Silencing transformer and transformer-2 in Zeugodacus cucurbitae causes defective sex determination with inviability of most pseudomales
Qin Ma ,Zizhen Fan ,Ping Wang ,Siya Ma ,Jian Wen ,Fengqin Cao, ,Xianwu Lin#,Rihui Yan,#
1 Key Laboratory of Green Prevention and Control of Tropical Plant Diseases and Pests of Ministry of Education, School of Plant Protection, Hainan University, Haikou 570228, China
2 School of Life Sciences, Hainan University, Haikou 570228, China
3 Hainan Yazhou Bay Seed Lab, Sanya 572000, China
Abstract transformer is a switch gene for sex determination in many insects,which cooperates with transformer-2 that is expressed in both sexes to regulate female differentiation,particularly in dipterans. Zeugodacus cucurbitae(Coquillett) is a very destructive pest worldwide,however,its sex determination pathway remains largely uncharacterized. Here,we show that the female sex ratio is sharply reduced with knockdown of either transformer or transformer-2 by RNA interference in early embryos of Z.cucurbitae. Most of the males grown from the embryos with transient transformer and transformer-2 suppression mated with wild-type females and produced mixed sex progeny,with one exception that produced only female progeny,and all of the few remaining males failed to mate with wild-type females and produced no progeny. The exceptional male and those males with mating failure were XX pseudomales as determined by the detection of Y chromosome-linked Maleness-on-the-Y,indicating that most XX pseudomales are not viable. The phenotypes of transformer and transformer-2 suggest that they play a key role in regulating sex determination and are required for female sexual development of Z.cucurbitae. Our results will be beneficial to the understanding of sex determination in Z.cucurbitae and can facilitate the development of genetic sexing strains for its biological control.
Keywords: Zeugodacus cucurbitae,transformer,transformer-2,sex determination,RNA interference,biological control
1.Introduction
The melon fly,Zeugodacuscucurbitae(Coquillett)(Diptera: Tephritidae),which was previously categorized asBactroceracucurbitae(Virgilioet al.2015),is one of the most destructive pests of cucurbitaceous crops throughout the Pacific,Asia,and Africa (Dhillonet al.2005;De Meyeret al.2015). This species has been reported to damage over 130 plant hosts representing over 30 families and can cause production losses of 30 to 100%,thus it is a serious threat to fruit and vegetable production and commerce (Dhillonet al.2005). To control and suppress the field populations ofZ.cucurbitae,several successful strategies have been employed such as pheromone traps,sticky yellow cards,fruit bagging,and insecticide sprays. However,there is an urgent need to develop improved pest control methods because of the high economic and environmental costs and the emergence of insecticide-resistant populations (Jinet al.2016).
New strategies have been developed and applied for pest control. The sterile insect technique (SIT) is an environmentally friendly and very successful method for controlling pests through the continuous release of massreared sterilized males that suppress wild populations by mating with wild females (Dycket al.2005;Antet al.2012). Previous studies inCeratitiscapitatashowed that one key to the success of its SIT programs was the translocation of selectable traits to the Y chromosome in genetic sexing strains that enabled male selection on a massive scale (Dycket al.2005). However,developing such a similar strain for other pests is not easy. With the development of molecular biology and genetics,insect genetic engineering,which requires an intensive understanding of the target pest at the molecular level,has shown great potential for pest control (Alphey and Andreasen 2002;Handler 2004;McFarlaneet al.2018;Papathanoset al.2018;Grilliet al.2021;Wuet al.2022).However,only limited knowledge about genetic and molecular mechanisms ofZ.cucurbitaeis available,and in particular,its sex determination mechanism remains to be defined.
Sex determination is a complex but highly programmed developmental process that exhibits a wide variety of mechanisms among different organisms,including insects(Sacconeet al.2002;Sanchez 2008;Hedigeret al.2010;Shukla and Palli 2012;Morrowet al.2014). In general,various less-conserved upstream signals regulate the expression of relatively conserved downstream factors,such astransformer(tra),transformer-2(tra-2),doublesex(dsx),andfruitless(fru),through sex-specific alternative splicing cascades. Among them,trais a central player in the evolution of sex determination in many insects by acting as a switch gene that determines sexual development at an early developmental stage and sustains the sexual identity throughout the whole lifespan(Verhulstet al.2010;Geuverink and Beukeboom 2014).Thetragene is regulated at the post-transcriptional level and its pre-mRNA is spliced in a sex-specific manner.In females,the upstream regulatory signals direct female-specific splicing of the primarytratranscript,yielding a mature transcript that can be translated into a functional TRA protein. TRA-2 is the product of the genetransformer-2that is expressed in both sexes,and it is a cofactor of TRA that is absolutely essential for the proper functions oftra(Thongsaiklainget al.2018).TRA and TRA-2 form a splicing regulatory complex that regulates the alternative splicing and the expression of its downstream target genesdsxandfruin a female-specific mode,thereby promoting female-specific development.By contrast,the primary transcript oftrain males is male-specifically spliced,yielding a mature transcript with premature stop codons,and therefore a truncated nonfunctional peptide. Consequently,its regulated downstream genes are also male-specifically spliced and the insect adopts a male-specific development mode(Lagoset al.2007;Ruizet al.2007;Liuet al.2015;Zhaoet al.2019). The master role of the TRA/TRA-2 complex in determining sexual dimorphism makestraandtra-2two potential target genes for the genetic sexing and control of pests (Fuet al.2007;Conchaet al.2016). Although the genestraandtra-2have been isolated inZ.cucurbitaeand the expression oftraat different developmental stages has been examined (Luoet al.2017),their exact functions in the sex determination ofZ.cucurbitaehave not been fully characterized.
In this study,we performed RNA interference(RNAi) to knockdown thetransformergene (Zctra) andtransformer-2gene (Zctra-2) ofZ.cucurbitaein newly laid eggs in order to examine their functions. When the expression of eitherZctraorZctra-2in early embryos was transiently suppressed by RNAi,the female development ofZ.cucurbitaewas significantly affected. The fertility test showed thatZctraorZctra-2depletion resulted in pseudomales,which were identified by failure to detect the Y chromosome-linked geneMaleness-on-the-Y(MoY) inZ.cucurbitae. Our results demonstrate thatZctraandZctra-2play key roles in the sex determination ofZ.cucurbitae. The isolation and characterization ofZctraandZctra-2will enrich our understanding of sex determination in this insect and raise the possibility of developing genetic control strategies for it in the future.
2.Materials and methods
2.1.lnsect culture
The melon flies used in this study were collected on the City West campus of Hainan University,China and maintained in the laboratory at 26°C,70% RH,and a photoperiod of 14 h:10 h (L:D). The larvae were reared on an artificial diet (Liuet al.2020),and the grown larvae were transferred into plastic boxes with sand before pupation. After 7 d,the pupae were transferred into insect cultivation cages for eclosion. The adults were fed water and a protein-rich food consisting of 1:3 brewer’s yeast powder/sugar (w/w).
2.2.RNA interference
The templates for the synthesis ofZctra,Zctra-2andGFPdsRNAs (Nagaiet al.2002;Yanet al.2010) were amplified from the recombinant plasmids containing theZctra,Zctra-2orGFPcDNAs. AZctrafragment of 738 bp,aZctra-2fragment of 417 bp,and aGFPfragment of 700 bp were obtained using the primers containing a T7 promoter at the 5´ ends (Appendix A).
The dsRNAs againstZctra,Zctra-2andGFPwere synthesized using a Megascript T7 transcription kit(Thermo Fisher Scientific,USA). The dsRNA ofGFPserved as a negative control. One μg μL–1of dsRNA was microinjected into eggs within 1 h after egg laying (AEL)according to Fanet al.(2023) with some modifications.Briefly,newly laid eggs were collected 15 min after egg laying and then dechorionated by 1% sodium hypochlorite for 10 s and 0.02% Triton X-100 for 15 s. After washing with deionized H2O,the eggs were aligned neatly on a slide with double-sided sticking tape and covered with a mixture of 1: 1 (v/v) halocarbon 700/27 oils (Sigma,USA)after air drying. Embryo injection was carried out using a FemtoJet 4i microinjector (Eppendorf,Germany) with the following parameters: pi=500 hPa,ti=2 s,and pc=1,500–2,000 hPa. After injection,the eggs were cultured at 26°C within a humid chamber. Larvae were then transferred to artificial diet to allow further development under the same conditions as wild-type flies. After eclosion,sexual dimorphism was examined,and the progeny were classified by sex.
2.3.Fertility test
The 10-day-old adult males fromZctraandZctra-2dsRNA silencing (traKD andtra-2KD) were used to perform fertility tests,and wild-type males and the males from theGFPdsRNA injection (GFPKD) were used as the control.Either 30 crosses (traKD) or 40 crosses (tra-2KD,noninjected andGFPKD) were set up in separate cages,each containing one male from each treatment and two virgin wild-type females. Mating behaviors were observed every 30 min from 7:00 p.m.to 10:30 p.m.,and eggs were collected from each cross for five consecutive days. After egg collection,the parental males were dissected and their internal sexual organs were examined. After egg hatching,larvae were transferred onto artificial diet and cultured with the same standard method as the wild-type flies. After eclosion,sexual dimorphism of the progeny was examined.
2.4.RNA isolation and RT-PCR analyses
Total RNA was isolated using TRI reagent (Sigma,USA) according to the manufacturer’s instructions. The concentration of extracted RNA was determined with an ultra-micro ultraviolet-visible spectrophotometer(MIULAB,China). A total of 1 μg of total RNA was used to synthesize cDNA using the PrimeScript™ RT reagent kit with gDNA eraser (TaKaRa,China) according to the manufacturer’s instructions. RT-PCR was performed to detectZctraandZctra-2expression at 8 and 12 h after dsRNA injection using gene-specific primers (Appendix A).α-Tubulinwas used as the reference gene. RT-PCR was carried out using the following parameters: 3 min at 94°C;35 cycles of 30 s at 94°C,30 s at 59°C,20 s at 72°C;and 5 min at 72°C. A PCR product of 242 bp forZctraand a PCR product of 252 bp forZctra-2would be obtained.
2.5.Genomic DNA isolation and MoY detection
Total DNA of thetraKD males andtra-2KD males used for the fertility test,as well as wild-type males and females were obtained from the DNA phase using the TIANamp genomic DNA kit (Tiangen,China) according to the manufacturer’s instructions.ZcMoY,the Y chromosomelinkedMoYortholog inZ.cucurbitae(Fanet al.2023),was detected with PCR usingZcMoY-specific primers(Appendix A). PCR was performed with the following parameters: 3 min at 94°C;35 cycles of 30 s at 94°C,30 s at 55°C,1 min 10 s at 72°C;and 5 min at 72°C. A PCR product of 1,280 bp would be obtained whenZcMoYexisted in the fly.
3.Results
3.1.Knockdown of Zctra in embryos by RNAi exerts a strong masculinizing effect
A previous study has shown thatZctrais expressed in early embryonic stages,and the female transcript appears earlier than the male transcript (Luoet al.2017). These results indicate thatZctramay participate in sex determination in early embryos. RNAi ofZctrawas performed to examine the role ofZctrain sex determination inZ.cucurbitae. A dsRNA targeting 738 bp ofZctrawas synthesized (Fig.1-A) and injected into the newly laid eggs within 1 h. The expression ofZctrawas significantly reduced at 8 and 12 h (Fig.1-B),and 96.21%of the embryos developed into males after they were injected withtradsRNA. The male ratio was significantly higher intraKD flies than in theGFPKD (54.01%) or noninjected (46.43%) flies,suggesting that the silencing ofZctramay exert a strong masculinizing effect (Table 1).

Table 1 Effects of Zctra and Zctra-2 knockdown on sexual development1)
Wild-type male adults have male external genitalia and a row of bristles on each side of the third tergum(Fig.2-A and B),while wild-type female adults have no bristles on the third tergum and possesses femalespecific external genitalia (Fig.2-C and D). Intersex individuals that exhibited abnormal female external genitalia with a row of bristles only on one side of the third tergum were observed inZctraRNAi flies (Fig.2-E and F). Conversely,there were no intersex individuals in the controls. These results suggested that the suppression ofZctraexpression in the early embryos disturbed female development,indicating thatZctraplays a key role insexual determination inZ.cucurbitae.

Fig. 2 Phenotypes of intersex individuals induced by Zctra and Zctra-2 knockdown by RNAi. The insets are enlarged views of the sexual characteristics marked by arrows. A and C,dorsal views of wild-type male (A) and female (C),showing the males exhibit short black pigmented bristles on both lower sides of the third tergum (arrows). B and D,ventral views of normal male genitalia(B) and female ovipositor (D). Intersex individuals from Zctra RNAi flies show abnormal female external genitalia (F) with a row of bristles (arrow) on only one side of the third tergum (E). Intersex individuals from Zctra-2 RNAi flies exhibit the exposed vas deferens (arrow head) (H),with a row of bristles on one side of the third tergum and two rows on the other side (arrows) (G). Scale bars: 500 μm.
We further examined the internal reproductive systems of the intersex individuals. Compared to wild-type adult testes and ovaries (Fig.3-A and B),intersex adults showed malformed interior reproductive organs,such as two testes of different sizes (Fig.3-C). Intriguingly,both aberrant testes and ovaries were found to coexist in the intersex individuals (Fig.3-D). Taken together,these results showed thatZctradepletion caused the formation of abnormal reproductive organs,suggesting that sex determination was disrupted byZctraRNAi.
3.2.Knockdown of Zctra-2 in embryos by RNAi causes abnormal female development
As TRA-2 is a component of the TRA/TRA-2 complex,we transiently silencedZctra-2in early embryos to explore its role in sex determination. For this purpose,a dsRNA that targets 417 bp ofZctra-2(Fig.1-A) was injected into the newly laid eggs within 1 h. The expression ofZctra-2was drastically reduced at 8 and 12 h aftertra-2dsRNA injection (Fig.1-B). In addition,the female rate inZctra-2RNAi flies was less than 6.00%,which was significantly lower than theGFPKD (45.99%) or non-injected (53.57%)flies (Table 1),implying that the depletion ofZctra-2results in a shift in gender and causes most females to turn into either intersexes or males. Intersex individuals were also observed inZctra-2RNAi flies and the proportion increased drastically to 39.55%,whereas no intersex individuals were found in the controls (Table 1).Unlike wild-type adults (Fig.2-A–D),we observed that intersex individuals had abnormal male external genitalia with a row of bristles on one side of the third tergum and two rows of bristles on the other side (Fig.2-G and H).These results suggested that the sexual development of the females was disrupted when early embryos were injected with dsRNA oftra-2.

Fig. 3 Development of internal genital organs affected by Zctra and Zctra-2 knockdown. A and B,normal testes (A)and ovaries (B) from wild-type male and female,respectively. C and D,abnormal testes (C) and abnormal ovaries with abnormal testes (D) of intersex individuals from Zctra knockdown. E and F,both testes being small in size (E) and the coexistence of both aberrant testes and ovaries (F) in the intersex adults from Zctra-2 knockdown. Scale bars: 500 μm.
We further dissected the internal reproductive systems of intersexes fromZctra-2RNAi treated individuals. As expected,aberrations occurred in the testes and ovaries compared to the wild-type adult testes and ovaries (Fig.3),such as abnormal testes (Fig.3-C),abnormal ovaries with abnormal testes (Fig.3-D),both testes being small in size (Fig.3-E),and the coexistence of both aberrant testes and ovaries in the intersex adults (Fig.3-F). These observations revealed that the intersexes had abnormal interior reproductive organs and were therefore incapable of reproduction.
3.3.Suppression of Zctra or Zctra-2 in embryos leads to masculinization of XX flies
To test whetherZctraorZctra-2depletion influences the fertility of RNAi males,we randomly selected G0 males grown from RNAi treatments and wild-type males,and set up about 40 crosses for each treatment to perform the fertility test. For each cross,one male that had developed from a dsRNA-injected embryo was crossed to two virgin wild-type females in one cage. The mating and emergence rates were both 100% for the non-injected andGFPdsRNA males. However,96.67% oftraKD males mated with wild-type females and the hatching rate was 83.33%,while 7.50% oftra-2KD males did not mate with wild-type females and the hatching rate was 87.50% (Table 2).

Table 2 Effects of Zctra and Zctra-2 knockdown on reproduction1)
We then investigated the progeny of all crosses. As expected,the crosses of non-injected andGFPdsRNA males yielded both male and female progeny. Surprisingly,both male and female progeny were also discovered from most of the crosses oftraKD andtra-2KD males that produced progeny,which suggests that most of the paternal males had the XY karyotype. Furthermore,it is worth noting that among the crosses ofZctraRNAi males,we observed only one cage (cage 17) in which the offspring were all females,indicating that this paternal “male” had the XX karyotype. This result is surprising,as we expected that there would be more surviving pseudomales within the crosses that would produce only female progeny because of the high male or intersex ratios after RNAi treatment fortraortra2. Considering the low rate of females (less than 6.00%,Table 1) in the G0 adults and the overwhelmingly high ratio of paternal XY males,our results suggest that most of the XX pseudomales were not viable and did not survive to the adult stage.
To examine whether the reproductive system was normally developed,which could be one of the possible reasons contributing to the mating failure of RNAi males in the fertility test,we dissected all the RNAi males in the fertility test to examine their internal genital structures and found they were normal compared to those from wildtype males. We further investigated the activity of sperm from males that were mated and unmated with wild-type females,and found that for all males in the fertility test the sperm movement showed no difference from that of wildtype males (data not shown).
3.4.Pseudomales were confirmed by detecting the MoY gene
Previous studies showed that transient suppression oftrain the early embryos of many dipteran flies reversed supposed-to-be females to XX pseudomales (Albaet al.2002;Liet al.2013;Raphaelet al.2014;Liuet al.2015).Thus,we speculated that the unmated males from the RNAi treatments might be pseudomales. Recently,the M factor on the Y chromosome,which is proposed to block the transcription or translation oftraand inhibit female development in XY individuals,has been identified asMaleness-on-the-Y(MoY) inCeratitiscapitata(Meccarielloet al.2019). We have isolatedMoYinZ.cucurbitae(ZcMoY) and found thatZcMoYis specifically located on the Y chromosome (Fanet al.2023). Thus,theZcMoYgene was examined to determine the karyotypes of RNAi flies. The results showed thatZcMoYwas detected in all males that produced progeny when they were crossed with wild-type females,whereas the males that produced no offspring when crossed with wild-type females did not contain theZcMoYgene in their genomes(Fig.4-A),suggesting that they were XX pseudomales.These results demonstrate that the XX embryos that aresupposed to develop into females can be reversed to XX pseudomales when the expression ofZctraorZctra-2is transiently knocked down by RNAi.
Since we got one male (from cage 17) afterZctrasilencing whose progeny were all females,we decided to determine its karyotype by examining theZcMoYgene.As we suspected,this parental “male” did not contain theZcMoYgene in its genome (Fig.4-B). Taken together,our results suggest that when XX embryos ofZ.cucuribitaeare reversed to XX pseudomales,most of them are incapable of mating and only a very few are fertile producing all female offspring due to the XX karyotype of the pseudomales.
4.Discussion
Insects are a tremendously successful group that account for the great majority of animal species and can be found in almost all terrestrial and freshwater habitats (Gullan and Cranston 2003). This diversity at the taxonomic level is matched by a wide variety of sex determining mechanisms,and insect model systems have provided important insights into the biology and mechanisms of sex determination (Sanchez 2008). To date,the genes and molecular pathways involved in sex determination have been identified in many insects (Blackmonet al.2017),and the properties of sex-specifically expressed genes are widely used in genetic pest control (Morrisonet al.2010;Schetelig and Handler 2012;Koukidou and Alphey 2014;Yan and Scott 2015;Conchaet al.2016;de Araújoet al.2018). For example,a CRISPR-Cas9 gene driver that targets the female-specific exon 5 ofdoublesexcauses complete population suppression in cagedAnophelesgambiaemosquitoes (Kyrouet al.2018),implying that the use of sex determining mechanisms has great potential for applications in pest control.
In this study,we examined the roles ofZctraandZctra-2in the sex determination ofZ.cucurbitaeby injecting their dsRNAs into early embryos to knockdown their expression. Generally,the sex ratio of wild-type progeny is nearly 1:1,as shown by the sex ratio ofGFPKD or non-injected flies in this study (Table 1). However,in the adults that emerged fromZctraorZctra-2dsRNA injected embryos,female development was significantly disrupted as only less than 6% of the individuals developed into females,and both pseudomales that were confirmed by the Y chromosome-specific geneZcMoYand intersex individuals were observed. In addition,the ratios of males were 96.21 and 54.48% after dsRNA oftraortra-2injection,respectively,indicating thatZctraandZctra-2likely play a distinct role in sex determination.Generally,female-specific splicing oftrainvolves an autoregulatory loop,in which the TRA protein is required for female-specific splicing of its own pre-mRNA (Siddallet al.2022). Once the auto-regulatory loop is disturbed by the transient suppression oftraexpression,female-specific splicing oftrais disrupted and the individuals develop into pseudomales. However,tra-2is a non-sex-specific auxiliary splicing factor that can be transcribed throughout development. Therefore,the expression oftra-2will return to the normal level after transient suppression to recover its function in development and produce more intersex individuals (Salveminiet al.2009). It will be of great interest to clarify the functional differences betweenZctraandZctra-2using CRISPR/Cas9 in the future. Our results clearly demonstrate that the depletion ofZctraorZctra-2causes XX embryos which are presumptively females to develop into XX pseudomales and intersexes.Our previous report has shown that the female-specificZctratranscript is expressed in the early embryonic stage from 0 to 3 h after egg laying (Luoet al.2017),indicating thatZctramay function as early as in newly laid eggs and that the transient suppression ofZctraexpression in early embryos may reverse female development,which was confirmed by our results in this study. Our results are also consistent with previous reports in which knockdown or knockout oftraresults in the reversal of females to males in many other dipteran flies (Lagoset al.2007;Concha and Scott 2009;Liuet al.2015;Laohakieatet al.2016;Zhaoet al.2019). Taken together,we suggest thatZctrahas structural,regulatory and functional conservation with its homologs in the tephritid flies.
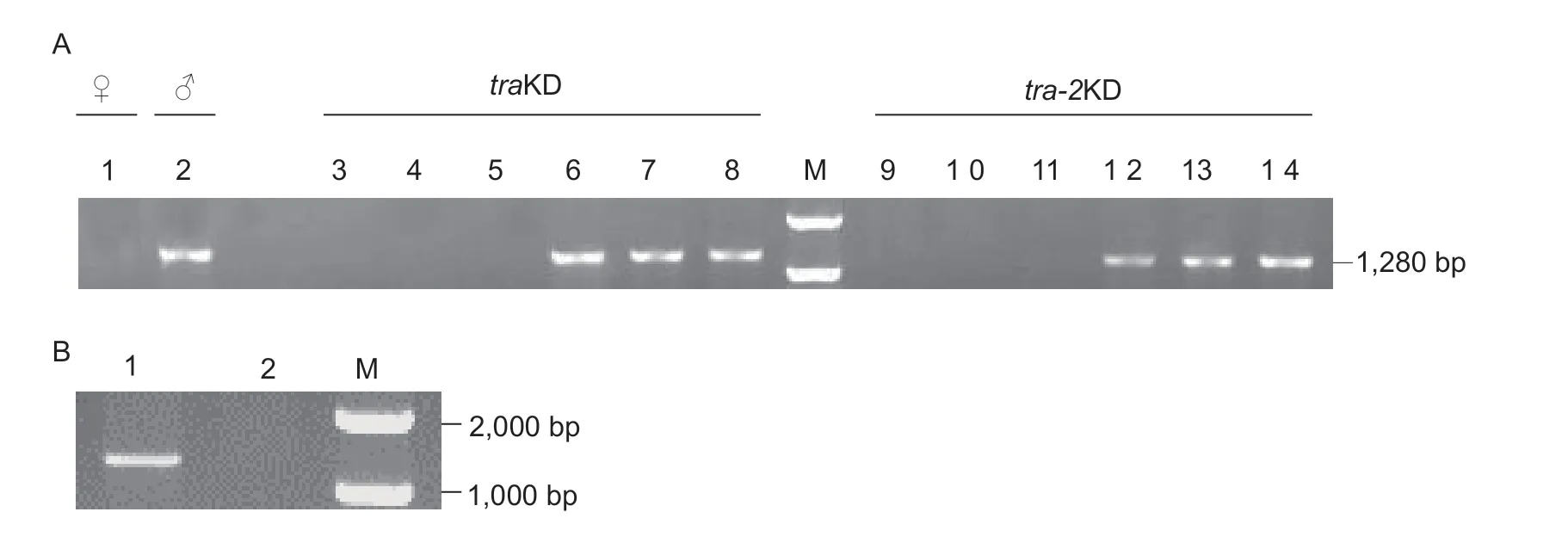
Fig. 4 MoY detection for paternal males in the genetic test. A,males that produced either progeny or no progeny when crossed with wild-type females in the fertility test. Female (1) and male (2) are the negative and positive controls,respectively. Numbers 3–5 denote the traKD males that produced no progeny when crossed with wild-type females;6–8 denote the traKD males that produced progeny when mated with wild-type females;9–11 denote the tra-2KD males that produced no progeny when crossed with wild-type females;12–14 denote the tra-2KD males that produced progeny when mated with wild-type females. B,ZcMoY detection in the Zctra RNAi male from cage 17 whose offspring were all females. 1 represents wild-type male adult;2 represents the paternal male from cage 17;M represents DL2,000 DNA marker.
Previous studies demonstrated thattraknockdown in early embryos produces XX pseudomales in many dipteran flies (Concha and Scott 2009;Liet al.2013;Koukidou and Alphey 2014;Liuet al.2015). Our results from genetic crosses showed the following effects oftradepletion inZ.cucurbitae: 1) All males that produced mixed progeny were XY karyotypes,which were confirmed by detection of the Y-linked M factorZcMoY;2) all males that produced no progeny were XX pseudomales as suggested by the lack ofZcMoYdetection in their genomes;and 3) only one “male”whose progeny were all females had the XX karyotype as determined byZcMoYdetection. A recent study found that knockdown oftramRNA could lead to hyperactivation of the X chromosomes and death of XX transformed males inLuciliacuprina(Williamsonet al.2021),which could offer an explanation for the low rate of XX transformed males in our study. Our data have confirmed thatZctraorZctra-2depletion can produce XX adult pseudomales at a low frequency,thereby suggesting that most XX pseudomales are not viable during development. The next steps will be to explore whethertraortra-2is involved in a dosage compensation pathway (Williamsonet al.2021) and to determine the mechanism underling the deaths of the XX pseudomales.
As key players in sex determination,TRA and TRA-2 form the TRA/TRA-2 complex to regulate the sex-specific alternative splicing of the pre-mRNAs ofdsxthat encodes the transcription factors involved in the establishment of almost all the morphological traits and courtship behaviors that differ between males and females (Hildreth 1965;Villella and Hall 1996;Kimuraet al.2008;Rideoutet al.2010;Yamamotoet al.2014;Shirangiet al.2016). Although thetra/tra-2-dsxaxis is conserved in regulating sexually dimorphic morphologies and behaviors,the downstream target genes in pests remain undefined. Therefore,the mechanism by which thetra/tra-2-dsxaxis participates in regulating the development of sexual organs in pests is unclear. InDrosophilamelanogaster,numerousdsxtarget genes that regulate sexual dimorphism have been identified,such asthickveinsandabdominal-A(Cloughet al.2014).The identification and functional analysis of these genes inZ.cucurbitaein the future may help us better understand sex determination in dipteran pests.
5.Conclusion
As an extension of our previous study,we have isolated and characterizedtrainZ.cucurbitae. Our data show thatZctrais conserved not only in its molecular characteristics but also in its functions in regulating sex determination.The ability oftraknockdown or knockout to reverse XX females to pseudomales can be highly advantageous for the application of SIT as the male-only release is much more efficient than the release of both sterile males and females. The specific characterization ofZctrahas therefore paved the way for the genetic control ofZ.cucurbitae. It will be also very important to study its upstream regulatory signals and downstream target genes in order to better understand the sex determining pathway,which will provide a substantial theoretical basis for the research and applications of insect sex determination.
Acknowledgements
This work was supported by the Hainan Provincial Natural Science Foundation of China (321CXTD435),the National Natural Science Foundation of China (31860523,31660339,31702059,and 32260665),and the National Key R&D Program of China (2022YFC2601400).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Ethical statement
All applicable international,national and institutional guidelines for the care and use of animals were followed.
Appendixassociated with this paper is available on https://doi.org/10.1016/j.jia.2023.06.019
 Journal of Integrative Agriculture2024年3期
Journal of Integrative Agriculture2024年3期
- Journal of Integrative Agriculture的其它文章
- Temporal and spatial evolution of global major grain trade patterns
- Does Green Food Certification promote agri-food export quality?Evidence from China
- Lateral root elongation in maize is related to auxin synthesis and transportation mediated by N metabolism under a mixed NO3– and NH4+ supply
- Calcium carbonate promotes the formation and stability of soil macroaggregates in mining areas of China
- Irrigation and nitrogen fertiliser optimisation in protected vegetable fields of northern China: Achieving environmental and agronomic sustainability
- Combining field data and modeling to better understand maize growth response to phosphorus (P) fertilizer application and soil P dynamics in calcareous soils
