Porcine enteric alphacoronavirus infection increases lipid droplet accumulation to facilitate the virus replication
Qi Gao ,Yongzhi Feng ,Ting Gong ,Dongdong Wu,Xiaoyu Zheng,Yizhuo LuoYunlong YangZebu Song,Lang Gong#,Guihong Zhang#
1 Guangdong Provincial Key Laboratory of Zoonosis Prevention and Control, College of Veterinary Medicine, South China Agricultural University, Guangzhou 510642, China
2 Maoming Branch, Guangdong Laboratory for Lingnan Modern Agriculture, Maoming 525000, China
3 Key Laboratory of Animal Vaccine Development, Ministry of Agriculture and Rural Affairs, Guangzhou 510000, China
4 Guangdong Laboratory for Lingnan Modern Agriculture, Guangzhou 510642, China
Abstract Coronaviruses are widely transmissible between humans and animals,causing diseases of varying severity. Porcine enteric alphacoronavirus (PEAV) is a newly-discovered pathogenic porcine enteric coronavirus in recent years,which causes watery diarrhea in newborn piglets. The host inflammatory responses to PEAV and its metabolic regulation mechanisms remain unclear,and no antiviral studies have been reported. Therefore,we investigated the pathogenic mechanism and antiviral drugs of PEAV. The transcriptomic analysis of PEAV-infected host cells revealed that PEAV could upregulate lipid metabolism pathways. In lipid metabolism,steady-state energy processes,which can be mediated by lipid droplets (LDs),are the main functions of organelles. LDs are also important in viral infection and inflammation.In infected cells,PEAV increased LD accumulation,upregulated NF-κB signaling,promoted the production of the inflammatory cytokines IL-1β and IL-8,and induced cell death. Inhibiting LD accumulation with a DGAT-1 inhibitor significantly inhibited PEAV replication,downregulated the NF-κB signaling pathway,reduced the production of IL-1β and IL-8,and inhibited cell death. The NF-κB signaling pathway inhibitor BAY11-7082 significantly inhibited LD accumulation and PEAV replication. Metformin hydrochloride also exerted anti-PEAV effects and significantly inhibited LD accumulation,downregulated the NF-κB signaling pathway,reduced the production of IL-1β and IL-8,and inhibited cell death. LD accumulation in the lipid metabolism pathway therefore plays an important role in the replication and pathogenesis of PEAV,and metformin hydrochloride inhibits LD accumulation and the inflammatory response to exert anti-PEAV activity and reducing pathological injury. These findings contribute new targets for developing treatments for PEAV infections.
Keywords: porcine enteric alphacoronavirus,NF-κB inflammatory pathway,lipid droplet,metformin hydrochloride
1.Introduction
Porcine enteric alphacoronavirus (PEAV) belongs to the Coronavirinae subfamily in the Coronaviridae family and Nidovirales order (Zhang 2016). The clinical symptoms of PEAV include vomiting,diarrhea,and dehydration,and the mortality rate following PEAV infection is about 90% (Gonget al.2017;Zhouet al.2018). PEAV can cause different degrees of pathological damage to the respiratory tract,liver,intestines,and nervous system in pigs (Weiss and Navas-Martin 2005;Wooet al.2006;Lauet al.2007;Gonget al.2017). However,there are no effective vaccines or drugs to prevent and treat PEAV infections. Therefore,there is an urgent need to develop novel anti-PEAV drugs that can target viral or host factors.
Lipid droplets (LDs) are involved in lipid metabolism and energy homeostasis. When nutrients are scarce,LDs store neutral lipids as an energy source (Boschet al.2020;Yan and Hong 2020). Sterol O-acyltransferase (SOAT1)and diacylglycerol acyltransferase 1 (DGAT-1/2) catalyze fatty acid and cholesterol levels in cells and are then converted to triglycerides and cholesteryl esters for storage in LDs,respectively. LDs not only play an important role in lipid metabolism,but also participate in physiological processes,such as cell signal transduction,autophagy,the inflammatory response,and viral replication. LDs can obtain energy and provide an assembly platform for viral replication (Olzmann and Carvalho 2019). When foreign microbes stimulate the host,lipids activate Tolllike receptors,thereby activating the nuclear factorkappa B (NF-κB) signaling pathway and upregulating the expression of inflammatory factors,such as tumor necrosis factor (TNF)-α,interleukin (IL)-1α,IL-1β,and IL-8 (Adachiet al.1998;Kawaiet al.1999;Pålsson-Mcdermott and O’Neill 2004;Luet al.2008;Miuraet al.2010;Gizziet al.2018). Studies have found that SARS-CoV-2 stresses the metabolic lipid synthesis and uptake pathways in hosts,thereby causing the accumulation of LDs and creating a lipid-rich intracellular environment conducive to the proliferation of SARS-CoV-2 and secretion of inflammatory factors (Diaset al.2020). Therefore,therapeutic strategies that target LD biosynthesis,distribution,transport,and metabolism may be effective in inhibiting LD accumulation.This approach may also provide new avenues for future antiviral research.
The NF-κB signaling pathway is widely regarded as a classical pro-inflammatory transduction pathway and can regulate many genes involved in immunity,inflammation,and cell survival (Lawrenceet al.2009). The expression of various pro-inflammatory cytokines,such as IL-1,IL-6,IL-8,interferon-β,and TNF-α,is affected by the regulation of the NF-κB signaling pathway (Beg and Baltimore 1996). Inflammation is a complex physiological process,and the inflammatory response is characterized by the regulation of the secretion of pro-inflammatory and antiinflammatory factors and the activity of various signaling pathways (Beg and Baltimore 1996;Dejardinet al.2002).Recently,the use of old drugs has become a hot topic in antiviral drug research. The 2019 outbreak of the highly contagious SARS-CoV-2 caused widespread global concern. Metformin hydrochloride was reported to reduce heart failure in patients with SARS-CoV-2 through antiinflammatory effects by inhibiting the NF-κB signaling pathway and downregulating the expression of inflammatory factors (Xianet al.2021). In this study,LD inhibitor DGAT-1 inhibitor was found to inhibit PEAV replication and the host NF-κB signaling pathway,downregulate the production of the inflammatory cytokines IL-1β and IL-8,and reduce cell death. The antiviral mechanisms of metformin hydrochloride in PEAV infection were further investigated. The study found that BAY11-7082 and metformin hydrochloride reduced LD accumulation by inhibiting the NF-κB signaling pathway,and the replication of PEAV and production of the inflammatory cytokines IL-1β and IL-8 were inhibited,and cell death was reduced. The above research provides new targets for further research on the prevention and control of PEAV infections.
2.Materials and methods
2.1.Cell culture,virus and reagents
Vero E6 and IPI-FX cells were grown in Dulbecco’s modified Eagle’s medium (C11995500BT;DMEM;Gibco,Waltham,MA,USA) supplemented with 10% fetal bovine serum (10099141C;FBS;Gibco,Waltham,MA,USA) at 37°C with 5% CO2. PEAV strain GDS04,a gift from Yongchang Cao of Sun Yat-sen University,China,was propagated in medium containing 0.3% tryptone phosphate broth (TPB) and 10 mg mL–1trypsin (Gibco)in Vero cells. And it was preserved at the Infectious Diseases Laboratory of South China Agricultural University. BAY11-7082 (HY-13453,MCE,Shanghai,China),the DGAT-1 inhibitor (HY-50670,MCE,Shanghai,China),and metformin hydrochloride (HY-17471A,MCE;Shanghai,China) were prepared as stock solutions of 100 mmol L–1in 100% DMSO (D8371,Solarbio,Beijing,China).
2.2.Antibodies
The α-Tubulin rabbit polyclonal antibody was purchased from Beyotime (AF5012,Shanghai,China). Rabbit monoclonal antibodies against NF-κB p65 (4764),phospho-NF-κB p65 (3033),IκBα (4812),phospho-IκBα (2859),and MyD88 (4283) were purchased from Cell Signaling Technology (Danvers,MA,USA). IRDye®800CW goat anti-rabbit IgG (926-32211) and goat antimouse IgG antibodies (highly cross-adsorbed) (925-32210) were purchased from LI-COR Biosciences(Lincoln,NE,USA). The PEAV N protein antibody was a murine monoclonal antibody that was prepared in our laboratory and used in both the Western blot and indirect immunofluorescence assay (IFA) experiments.
2.3.Viral inoculation of the Vero E6 and IPI-FX cell lines
The Vero E6 and IPI-FX cells were infected with PEAV at a multiplicity of infection (MOI) of 0.1 and incubated for 6,12,24,36,and 48 h at 37°C with 5% CO2. Following incubation for 1 h at 37°C with 5% CO2,the culture medium was discarded,and the cells were washed twice with phosphate-buffered saline (PBS) and incubated at 37°C with 5% CO2. The cell culture supernatants or cells were collected and stored at–80°C and analyzed to detect extracellular and intracellular virions. Viral genome copy numbers were analyzed using a quantitative realtime PCR (RT-qPCR). Viral N protein expression was analyzed using Western blotting and IFA.
2.4.RNA isolation,cDNA library preparation,and sequencing
The Vero E6 cells were infected with PEAV (0.1 MOI),incubated for 24 h at 37°C with 5% CO2,and harvested from the T75 flasks. Total RNA was extracted from the cells using RNAiso Plus (9180,TaKaRa,Kyoto,Japan)according to the manufacturer’s instructions. RNA quantity and purity were assessed using a Thermo NanoDrop Lite spectrophotometer (ND-LITE,Thermo Fisher Scientific,Waltham,MA,USA)). The samples were sent to Novogene(Beijing,China) for the removal of ribosomal RNA and construction of strand-specific libraries. First,the ribosomal RNA was removed from the total RNA,which was then fragmented into 250–300 bp sections. The fragmented RNA was used as a template,and a random oligonucleotide was used as a primer to synthesize the first strand of cDNA. RNase H was used to synthesize the first strand of cDNA. The RNA strand was degraded,and the second strand of cDNA was synthesized using dNTPs (dUTP,dATP,dGTP,and dCTP) as raw materials in the DNA polymerase I system. The purified double-stranded cDNA was end-repaired,A-tailed,and connected to sequencing adapters. AMPure XP beads were used to screen cDNAs of approximately 200 bp. The U-containing second strand of cDNA was degraded using the USER enzyme,and PCR amplification was performed to obtain a library.
2.5.RNA-seq data analysis
The standard RNA-sequencing (RNA-seq) analysis process mainly includes quality control,alignment,splicing,screening,quantification,a differential significance analysis,and functional enrichment. The most important results of an RNA-seq analysis are the levels of significance of the differences in gene expression. Statistical methods were used to compare the differential gene expression under two or more conditions,identify differential genes associated with certain conditions,and further analyze the biology and significance of these differentially-expressed genes(DEGs). Unigenes with a fold change>2 and Q value≤0.05 were considered significantly differentially expressed.The DEGs were analyzed using the Metascape analysis system (https://metascape.org/gp/index.html) and Kyoto Encyclopedia of Genes and Genome (KEGG). Functional annotation and pathway analyses of the DEGs were performed using the KEGG database. Enriched ontology clustering was performed using Metascape (http://metascape.org/gp/index.html#/main/step1) to explore the possible shared functional and mechanistic associations and pathways between the identified genes (Liet al.2020;Yuet al.2022).
2.6.Lipid droplet staining
Vero E6 cells were seeded onto coverslips. The infected cells were fixed with 4% formaldehyde. LDs were stained with 0.3% Oil Red O (diluted in 60% isopropanol) for 2 min at 25°C. The coverslips were mounted onto slides using antifade mounting medium (VECTASHIELD®).Nuclear recognition was assessed using 4´,6-diamino-2-phenylindole (DAPI) staining (1 μg mL–1) for 5 min.Fluorescence was analyzed using a confocal scanning microscope (Olympus,Tokyo,Japan).
2.7.Cell viability assay
Vero E6 and IPI-FX cells were seeded in 96-well plates.The cells were supplemented with maintenance medium and different concentrations of BAY11-7082,the DGAT-1 inhibitor,and metformin hydrochloride and incubated at 37°C for 48 h. DMSO was used as a control (vehicle). CCK-8 solution (C0037,10 μL;Beyotime,Shanghai,China) was then added,and incubation was continued for 1 h. After incubation,the absorbance was read at 450 nm using an enzyme-labeled instrument,and cell viability was calculated.
2.8.lmmunofluorescence staining
The Vero E6 cells infected with PEAV (0.1 MOI) were seeded on a 24-well plate. After 24 h of being infected,the cells were washed five times with PBS (1 mL each time),fixed in 500 μL of 3.7% paraformaldehyde for 30 min at 25°C,permeabilized in 1 mL of 0.1% (w/v) Triton X-100 for 20 min at 25°C,washing with PBS three times for 5 min each time,and incubated with the PEAV N protein monoclonal antibody,which had previously been diluted with 2% bovine serum albumin (BSA) at a ratio of 1:500,and incubated in the dark with a secondary antibody diluted with 2% BSA (1:200) for 1 h at 37°C in a humid chamber. The nuclei were then stained with DAPI at 25°C for 5 min and washed three times with PBS. Cellular fluorescence was observed under an immunofluorescence microscope (Nikon,Tokyo,Japan).
2.9.Western blotting
For the Western blot analysis,the cells were lysed in RIPA buffer (P0013B,Beyotime,Shanghai,China) and denatured by adding 4× Laemmli sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) buffer(containing DL-dithiothreitol),followed by heating for 15 min at 100°C. The proteins were then separated on SDSPAGE gels and transferred onto nitrocellulose membranes using a Trans-Blot Turbo Rapid Transfer System (Bio-Rad,Hercules,CA,USA) according to the manufacturer’s instructions. The membranes were blocked with 5%defatted milk (dissolved in Tris-buffered saline (TBS))for 1 h at 37°C,washing with PBS three times for 5 min each time,and then incubated with a primary antibody for 1 h at 25°C or 12 h at 4°C. The membranes were then washed three times (5 min per wash) using wash buffer(TBS containing 0.1% Tween-20) and incubated with an IRDye®800CW secondary antibody for 1 h at 37°C.The membranes were washed thrice in wash buffer and imaged using an Odyssey Imaging System (926-73000,LI-COR,Nebraska,USA) to visualize the protein bands.α-Tubulin was used as a loading control.
2.10.Real-time quantitative PCR analysis
PEAV genomic RNA was extracted from the cell supernatants or cells using the AxyPrep™ Body Fluid Viral DNA/RNA Kit. The RNA was reverse-transcribed into cDNA using the HiScript II 1st Strand cDNA Synthesis Kit (+gDNA wiper) (R212-02;Vazyme,China). The cDNA (1 μL) was used for a real-time PCR assay using AceQ Universal U+Probe Master Mix V2 (Q513-02,Vazyme,Nanjing,China). The relative quantity of viral cDNA was determined using N-gene primers and a probe experiment. Total RNA was isolated using RNAiso Plus(9108;TaKaRa). Another 1 μL of the cDNA was used for a real-time PCR assay using the ChamQ Universal SYBR qPCR Master Mix (Q711-02;Vazyme,China).The relative quantity of cellular RNA was determined by performing a comparative Ct (ΔΔCt) experiment using GAPDH as an endogenous control. The qPCR assays were performed on a Bio-Rad CFX96 real-time PCR machine (Bio-Rad,Hercules,CA,USA) according to the manufacturer’s instructions. The gene-specific primer and probe sequences are listed in Table 1.

Table 1 Primer and probe sequences used for RT-qPCR
2.11.Flow cytometry
A propidium iodide (PI)/RNase staining buffer kit (550825,BD,New York,USA) was used to stain the Vero E6 cells infected with PEAV that were treated with the DGAT-1 inhibitor or metformin hydrochloride. The fluorescence of the cells was detected using flow cytometry. PI was detected in the orange range of the spectrum using a 562–588 nm band pass filter.
2.12.Statistical analysis
The results are presented as the mean±standard deviation (SD) of at least three independent experiments.The collected data were analyzed using GraphPad Prism version 8 (GraphPad Software,La Jolla,CA,USA). The data were compared using Student’st-tests,with*P<0.05,**P<0.01,and***P<0.001 indicating statistically-significant differences.
3.Results
3.1.PEAV infection upregulates the lipid metabolism pathway
RNA viruses,such as SARS-CoV-2 can regulate cellular lipid metabolic levels and contribute to their replication,and demonstrate the essential role of lipid metabolic reprograming and LD formation in SARSCoV-2 replication and pathogenesis (Diaset al.2020).Therefore,the purpose of this study was to explore whether PEAV,which also belongs to coronaviruses,can also activate lipid metabolic pathways. First,we investigated the transcriptomic changes after PEAVinfection with Vero E6 cells. Our results showed that 15,720 DEGs were shared between the PEAVinfected and uninfected groups after 24 h,which may be indicative of their alterations or role in the pathogenesis of the disease. These DEGs may therefore be potential diagnostic biomarkers of the disease or contribute to its development. In total,9,459 DEGs were up-regulated and 6,261 DEGs were down-regulated. The logFC,q value,andP-value of the DEGs are presented in Appendix A. To understand the pathways in which most of the 9,459 up-regulated DEGs are involved,the genes were uploaded to Metascape. Most of the DEGs were involved in herpes simplex virus 1 infections,signaling pathways that regulate the pluripotency of stem cells,cancer pathways,transcriptional misregulation in cancer,the MAPK signaling pathway,TNF signaling pathway,FoxO signaling pathway,circadian rhythm,axon guidance,autophagy-animal,p53 signaling pathway,PI3K-Akt signaling pathway,lipid and atherosclerosis,parathyroid hormone synthesis,secretion and action,microRNAs in cancer,apoptosis,JAK-STAT signaling pathway,renal cell carcinoma,acute myeloid leukemia,and longevity regulating pathway (Fig.1-A). The subset of representative terms of the functional analysis of the genes was converted into a network layout in Metascape,as shown in Fig.1-B. The significant terms from the gene function analysis were hierarchically clustered into a tree based on kappa statistics similarities. Each term was represented by a circular node,where their sizes were proportional to the number of input genes corresponding with that term. The colors represented the cluster identities;the statistically-significant range of the nodes were marked by the color range;and the terms with a kappa score>0.3 were linked by an edge.These results suggest that PEAV can up-regulate the host lipid metabolic pathway.

Fig. 1 Differentially-expressed genes (DEGs) and the KEGG pathway enrichment analysis. A,functional annotation of the DEGs using Metascape. The top-20 terms are displayed in a bar chart based on the P-values (log10 scale). B,network plot of the DEGs using Metascape.
3.2.PEAV infections increase LD accumulation in lipid metabolism pathways
Viruses can regulate cellular metabolic levels and contribute to their replication. RNA viruses,such as SARS-CoV-2 (Diaset al.2020),dengue virus (DENV)(Carvalhoet al.2012;Samsaet al.2020),hepatitis C virus (HCV) (Boulantet al.2007;Lynet al.2013;Leeet al.2019),and reovirus (Coffeyet al.2006),have been reported to regulate lipid metabolism pathways and trigger LD formation,thereby providing sites and energy for viral replication. The accumulation of the LDs was increased in the Vero E6 cells infected with PEAV for 24 h,as observed by transmission electron and confocal microscopy (Fig.2-A–C). Moreover,transmission electron microscope revealed that there were PEAV virions in the LDs,and confocal microscopy revealed that the PEAV co-localized with the LDs (Fig.2-D). These results suggest that LDs may provide energy and sites for PEAV replication.
3.3.Inhibition of LD accumulation inhibits PEAV replication
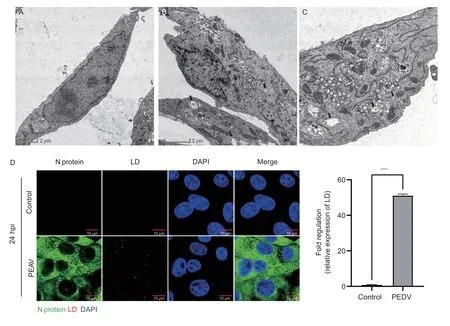
Fig. 2 The replication of porcine enteric alphacoronavirus (PEAV) is associated with lipid droplet (LD) accumulation in Vero E6 cells. A,B and C,transmission electron microscopy-based ultrastructural analyses of uninfected Vero E6 cells and Vero E6 cells 24 h post-infection (hpi) with PEAV (MOI 0.1). Arrows mean LDs. D,LDs and the PEAV N protein were imaged using fluorescent microscopy.Red,oil red O staining;green,PEAV N protein;blue,nuclei were stained with DAPI. Each datum represents the results of three independent experiments (mean±SD,n=3). Data are compared using Student’s t-tests,with ****P<0.0001 indicating statistically significant differences.

Fig. 3 The DGAT-1 inhibitor can inhibit LD accumulation and porcine enteric alphacoronavirus (PEAV) replication. A and B,the activity of Vero E6 and IPI-FX cells were detected using a CCK8 cell activity detection kit after treatment with the DGAT-1 inhibitor at concentrations of 10,25,50,100,and 200 μmol L–1 for 48 h. C,the LDs were imaged using fluorescence microscopy. Red,oil red O staining;green,PEAV N protein;blue,nuclei were stained with DAPI. D and E,after the Vero E6 and IPI-FX cells had been treated with 80 μmol L–1 DGAT-1 inhibitor for 3,6,9,12,18,24,and 36 h,the CT value of the PEAV N gene was detected by RT-qPCR. F and G,Vero E6 and IPI-FX cells were treated with 50 μmol L–1 DGAT-1 inhibitor for 6,12,24,36,and 48 h,and the expression level of the PEAV N protein was detected by Western blotting. H,Vero E6 cells were treated with 25 μmol L–1 DGAT-1 inhibitor for 24 h,and the expression level of the PEAV N protein was detected by an IFA. The expression of tubulin was used as a positive control. I,DGAT-1 inhibitor anti-PEAV replication ability in Vero E6 cells determined with the TCID50 assay. Each datum represents the results of three independent experiments (mean±SD,n=3). Data are compared using Student’s t-tests,with* P<0.05,**P<0.01,*** P<0.001 and **** P<0.0001 indicating statistically significant differences.
DGAT-1 is a key enzyme involved in the final step of triacylglycerol synthesis;therefore,it is essential for LD formation and accumulation (Chitrajuet al.2017). To investigate the effects of the DGAT-1 inhibitor on LD accumulation following PEAV infection,Vero E6 and IPIFX cells were treated with different concentrations of the DGAT-1 inhibitor. Cell activity was assessed 48 h after the treatments. Optimal concentrations of 50 and 25 μmol L–1DGAT-1 inhibitor were selected to treat the Vero E6 and IPI-FX cells,respectively (Fig.3-A and B).The LD assay of the Vero E6 cells infected with PEAV after the treatment with the DGAT-1 inhibitor showed that the DGAT-1 inhibitor significantly inhibited the accumulation of LDs that had been caused by the PEAV infection (Fig.3-C). To understand the role of LDs in PEAV infection,PEAV-infected Vero E6 and IPI-FX cells were treated with the DGAT-1 inhibitor. The changes in the viral content of the cells were detected using RTqPCR,Western blotting,IFA and TCID50in Vero E6(Fig.3-D,F,H,and I) and IPI-FX cells (Fig.3-E and G).The results showed that the DGAT-1 inhibitor inhibited LD accumulation significantly and further inhibited PEAV replication.
3.4.Inhibition of LD accumulation inhibits the NFκB signaling pathway that is up-regulated by PEAV infection
LDs are organelles with important functions in the inflammatory response and innate signaling (Bozzaet al.2011;Pereira-Dutraet al.2019;Boschet al.2020),which can produce inflammatory mediators in response to external stimuli and regulate immune responses (Gizziet al.2018). To investigate whether LDs contribute to the PEAV-induced host inflammatory response,Vero E6 and IPI-FX cells infected with PEAV were treated with the DGAT-1 inhibitor to detect the expression of p65,IκB,pp65 and pIκB,which are key proteins in the NFκB inflammatory pathway. The expression levels of p65 and IκB proteins remained stable,the inhibition of LD accumulation significantly inhibited the phosphorylation of p65 and the IκB protein in Vero E6 (Fig.4-A) and IPIFX cells (Fig.4-B),which had been activated by the PEAV infection. The NF-κB signaling pathway was down-regulated,which indicated that the inhibition of LD accumulation may reduce the inflammatory response caused by PEAV infection.

Fig. 4 Effects of the DGAT-1 inhibitor on porcine enteric alphacoronavirus (PEAV)-induced NF-κB signaling pathway activation.A,effects of the DGAT-1 inhibitor on the NF-κB p65,phospho-NF-κB p65,IκB and phospho-IκB protein after PEAV infection.Western blotting was used to measure the expression of the NF-κB p65,phospho-NF-κB p65,IκB and phospho-IκB protein after 0,6,12,24,36,and 48 h in each group of Vero E6 cells. B,effects of the DGAT-1 inhibitor on the NF-κB p65,phospho-NF-κB p65,IκB and phospho-IκB protein after PEAV infection. Western blotting was used to measure the expression of the NF-κB p65,phospho-NF-κB p65,IκB and phospho-IκB protein after 0,6,12,24,36,and 48 h in each group of IPI-FX cells. The expression of tubulin is used as a positive control. Each datum represents the results of three independent experiments (mean±SD,n=3).
3.5.Inhibition of LD accumulation inhibits the production of the inflammatory cytokines lL-1β and IL-8 induced by PEAV infection
Increases in the lipid content of cells can not only promote the proliferation of viruses,but also produce lipotoxicity,which leads to further cell damage. Moreover,various viral infections can severely dysregulate the immune response,increase the production of pro-inflammatory cytokines and chemokines,and lead to different degrees of pathological damage (Bohmwaldet al.2019;Yan and Hong 2020). The RT-qPCR results showed that PEAV infections can up-regulate the production of the inflammatory factors IL-1β and IL-8. The Vero E6 (Fig.5-A and C) and IPI-FX (Fig.5-B and D) cells infected with PEAV were treated with the DGAT-1 inhibitor,and the production of the inflammatory factors was detected. The inhibition of LD accumulation significantly inhibited the production of IL-1β and IL-8.
3.6.lnhibition of NF-κB signaling pathway inhibits LD accumulation and PEAV replication
The activation of NF-κB signaling regulates lipid accumulation and metabolic pathways (Berardoet al.2020;Lonardoet al.2020;Shaoet al.2020). To explore the effect of the NF-κB signaling pathway inhibitor BAY11-7082 on LD accumulation during PEAV infection,Vero E6 and IPI-FX cells were treated with different concentrations of BAY11-7082. Cell activity was detected after 48 h of treatment. Optimal concentrations of 10 and 20 μmol L–1were selected to treat the Vero E6 and IPI-FX cells,respectively (Fig.6-A and B). An assessment of the LDs in the Vero E6 cells infected with PEAV and treated with BAY11-7082 showed that BAY11-7082 significantly inhibited the PEAV infection-induced accumulation of LDs(Fig.6-C). To understand the role of the NF-κB signaling pathway in PEAV infection,the Vero E6 and IPI-FX cells infected with PEAV were treated with BAY11-7082. The changes in the viral content of the cells were detected by RT-qPCR (Fig.6-D and E),Western blotting (Fig.6-F and G),IFA (Fig.6-H),and TCID50(Fig.6-I). The inhibition of the NF-κB signaling pathway by BAY11-7082 significantly inhibited PEAV replication. These results suggest that the inhibition of the PEAV infection-activated NF-κB signaling pathway by BAY11-7082 reduces LD accumulation and inhibits PEAV replication.

Fig. 5 Effects of the DGAT-1 inhibitor on porcine enteric alphacoronavirus (PEAV)-induced IL-1β and IL-8 production. RT-qPCR was used to assess the expression of IL-1β and IL-8 at the mRNA level in Vero E6 and IPI-FX cells infected with PEAV following treatment with the DGAT-1 inhibitor after 0,12,18,and 24 h. A and C,increased IL-1β expression was observed in the PEAV-infected cells,and this was reversed by the DGAT-1 inhibitor. B and D,increased IL-8 expression was observed in the PEAV-infected cells,and this was reversed by the DGAT-1 inhibitor. Each datum represents the results of three independent experiments (mean±SD).The data were compared using Student’s t-tests,with *P<0.05,**P<0.01,and ***P<0.001 indicating statistically-significant differences.

Fig. 6 BAY11-7082 can inhibit LD accumulation and porcine enteric alphacoronavirus (PEAV) replication. A and B,the activity of Vero E6 and IPI-FX cells was detected using a CCK8 cell activity detection kit after treatment with BAY11-7082 at concentrations of 2,5,10,20,and 40 μmol L–1 for 48 h. C,LDs were imaged using fluorescence microscopy. Red,oil red O staining;green,PEAV N protein;blue,nuclei were stained with DAPI. D and E,after the Vero E6 and IPI-FX cells had been treated with BAY11-7082 for 3,6,9,12,18,24,and 36 h,the CT value of the PEAV N gene was detected using RT-qPCR. The mRNA level of GAPDH was used as a positive control. F and G,Vero E6 and IPI-FX cells were treated with BAY11-7082 for 6,12,24,36,and 48 h.The expression level of the PEAV N protein was detected using Western blotting. H,Vero E6 cells were treated with 25 μmol L–1 BAY11-7082 for 24 h,and the expression level of the PEAV N protein was detected by an IFA. The expression of tubulin was used as a positive control. I,BAY11-7082 anti-PEAV replication ability in Vero E6 cells determined with the TCID50 assay. Each datum represents the results of three independent experiments (mean±SD,n=3). Data are compared using Student’s t-tests,with*P<0.05,**P<0.01,***P<0.001 and ****P<0.0001 indicating statistically-significant differences.
3.7.Metformin hydrochloride inhibits PEAV replication
To investigate the antiviral activity of metformin hydrochloride,its cytotoxicity in Vero E6 and IPI-FX cells was assessed. Metformin hydrochloride at the optimum concentration of 80 μmol L–1for the Vero E6 and IPI-FX cells was selected for the experiments (Fig.7-A and B).The cells from both cell lines were infected with PEAV and incubated with 80 μmol L–1metformin hydrochloride.After the treatment,the RNA and N protein expression of the virus were determined using RT-qPCR,Western blotting,IFA,and TCID50. The results show that metformin hydrochloride treatment significantly reduced the RNA,N protein expression levels and virus titer of PEAV in the Vero E6 (Fig.7-C,E,G,and I) and IPI-FX cells (Fig.7-D and F).

Fig. 7 Metformin hydrochloride can inhibit porcine enteric alphacoronavirus (PEAV) replication. A and B,the activity of Vero E6 and IPI-FX cells was detected using a CCK8 cell activity detection kit after treatment with metformin hydrochloride for 48 h. C and D,after the Vero E6 and IPI-FX cells had been treated with 80 μmol L–1 metformin hydrochloride,the CT value of the PEAV N gene was detected by RT-qPCR. The mRNA level of GAPDH was used as a positive control. E and F,the Vero E6 and IPIFX cells were treated with 80 μmol L–1 metformin hydrochloride,and the expression level of the PEAV N protein was detected by Western blotting. G,the Vero E6 cells were treated with 80 μmol L–1 metformin hydrochloride for 24 h,and the expression level of the PEAV N protein was detected by an IFA. The expression of tubulin was used as a positive control. H,metformin HCl anti-PEAV replication ability in Vero E6 cells determined with the TCID50 assay. Each datum represents the results of three independent experiments (mean±SD,n=3). Data are compared using Student’s t-tests,with *P<0.05,**P<0.01,and ***P<0.001 indicating statistically-significant differences.
3.8.Metformin hydrochloride can inhibit PEAV infection-induced LD accumulation
We further confirmed that the inhibitory effects of metformin hydrochloride on PEAV replication are related to LD accumulation. The Vero E6 cells were infected with PEAV and incubated with 80 μmol L–1metformin hydrochloride. LD accumulation in the Vero E6 cells was observed using confocal microscopy after 24 h of treatment (Fig.8). The metformin hydrochloride treatment significantly reduced the PEAV infection-induced accumulation of LDs.
3.9.Metformin hydrochloride can inhibit the PEAV infection-induced up-regulation of the NF-κB signaling pathway
To further investigate the antiviral mechanisms of metformin hydrochloride,its effects on the expression of the p65,IκB,pp65 and pIκB protein in the NF-κB signaling pathway of the PEAV-infected cells were analyzed. Vero E6 and IPI-FX cells infected with PEAV were treated with 80 μmol L–1metformin hydrochloride to assess whether metformin hydrochloride could reduce LD accumulation by inhibiting the NF-κB signaling pathway. The expression of pp65 and pIKB was detected in the NF-κB inflammatory pathway. After treatment with metformin hydrochloride,the expression levels of p65 and IκB proteins remained stable.Metformin hydrochloride significantly inhibited the PEAV infection-activated phosphorylation of p65 and the IκB protein in Vero E6 (Fig.9-A) and IPI-FX (Fig.9-B),thereby down-regulating the NF-κB signaling pathway.Metformin hydrochloride may therefore reduce the inflammatory response caused by PEAV infections.
3.10.Metformin hydrochloride can inhibit the production of the inflammatory cytokines lL-1β and IL-8 following PEAV infection
Metformin hydrochloride is an anti-inflammatory agent that can inhibit the production of several inflammatory cytokines and chemokines (Yeet al.2018). The RTqPCR analysis revealed that metformin hydrochloride significantly inhibited the production of the inflammatory cytokines IL-1β (Fig.10-A and C) and IL-8 (Fig.10-B and D) following the PEAV infection in Vero E6 and IPI-FX cells.
3.11.DGAT-1 inhibitor and metformin hydrochloride can inhibit the production of the cell death induced by PEAV infection

Fig. 8 Metformin hydrochloride can significantly inhibit LD accumulation. LDs were imaged using fluorescence microscopy. Red,oil red O staining;green,porcine enteric alphacoronavirus (PEAV) N protein;blue,nuclei stained with DAPI. Each datum represents the results of three independent experiments (mean±SD,n=3). Data are compared using Student’s t-tests,with ****P<0.0001 indicating statistically-significant differences.

Fig. 9 Effects of metformin hydrochloride on PEAV-induced NF-κB signaling pathway activation. A and B,effects of metformin hydrochloride on the NF-κB p65,phospho-NF-κB p65,IκB and phospho-IκB protein after PEAV infection. Western blotting was used to measure the expression of the phospho-NF-κB p65 and phospho-IκB protein after 0,6,12,24,36,and 48 h in each group of Vero E6 and IPI-FX cells. The expression of tubulin was used as a positive control.

Fig. 10 Effects of metformin hydrochloride on PEAV-induced IL-1β and IL-8 production. A and C,increased IL-1β expression was observed in the PEAV-infected cells,and this was reversed by the metformin hydrochloride inhibitor. B and D,increased IL-8 expression was observed in the PEAV-infected cells,and this was reversed by the metformin hydrochloride. Each datum represents the results of three independent experiments (mean±SD,n=3). Data are compared using Student’s t-tests,with * P<0.05,** P<0.01 and *** P<0.001 indicating statistically-significant differences.
Since the inflammatory response caused by the high production of IL-1β and IL-8 can induce cell death following viral infection (Montgomeryet al.2020),the IPI-FX cells infected with PEAV and treated with the DGAT-1 inhibitor and metformin hydrochloride were detected using flow cytometry. The PI staining and detection of the PEAV-infected IPI-FX cells by flow cytometry revealed that the PEAV infection had induced cell death (Fig.11-A and B). The detection of the PEAVinfected IPI-FX cells treated with the DGAT-1 inhibitor further showed that the inhibition of LD accumulation significantly inhibited PEAV-induced cell death (Fig.11-C). Metformin hydrochloride significantly inhibited PEAVinduced cell death in IPI-FX cells (Fig.11-D). The above results show that DGAT-1 inhibitor and Metformin HCl significantly inhibited the number of cell death induced by PEAV infection (Fig.11-E). These findings suggest that metformin hydrochloride is highly suitable for anti-PEAV activity,which also depends on inflammatory response-mediated cell death. Therefore,these results further support the theory that metformin hydrochloride is a potential candidate drug for the development of anti-PEAV therapies.
4.Discussion

Fig. 11 Effects of the DGAT-1 inhibitor and Metformin HCL on porcine enteric alphacoronavirus (PEAV)-induced cell death. IPI-FX cells infected with PEAV following treatment with the DGAT-1 inhibitor and Metformin HCL after 24 h. A,cell death was detected in the control group using PI staining. B,cell death was detected in the PEAV infected group using PI staining. C,the DGAT-1 inhibitor inhibited cell death induced by PEAV infection. D,the Metformin HCL inhibited cell death induced by PEAV infection.E,DGAT-1 inhibitor and Metformin HCl significantly inhibited the number of cell death induced by PEAV infection. Each datum represents the results of three independent experiments (mean±SD,n=3). Data are compared using Student’s t-tests,with ****P<0.0001 indicating statistically-significant differences.
The replication of most viruses can dependent on the LDs produced by host cells and use LDs for various physiological processes conducive to viral infection,such as biosynthesis,transport,and metabolism (Herker and Ott 2012;Pereira-Dutraet al.2019). PEAV is the porcine coronavirus that was reported in China in recent years. However,there are still many gaps in the understanding of the pathogenesis,host inflammatory response and metabolic regulation mechanism of PEAV infection. Therefore,it is urgent to study the pathogenesis of PEAV. In this study,PEAV infection increased LD accumulation and activated the inflammatory pathways to release inflammatory factors,thus causing a certain degree of pathological damage. Metformin hydrochloride reduced LD accumulation,inhibited the production of the inflammatory pathways and inflammatory factors,reduced disease-induced injury,and significantly reduced PEAV replication,thereby exerting antiviral effects. Metformin hydrochloride has been reported to have antiviral effects on SARS-CoV-2 and hepatitis C virus (HCV),as well as therapeutic effects on the acute inflammatory response and thrombosis (Xinet al.2016;Del Campoet al.2018;Karamet al.2021;Postleret al.2021). Metformin hydrochloride may therefore be a potential antiviral drug against PEAV infections,and its protective effects against PEAV pathogenic mechanisms should be further investigated. Previous reports have shown that after a virus infects the host,it can interact with and use LDs to obtain energy and provide an assembly platform for viral replication. Inhibiting LD accumulation can significantly inhibit viral replication,including that of DENV,HCV,and SARS-CoV-2 (Herkeret al.2012;Nevo-Yassafet al.2017;Zhanget al.2018;Diaset al.2020). In this study,PEAV infection increased LD accumulation,and the inhibition of LD accumulation effectively inhibited PEAV replication. LDs may therefore be potential antiviral targets for coronavirus.
LDs not only play an important role in lipid metabolism pathways,but also participate in signal transduction,autophagy,the inflammatory response,and other cellular processes (Olzmann and Carvalho 2019). However,the molecular mechanisms of action of LDs in the host inflammatory response triggered by porcine coronavirus infection have not yet been studied thoroughly. NFκB signaling pathway has been identified as a typical proinflammatory signaling pathway (Beget al.1996) that regulates many genes involved in immunity,inflammation,and cell survival (Lawrenceet al.2009). Here,we showed that the phosphorylation level of p65 and IκB proteins in the NF-κB signaling pathway increased after PEAV infection and promoted the production of the inflammatory factors IL1-β and IL-8. The inhibition of the NF-κB signaling pathway further inhibited LD accumulation and thus effectively inhibited PEAV replication,which not only promotes viral replication,but also produces lipovirus-induced cell damage. Viral infections can lead to dysregulations in the immune response and promote the production of proinflammatory factors and chemokines,thereby resulting in pathological damage (Bohmwaldet al.2019;Yan and Hong 2020). In this study,we found that PEAV infection can induce cell death and that the inhibition of LD accumulation can significantly inhibit the production of IL-1β and IL-8 and reduce cell death.Metformin hydrochloride was further found to inhibit the activation of the NF-κB signaling pathway following PEAV infection,thereby reducing the production of IL-1β and IL-8 and cell death. These results indicate that LDs are key organelles in PEAV-activated inflammatory responses and cell death,and metformin hydrochloride can effectively inhibit the inflammatory response and pathological injury caused by PEAV infection and exert antiviral effects.
Many studies have assessed the development of antiviral drugs that target the direct effects of intracellular LD formation,including viral,bacterial,and parasitic drugs,to treat infections,such as that caused by SARS-CoV-2(Baeket al.2022;Fonnesuet al.2022),the influenza A virus (Yuet al.2022),porcine reproductive and respiratory syndrome virus (Yuet al.2022),rotavirus (Criglaret al.2022) and other viruses. However,the main challenge in the development of antiviral agents against PEAV is the continuously-occurring variations in the PEAV genome.Therefore,it is important to find a drug that can act on the host to achieve antiviral effects. In this study,metformin hydrochloride effectively inhibited LD accumulation in host cells and exerted anti-PEAV effects. It further effectively inhibited the inflammatory response and pathological injury caused by PEAV infection. These results indicate that metformin hydrochloride may be a potential anticoronavirus drug. This study further provides a theoretical basis for future studies on the pathogenic mechanisms of PEAV and expanding the reserve of antiviral drugs.
5.Conclusion
LDs are organelles that are conducive to viral replication during PEAV infection. LDs interact with the NF-κB inflammatory pathway to promote the production of the inflammatory factors IL-1β and IL-8,thereby inducing cell death and causing severe pathological damage in the host. Metformin hydrochloride inhibits viral replication and cell damage by inhibiting LD accumulation,inhibiting NF-κB signaling,and reducing the production of IL-1β and IL-8,thereby exerting antiviral effects (Fig.12). These findings provide insights into the pathogenesis of PEAV and the antiviral mechanism of metformin hydrochloride against PEAV.
Acknowledgements
This research was funded by the National Natural Science Foundation of China (32102646),the Natural Science Foundation of Guangdong Province,China(2020A1515110315),the Start-up Research Project of Maoming Laboratory,China (2021TDQD002),and the China Agriculture Research System of MOF and MARA (cars-35).
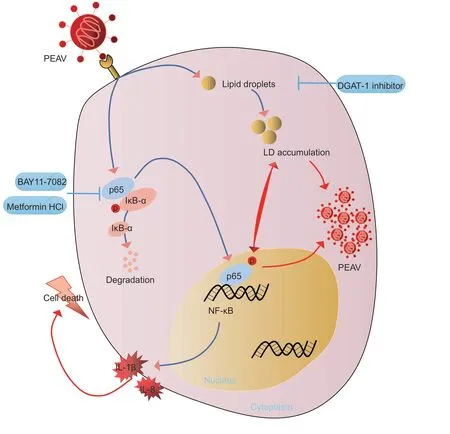
Fig. 12 Model of porcine enteric alphacoronavirus (PEAV) regulating LD accumulation and the NF-κB inflammatory pathway. PEAV infections can increase the accumulation of LDs in host cells and activate the NF-κB signaling pathway,thereby releasing a large amount of the inflammatory factors IL-1β and IL-8 and eventually causing serious disease-related injury. The DGAT-1 inhibitor,BAY11-7082,and metformin hydrochloride can inhibit LD accumulation and the NF-κB signaling pathway,thereby inhibiting the production of IL-1β and IL-8,reducing pathological injury,and exerting antiviral effects.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Ethical approval
This research did not involve animal ethics experiments.
Appendixassociated with this paper is available on https://doi.org/10.1016/j.jia.2023.10.010
 Journal of Integrative Agriculture2024年3期
Journal of Integrative Agriculture2024年3期
- Journal of Integrative Agriculture的其它文章
- Temporal and spatial evolution of global major grain trade patterns
- Does Green Food Certification promote agri-food export quality?Evidence from China
- Lateral root elongation in maize is related to auxin synthesis and transportation mediated by N metabolism under a mixed NO3– and NH4+ supply
- Calcium carbonate promotes the formation and stability of soil macroaggregates in mining areas of China
- Irrigation and nitrogen fertiliser optimisation in protected vegetable fields of northern China: Achieving environmental and agronomic sustainability
- Combining field data and modeling to better understand maize growth response to phosphorus (P) fertilizer application and soil P dynamics in calcareous soils
