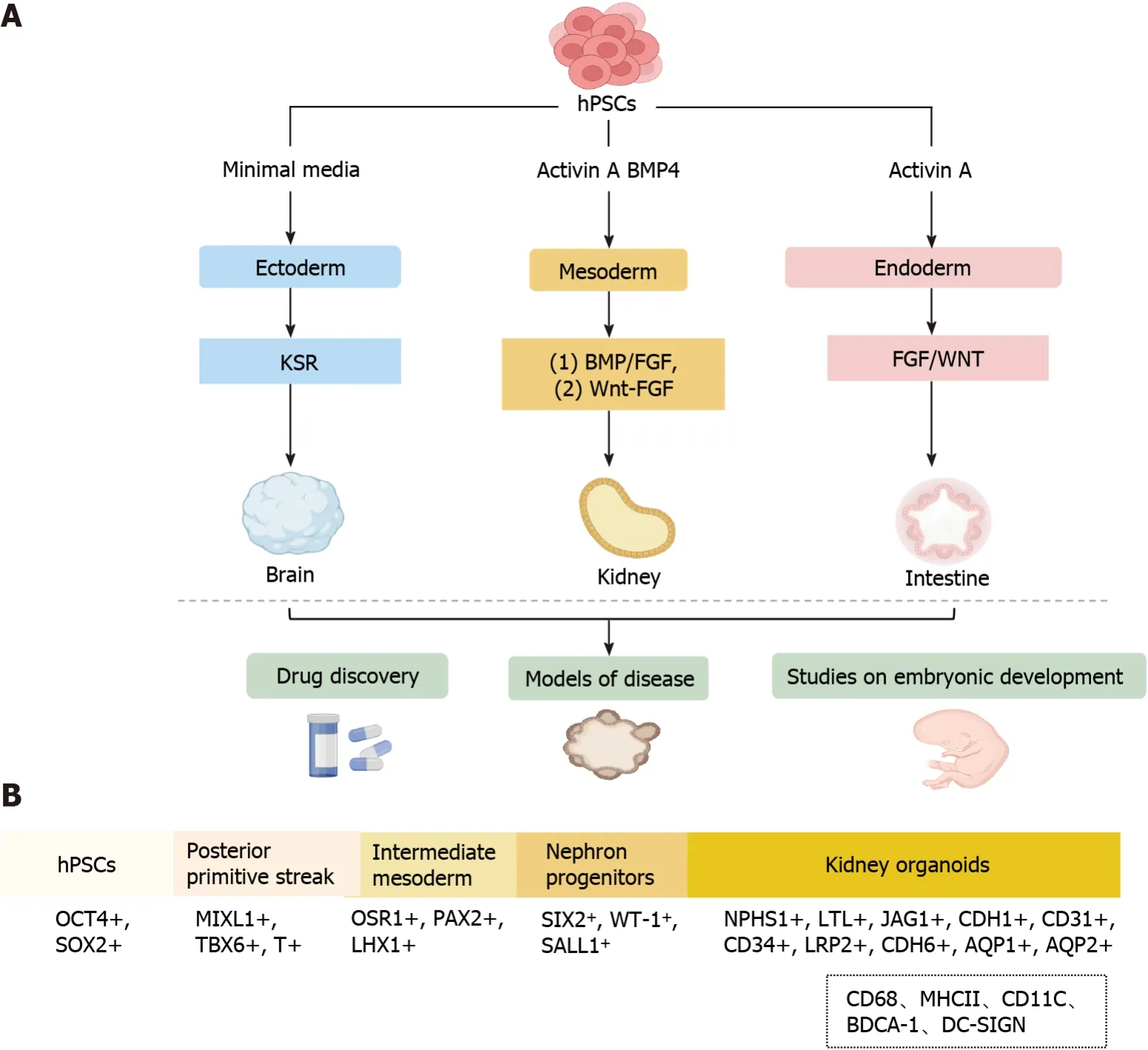
Figure 1 Overview of human pluripotent stem cell-derived organoids. A: Schematic signaling of endodermal,mesodermal,and ectodermal triodermal organoids derived from human pluripotent stem cells (hPSCs) by addition of different inducing factors;B: The process of generating hPSCs-derived kidney organoids involves the following consecutive stages: Generation of the posterior primitive streak,intermediate mesoderm,nephron progenitor cells,and kidney organoids.The dotted box shows immune cells not currently induced in kidney organoids in vitro.hPSCs: Human pluripotent stem cells;HSR: Knockout serum replacement;MHCII:Major histocompatibility complex;BDCA: Blood dendritic cell antigen;BMP: Bone morphogenetic protein;FGF: Fibroblast growth factor.
In this review,we discussed recent advances in the generation of human PSC (hPSC)-derived kidney organoids,with emphasis on the contribution of these advances to the understanding of human kidney development and research in disease modeling.Additionally,we discussed the limitations,future research focus,and applications of hPSC-derived kidney organoids;particularly,methods to produce highly complex hPSC-derived kidney organoids with vascular components were highlighted.
GENERATION OF HPSC-DERIVED KIDNEY ORGANOIDS
Since the establishment of embryonic stem and induced pluripotent stem cell lines,scientists have employed insights from developmental biology to derive differentiated cell types from these stem cells (Figure 1A),PSCs have high proliferative capacity and can differentiate into all three germ layers: Ectoderm,endoderm,and mesoderm[12-16].The process of generating kidney organoids exactly mimics the normal development conditions of the kidneyin vivo.Specifically,the generation of hPSC-derived organoids is performed using varying growth factors and culture substrates under precise timing to generate primitive streak,intermediate mesoderm (IM),renal unit progenitor cells,and kidney organoids(Figure 1B)[7,8,17-21].
During the embryonic period,the complex kidney develops from the relatively simple posterior kidney,and the embryonic kidney develops from different regions of the mesoderm.Specifically,nephron progenitor cells (NPCs)originate from the posterior IM (PIM) and differentiate into renal unit-like organs through Wnt and fibroblast growth factor (FGF) stimulation.In contrast,the ureteric bud originates from the anterior IM.FGF is a central regulator of the differentiation of the presomitic mesoderm into somatic tissue,which was the initially identified mesoderm-inducing signal[22,23].Nephron progenitors self-assemble to form multi-compartmentalized kidney organoids composed of different cellular components,including a segmentally patterned epithelium,interstitium,and endothelium,in response to the activation of FGF and/or Wnt signaling[20,21,24].FGFs are potential mesoderm inducers,while activin A,the first acting factor of transforming growth factor betas,can induce cell transition to the IM stage[25,26].However,these methods are expensive and time-consuming;therefore,recent efforts have been made to improve scalability and reduce the cost of iPSC-derived renal organoid generation.Morizane and Bonventre[27] induced the differentiation of H9-hESC and HDF-hIPSC cell lines to the primitive streak stage using a Wnt signaling pathway activator (CHIR99021) for 4 d in a planar environment,followed by sequential induction with activin A and FGF9 to bring them to the PIM stage and automatic development of PIM into MM,which further developed towards the renal lineage[27].Specifically,the cells reached the NPC stage at 9 d,as evidenced by the high expression of four NPC markers (CSIX2,WT1,CPAX2,and SALL1),and the NPCs were capable of subsequently differentiating into foot cells (NPHS1+,WT1+),proximal tubules(LTL+,APQ1+),medullary collaterals (CDH1+,AQP1+),and distal tubules (CDH1+) in the 3D environment[27].Overall,the main advantage of the protocol developed by Morizane and Bonventre[27] high (90%) efficiency of generating NPCs from H9-hESC and HDF-hIPSC cell lines.Moreover,the culture conditions were modified to allow for the formation of renal organoids in suspension culture,which increased the yield of cellular structures and reduced the overall cost,making the protocol more cost-effective than that developed by Takasatoet al[21].
Primitive streak development and subsequent mesoderm formation are highly ordered and spatiotemporally regulated by the interplay of several internal (transcription factors,epigenetic regulators,and chromatin remodeling factors) and external variables.In recent years,numerous protocols have been published on the differentiation of PSCs into kidney organoids (Table 1).Current strategies for the generation of renal organoids include the bone morphogenetic protein(BMP)/FGF pathway and the CHIR99021-FGF pathway.In the BMP/FGF pathway,hPSC differentiation into primitive streak mesoderm (PSM) is induced using BMP4/activin A,followed by stimulation of PSM with FGF9 to differentiate into IM and subsequent stimulation of IM with FGF9/BMP7/RA to differentiate into metanephric mesenchyme (MM)and ureteric bud (UB).Finally,cells in the MM and UB stages can further differentiate into organoids,including collecting ducts,proximal tubules,and foot cells.In the CHIR99021-FGF pathway,PSCs differentiation into PSM is induced using the Wnt agonist CHIR99021,followed by stimulation of PSM with FGF9 to differentiate into IM and subsequent differentiation of IM into MM and UB,both of which can differentiate into kidney organs,including collecting ducts,renal tubules,and glomeruli.
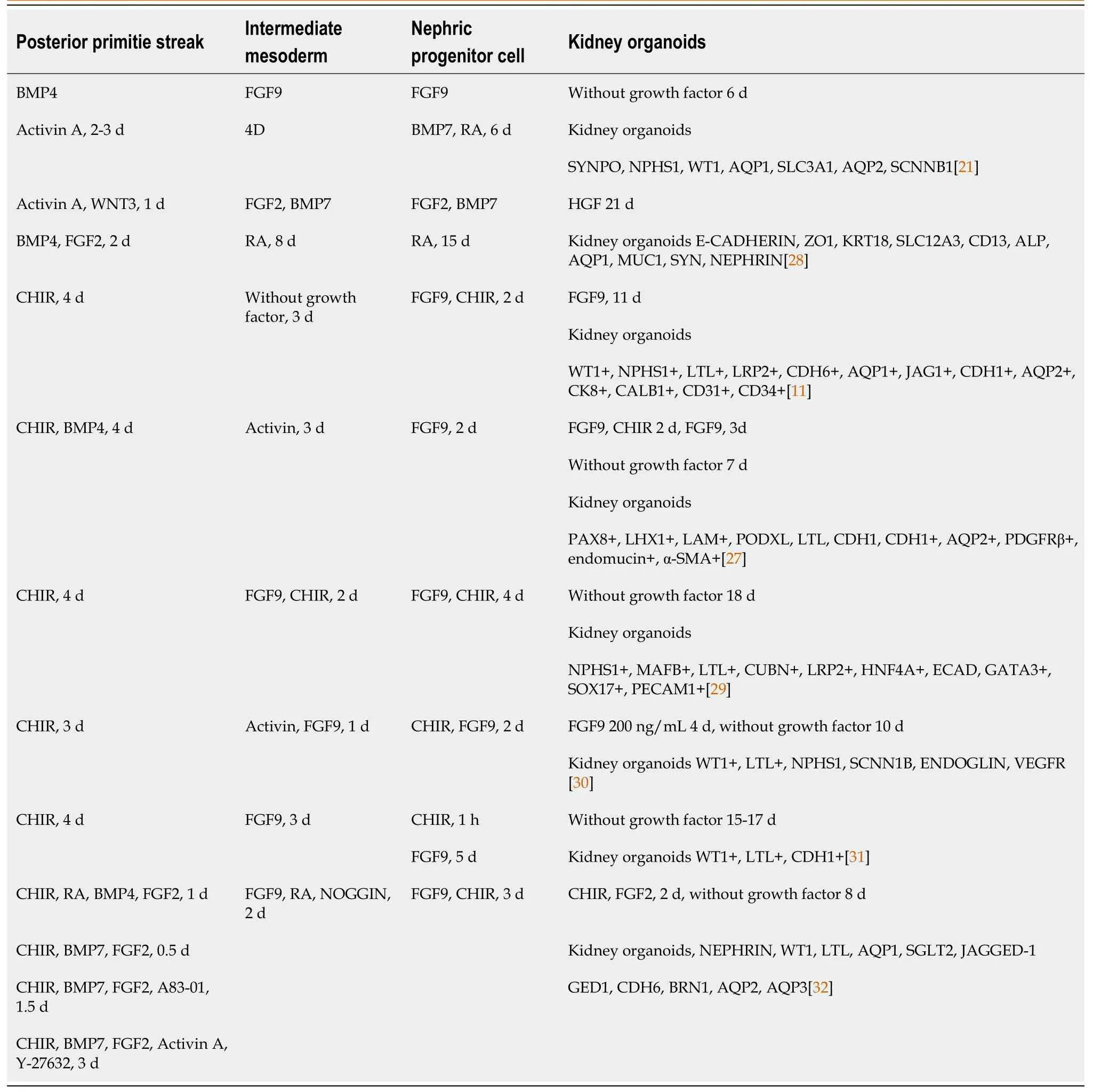
Table 1 Overview of human pluripotent stem cells derived kidney organoid model systems
Recently,the protocols for generating kidney organoids have attracted considerable attention (Table 1).However,the generated kidney organoids are incomplete and lack the complete vascular network structure,and the nephron organoids mainly contain glomeruli and proximal tubules,and protocols to effectively induce distal tubule formation are needed.Taguchiet al[33] developed a new protocol for inducing ureteric bud-like organs from PSCs,and although this class of organs has the properties of the collecting duct principal cells,the generation of intercalary cells remains a challenge.
HPSC-DERIVED KIDNEY ORGANOIDS FOR THE STUDY OF HUMAN KIDNEY DEVELOPMENT
Kidney development originates from the primary stripe of the embryo,which differentiatesviathe mesoderm into the anterior mesoderm.The anterior mesoderm develops into renal tubes and branching ureteric buds and eventually differentiates into collecting ducts.The posterior renal mesenchyme develops into renal units with renal distal progenitors and collecting duct progenitors that induce and differentiate from each other,ultimately forming a complex renal unitcollecting duct network structure[34].Kidney developmentin vivoinvolves four stages: Stage I,differentiation of PSCs into cells of the posterior primitive streak;stage II,cells of the posterior primitive streak are designated to differentiate into cells of the PIM;stage III,transition of PIM to NPCs of the posterior renal mesenchyme;and stage IV,mesenchymeto-epithelial transition from the posterior mesenchyme to the renal unit (Figure 2)[17,35].Organ development events may be studied using hPSC-derived organoids owing to their similarities with fetal tissues[36,37].Several important steps in human kidney development have been replicated in hPSC-derived kidney organoids.

Figure 2 Embryonic kidney development in vivo. The origins of metanephric mesenchyme (MM) and ureteric bud (UB) are spatially and temporally distinct(formed at E8.5).The UB is formed by the anterior intermediate mesoderm extending caudally forwards,whereas the MM is formed by the posterior intermediate mesoderm retaining its caudal tail backwards (formed at E10.5).
Recently,kidney organoids have become an important topic in renal development and regenerative medicine[38].Researchers can use kidney organs derived from hPSCs to model human kidney development and study the biological and molecular mechanisms involved.Kidney organoids are not only structurally similar to the human kidneys but also recapitulate some of the specific functions of the kidneys,including reabsorption[39].Various cell populations are generated during kidney development,which forms self-assembled tissues through signal transduction[27].Takasatoet al[36] generated kidney organoids from hESC using the CHIR99021-FGF9 induction procedure.Specifically,growth factors were withdrawn from thein vitroculture at 12-18 d,and the elongated ECAD+ureteral epithelium was surrounded by several clusters of mesenchyme (expressing the molecular hallmarks of the MM,WT1,SIX2,and PAX2) by day 18.The MM formation looked like a renal unit,expressing JAG1 and CDH6 proteins,with a visible connection between the ureteral epithelium and the renal vesicle lumen.The stepwise differentiation protocol for generating human renal tissue from PSCs includes the progression from an initial pattern to a posterior primitive streak,followed by differentiation to IM and then posterior renal mesenchyme.In the Takasato protocol for the differentiation of hPSCs,the mesoderm was induced by the addition of a classical Wnt agonist,followed by the addition of FGF9.Thereafter,the cells were placed on a Transwell filter on day 7 of culture to allow spontaneous tissue formation.A comparative analysis of the single-cell profile of human kidney-like organs and cells of the fetal renal cortex showed that the key renal cell types in the two organs were the same[36].
Notably,it is important to understand the cellular lineage relationships during nephrogenesis to better define them in the human kidney.In the past ten years,sophisticated methods have been developed to track cell fate in mice[40].Although the genealogical relationships in mouse kidney development have been extensively elucidated,this information cannot be fully applied to humans due to interspecies differences.However,genetic lineage analysis may be utilized in hPSCs to monitor the development of human progenitor cells into kidney organoids[41].Additionally,the homeodomain transcriptional regulator SIX2 is a crucial element in the kidney mesenchyme[42].SIX2+NPCs are pluripotent and can differentiate into multinodular epithelial cells,including the peduncle and wall epithelium of the glomerulus as well as the epithelium of the proximal tubule,Henle’s loop,and distal tubule.Additionally,SIX2+NPCs can self-renew in the interstitial space in the embryonic kidney[42].Howdenet al[41] investigated whether self-renewing SIX2+NPCs can give rise to renal unit cell types during human renal organ culture and found that SIX2-expressing cells can differentiate into proximal tubule renal unit segments but not into the collecting duct lineage,which is consistent with findings in mice.Collectively,these results provide proof-of-principle for tracking cell fates in human renal organoids to understand renal developmentin vivo.The origin of the renal vascular system has remained unclear in previous genetic studies on model organisms.Takasatoet al[36] showed that kidney organoids featured PDGFRA+early mesangial cells invading the glomeruli and PDGFRA+perivascular cells that lie along the KDR1 endothelia.In the growing kidney,the renal interstitium develops into pericytes and mesangial cells[43].Lowet al[11] reported an increased percentage of vascular progenitor cells,including SIX1+/KDR+/PECAM1-cells,in renal organoids by analyzing the transcriptomes of 62506 single cells on days 10,12,and 14 of renal differentiation,with the NPC cluster possessing the highest percentage of SIX1+/KDR+/PECAM1-cells.Additionally,there was an increase in the percentage of SIX1+/KDR+/PECAM1+and SIX1+/KDR-/PECAM1+mature endothelial cells.These results indicate that NPCs are an unconventional source of renal vasculature,which is consistent with the sequencing data of fetal kidney samples obtained during mid-pregnancy[44].Additionally,single-cell analysis of vascular endothelial cell subclusters revealed that the vascular endothelial cells of a kidney-like organ form a well-established genetic network and could specifically generate different endothelial subtype structures,such as arterioles and venules.
The establishment of these kidney organoid models has provided novel insights into the process of renal development and may advance the study of renal physiology and pathology.Kidney organoids are used to evaluate developmental and genetic diseases,and despite these promising advances in currently developed protocols,challenges remain.Under physiological conditions,the intrarenal immune system consists mainly of macrophages (CD68,major histocompatibility complex II) and dendritic cells (CD11C,blood dendritic cell antigen 1,DC-SIGN) (Figure 1B)[45,46].Immune cells have been detected in human tissues,including the kidney,but almost all currentin vitroorganoid models lack immune cells,although protocols for co-culturing organoids with immune cells have now been developed[47,48],and these protocols provide a new platform for studying complex epithelial-immune cell crosstalk.However,due to the relative novelty of kidney organoids,co-culture with immune cells has not yet been established,and in the future,this could potentially be a new way for kidney organoids to generate immune cells.
HPSC-DERIVED KIDNEY ORGANOIDS FOR HUMAN KIDNEY DISEASE MODELING
Organoids are superior to 2D cell cultures and animal models forin vitroexperiments (Figure 3),and may provide comprehensive insights into development,homeostasis,and pathogenesis.For example,more than 300 genes associated with childhood kidney disease[49] are not present in the mouse genome,and human pathology genes are differentially expressed in mice[50,51].Targeted differentiation of hPSCs into kidney organoids,combined with single-cell RNA sequencing technology can be used to explore disease mechanisms and identify novel directions for disease modeling[7,52,53].Additionally,the use of CRISPR/Cas9 to edit hPSCs,introduce specific mutations,and compare them with samples from homogeneous genetic backgrounds is another approach for studying inherited kidney diseases[54,55].
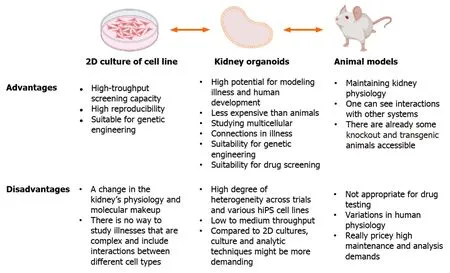
Figure 3 The benefits and drawbacks of using kidney organoids to simulate human illness are underlined. 2D: Two-dimensional;hiPS: Human induced pluripotent stem.
Polycystic kidney disease
Polycystic kidney disease (PKD) is one of the most prevalent monogenic kidney diseases worldwide and is classified into two types: Autosomal dominant (ADPKD) and autosomal recessive (ARPKD)[56,57].ADPKD is frequently caused by defects in polycystin-1 (PC1) and PC2,which are encoded by PKD1 and PKD2,respectively[58-60].Freedmanet al[7]knocked down the PKD1 and PKD2 genes in human-induced PSCs (hiPSCs) using CRISPR/Cas9 genome editing technology and utilized these cells to generate kidney organoids,providing new insights into the role of the microenvironment in the PKD process.PKD cysts were visible approximately 35 d after the first plating,and continued to grow during the culture period;moreover,the cysts showed a significant affinity forLotus tetragonolobuslectin.Importantly,isogenic control hPSCs that were plated and differentiated alongside the edited cells did not develop cysts under these circumstances[7],successfully validating the PKD model for organoid culture.In subsequent studies with improved culture conditions,suspension-cultured organoids promoted the formation of cysts (up to 75% of organoids developed as cysts) on day 21 of differentiation compared with those cultured under apposed conditions.Under these circumstances,control organoids with the same genetic background seldom developed cysts,demonstrating that cystogenesis is a unique result of PKD mutations[61].In a recent study,gene-edited kidney organoids were used to screen potential therapeutic agents (247 enzyme inhibitors) for polycystic kidneys,and it was observed that nine compounds inhibited cyst growth without hindering the overall growth of the organoids[60].The establishment of these disease models has improved the understanding of complex molecular and cellular events underlying the pathogenesis of PKD,thus helping to identify new targets for disease regulation and therapy[62].
Fabry nephropathy
A rare X-linked hereditary ailment called Fabry disease results in abnormalities in the glycosphingolipid metabolic pathway owing to the absence or low activity of the lysosomal enzyme α-galactosidase A (α-Gal A)[63].Cell morphology and function are impaired by the accumulation of globular triacyl ceramide (Gb3) and related neutral sphingolipids in lysosomes due to α-Gal A[63,64].Gb3 accumulates in renal cells,such as podocytes,glomerular endothelial cells,mesangial cells,tubular epithelial cells,and vascular endothelial cells,resulting in Fabry nephropathy[65].Fabry nephropathy is characterized by microalbuminuria and proteinuria.As the disease progresses,renal function deteriorates leading to end stage renal disease,which is the leading cause of Fabry nephropathy-related deaths[66].Animal experiments are not suitable for large-scale screening because animals are not genetically identical to humans.To overcome this limitation and develop new therapeutic strategies,researchers have generated GLA-knockout (KO) kidney organoids by knocking down GLA in hiPSCs using CRISPR/Cas9-mediated gene editing,followed by differentiation to generate the organoids (GLA-KO hiPSC kidney-like organs).Research evidence suggests that GLA-KO kidney organoids are a valuable tool for simulating human Fabry disease.GLA-KO kidney organoids that closely mimic human renal Fabry disease can facilitate the development of innovative treatment alternatives[67].
Congenital nephrotic syndrome
Most cases of congenital nephrotic syndrome (CNS) are caused by mutations in the NPHS1 (NEPHRIN) and NPHS2(PODOCIN) genes[68],which encode important podocyte proteins.Podocytes in the glomeruli of kidneys maintain the filtration barrier by creating interdigitating foot processes with intervening slit diaphragms,the disruption of which causes proteinuria.Patient-derived organoids have been employed to understand hereditary illnesses that affect the glomerulus[69].Specifically,patient cell lines have been used to develop 3D organoid-derived human glomeruli(OrgGloms) to simulate CNS.Expectedly,there was a decrease in NEPHRIN protein in OrgGloms,supporting the hypothesized degradation of the shortened protein encoded by the exon 10 NPHS1 variation.NEPHRIN interacts intracellularly with the adaptor protein PODOCIN,facilitating PODOCIN signaling activity and appropriate localization.Additionally,PODOCIN and NEPHRIN proteins were downregulated in CNS OrgGloms generated from patients with CNS[69].Overall,these findings demonstrate that the use of human kidney organoids may provide new perspectives on the cellular causes of glomerular disease.
Tuberous sclerosis complex
Tuberous sclerosis complex (TSC) is a multisystem tumor-forming disease caused by the absence of TSC1 or TSC2[70,71].Renal manifestations of TSC include mainly cysts and angiomyolipoma[72].However,the cellular pathogenesis of TSC remains unknown despite the well-described single-gene etiology[73].Pietrobonet al[74] utilized a genetically engineered human kidney organoid model that encapsulated the pleiotropic features of TSC kidney disease inin vitroandin situxenografts,and temporal single-cell RNA sequencing indicated that deletion of TSC1 or TSC2 affects multiple developmental processes in stromal,nephron (epithelial) and neural-glial cells.Deletion of TSC1 or TSC2 results in early upregulation of matrix-associated genes;additionally,epithelial cells in TSC1-/-and TSC2-/-organoids exhibit an epithelial-tomesenchymal transition that is insensitive to rapamycin.Moreover,melanocytic differentiation from MITF+SCPs was induced by the loss of TSC1 or TSC2.Conclusively,these results illustrate the pleiotropic developmental consequences of TSC1 or TSC2 double-allele inactivation and provide insights into the pathogenesis of TSC[74].
Nephronophthisis
Nephronophthisis (NPHP) is an autosomal recessive interstitial nephritis that manifests as tubular basement membrane damage,tubular dilatation,and interstitial fibrosis,and its pathogenesis is unclear[75].Forbeset al[76] performed wholeexome sequencing of NPHP1 and its core family line and identified compound heterozygous mutations in the IFT140 gene.Predecessor mutant iPSCs have been generated using reprogramming and gene editing and were induced to form renal organoids.The organoid tubules from the proband had primary cilia that were truncated and shaped like clubs;however,gene repair reversed this feature.Additionally,epithelial cells collected from organoids and subjected to differential expression analysis revealed the downregulation of genes related to apicobasal polarity,cell-cell junctions,and dynein motor assembly.Polarization deficiency was verified in the proband using Matrigel cyst cultures.Tanigawaet al[53] showed that gene repair could restore the deficit in slit diaphragm development in NPHS1 mutant podocytes using a PSC line from a patient with an NPHS1 missense mutation.
Kidney organoids as models for other disease
In addition to the aforementioned disease models,several hPSC-derived kidney-like organs have been used as disease models in recent years,including BRD4780,a small molecule that clears MUC1-fs from patient cells,knock-in mouse kidneys,and renal organoids.BRD4780 is promising for the treatment of MKD and other toxic protein diseases[77].Jansenet al[78] assessed the effects of severe acute respiratory syndrome coronavirus 2 on human kidneys using kidney organoids and found that coronavirus disease 2019-induced kidney damage and fibrosis were related to increased collagen-I expression and accumulation.Additionally,Guptaet al[79] used hPSC-derived kidney organoids to simulate the transition from intrinsic to incomplete repair of acute kidney injury.A single exposure to cisplatin resulted in intrinsic repair,preservation of tubular structure,and upregulation of genes associated with homologous-directed repair.Targeted drug screening was used to identify the DNA ligase IV inhibitor SCR7,which increases FANCD2-mediated repair and delays the progression of chronic injury in organoids.Conclusively,these results suggest that targeting the FANCD2/RAD51 pathway may have therapeutic potential for kidney disease,and highlight the important role of kidney organoids in exploring potential treatments.
However,the currently investigated protocols can only produce immature kidney organoids.Therefore,since only diseases that exhibit abnormalities early in embryonic development can be established by kidney organoids,more mature organoids will need to be developed in the future to be able to model and study late-onset diseases.
CONCLUSION
Despite several remarkable progress in the development of kidney organoids,there are still several challenges.Current challenges hindering the development of kidney organoids and their future applications include immature tissue development,scanty blood vessel formation,inability to form effective collecting tubes,and the emergence of several non-kidney cells (e.g.,myocytes and neurons) during the process of induced differentiation.More detailed analyses of the developing kidney at the individual cell level are required.Ultimately,organotypic renal structures need to be equipped with renal elements,collecting ducts,ureters,interstitia,and vascular flow in order to generate transplantable kidneys.However,recent studies have made important breakthroughs in addressing these issues.Regarding vascularization,planting the renal organoid under the peritoneum of thein situkidney generates abundant blood vessels and matures.
Generally,research on kidney organoids is continuously progressing and better results are expected in the future.Addressing the above limitations would facilitate the development of functional renal organoids and possible artificial kidneys for patients in need of transplants to treat CKD.However,extensive studies are still necessary to address these issues.
ACKNOWLEDGEMENTS
We thank Dr.Yong Chen,Central Laboratory of Guizhou Provincial People’s Hospital,for his guidance on the manuscript.
FOOTNOTES
Author contributions:All authors listed have significantly contributed to the development and the writing of this article.
Supported bythe National Natural Science Foundation of China,No.82 360148;and Guizhou Science &Technology Department,No.QKHPTRC2018-5636-2 and No.QKHPTRC2020-2201.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Hong-Yan Long 0009-0009-2291-2741;Yan Zha 0000-0002-8713-1665.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Yuan YY
 World Journal of Stem Cells2024年2期
World Journal of Stem Cells2024年2期
- World Journal of Stem Cells的其它文章
- Multiple pretreatments can effectively improve the functionality of mesenchymal stem cells
- Cellular preconditioning and mesenchymal stem cell ferroptosis
- Therapeutic utility of human umbilical cord-derived mesenchymal stem cells-based approaches in pulmonary diseases: Recent advancements and prospects
- Unlocking the versatile potential: Adipose-derived mesenchymal stem cells in ocular surface reconstruction and oculoplastics
- Crosstalk between Wnt and bone morphogenetic protein signaling during osteogenic differentiation
- Recent progress in hair follicle stem cell markers and their regulatory roles
