Effects of Exogenous Plant Hormones on Growth Status and Secondary Metabolism of Houttuynia cordata Thunb.
Xitao WANG, Kai YAN, Tianhua YU, Zhannan YANG*, Shiqiong LUO
1. Key Laboratory for Information System of Mountainous Areas and Protection of Ecological Environment of Guizhou Province/School of Life Sciences, Guizhou Normal University, Guiyang 550081, China; 2. Liupanshui Normal University, Liupanshui 553004, China
Abstract [Objectives] To improve the yield and secondary metabolite content of medicinal plants and to further develop and utilize the medicinal and other functions of medicinal plants. [Methods] We used the sterile tissue culture method with Houttuynia cordata Thunb. as the research object. Different concentrations of 1-naphthalene acetic acid (NAA), auxin (indole-3-acetic acid, IAA) and gibberellin acid (GA3) were added to the group culture medium of H. cordata to investigate the effects of exogenous plant hormones on plant height, root length, fresh weight, morphological characteristics, four phenolics and 20 volatile compounds. [Results] The results showed that the exogenous plant hormone of 3 mg/L GA3 significantly increased plant height by 79.9% over the control; the exogenous plant hormone of 3 mg/L IAA significantly increased root length by 52.6% over the control; and the exogenous plant hormone of 1 mg/L GA3 significantly increased fresh weight of single plant by 458.2% over the control. In the treatment group of 1 mg/L NAA, chlorogenic acid content was significantly increased by 52.6% compared with the control; in the treatment group of 1 mg/L IAA, chlorogenic acid, rutin, isodendrin and quercetin content were significantly increased by 109.1%, 100.6%, 173.8%, and 198.7% compared with the control, respectively; in the treatment of 3 mg/L GA3, chlorogenic acid, rutin, isoquercitin, and quercitin content were significantly increased by 65.3%, 104.9%, 139.0% and 191.2% over the control. In addition, the content of volatile compounds was significantly higher in all H. cordata treated with exogenous plant hormones of 2 mg/L NAA, 1 mg/L IAA, and 3 mg/L GA3; however, the content of volatile compounds was lower in all of the treatments with 2 mg/L GA3. [Conclusions] Different exogenous plant hormones have certain effects on the growth morphology and secondary metabolic content of H. cordata, which provides theoretical basis and technical support for the development and utilization of medicinal plants.
Key words 1-Naphthaleneacetic acid (NAA), Auxin (IAA), Gibberellic Acid (GA3), Phenols, Volatiles
1 Introduction
Plant hormones are naturally present in plants in tiny amounts but significantly affect plant growth and development[1]. There is a wide variety of hormones in plants, which are mainly divided into six categories: auxin (indole-3-acetic acid, IAA), cytokinin (CK), abscisic acid (ABA), gibberellin (GA), ethylene (ET), and brassinosteroids (BR), and the various hormones promote each other or antagonize each other to regulate the growth and development of plants[2-3]. Plant hormones are important nutrients that regulate the growth and development of plants under non-suitable conditions, and are involved in the growth and development of various organs such as roots, stems, and leaves of plants, and are often applied to rooting of plant spikes and promotion of seedling growth[4]. Studies have shown that exogenous plant hormones IAA, ET, CK, and GA3have obvious effects on seedling morphology, physiological and biochemical traits, including root growth, stem growth, promotion of adventitious root generation in plant branches, and lateral bud formation, which is an ideal regulator for the strengthening of seedlings[5-6]. The 1-naphthaleneacetic acid (NAA) has the effect of promoting cell division and expansion and inducing the formation of adventitious roots, for example, Li[7]etal.found that 10 mg/L NAA had the best performance in promoting the rooting and root quality in chrysanthemums. Auxin (IAA) is also involved in several developmental processes, such as vascular tissue development, cell elongation, and apical dominance significantly promotes the elongation of primary roots and increases the specific surface area of the root system in cucumber seedlings[8]. In addition, relevant studies have reported that gibberellin (GA) not only increases plant growth, leaf number, shoot formation, cell division and elongation, and flowering, but also promotes the increase in the content of metabolites soluble sugars, cell wall polysaccharides, and starch in the plant[9].
Plant hormones play an important role in regulating plant development and secondary metabolism at various stages of plant growth and development[10]. In recent years, many new plant hormones have been discovered that they can not only regulate physiological processes such as development, metabolism, and senescence by affecting nucleic acids, proteins, and enzymes in the plant, but also regulate the synthesis of secondary metabolites such as flavonoids, terpenoids, and alkaloids in the plant[11-12]. For example, appropriate concentrations of exogenous ABA can promote the accumulation of major bioactive components (glycyrrhizin, isoglycyrrhizin, and isoglycyrrhizin) inGlycyrrhizaglabra[13-14]. Exogenous ABA can also regulate the synthesis ofChaihusaponins under drought stress[15]. Szymczyk[16]found that salicylic acid (SA) and the synthetic auxin NAA acted as an exciter to stimulate the accumulation of total tanshinone level and dihydrotanshinone, cryptotanshinone, tanshinine I and tanshinone IIA level inSalviamiltiorrhizacallus cultures growing on solid MS medium. Therefore, it is not clear whether exogenous plant hormones can influence the synthesis of secondary plant metabolites by altering the uptake and enrichment of nutrients in plants. Therefore, exogenous plant hormones are one of the important factors affecting the growth status and secondary metabolite content of plants, and the study of the effects of exogenous plant hormones on medicinal plants has also deepened our understanding of the mechanisms of medicinal plants- hormones interactions.
HouttuyniacordataThunb. is commonly used for dietary and medicinal development as a traditional medicine and food plant[17].H.cordataitself contains a variety of secondary metabolites, such as volatile substances, alkaloids, flavonoids, polysaccharides, and sterols,etc.It not only has antioxidant, antibacterial, anti-inflammatory, viral inhibition, and immunity enhancement, and other medicinal value, but also contains a wealth of nutritive value, so it is a purely natural health care product, and is extremely valuable for the development and utilization of the value of the broad market outlook[18-20].
The aim of our study is to help explain effects of exogenous plant hormones on the growth status and secondary metabolism ofH.cordata. Therefore, in this experiment, we choseH.cordatagroup-cultivated seedlings as the test material and explored the effects on the growth and secondary metabolites ofH.cordatathrough the addition of exogenous plant hormones NAA, IAA, and GA3, to provide a theoretical basis for the development and utilization ofH.cordatafor improving its edible value and medicinal efficacy, as well as providing a new idea for the mechanism of the growth hormone regulation on the secondary metabolites of the natural products.
2 Materials and methods
2.1 Materials and treatmentsThe asepticH.cordataseedlings cultured in the laboratory were selected as the experimental materials, and the stem node segments with 1 leaf blade cut from the same batch ofH.cordatawere used as the explants and inoculated onto MS basal medium (MS 4.76 g/L, sucrose 30 g/L and agar 8 g/L) and different plant hormones were added. The tissue-culture seedlings were divided into three groups for cultivation, the treatment groups were A (NAA), B (IAA), and C (GA3), and each group was divided into four concentration gradients (0, 1, 2, and 3 mg/L), and 0 mg/L was the control (CK), and each gradient was inoculated with 10 vials, and each vial was inoculated with one explant (Fig.1). The culture containers were glass culture flasks, the culture period was 50 d, and the culture conditions were temperature (25 ± 1)℃, light time 12 h/d, and light intensity 2 000 IX; the culture environment used was the BIC intelligent artificial climate incubator.

Note: CK-A3, 3 mg/L NAA, B2, 2 mg/L IAA, C3, 3 mg/L GA3.
2.2 Morphological analysisAll the grouped seedlings cultured for 45 d were taken out, and the number of leaves, plant height, fresh weight, and primary root length of each of the five grouped seedlings were measured randomly. The number of leaves was recorded as the one with the highest number of leaves; plant height was measured from the highest part of the top of the main stem of the plant to the base of the plant using vernier calipers; the fresh weight of all the leaves, stems, and roots of the plant was measured using an electronic balance (accurate to 0.000 1).
2.3 Determination of secondary metabolitesThe content of phenolics (chlorogenic acid, rutin, kaempferol-3-oglucorhamnoside, quercitin, isoquercetin, and quercetin) ofH.cordatawas determined using the high-performance liquid chromatography-methanol ultrasonic extraction method of Ye[21]. The chromatographic conditions were as follows: LC-20AT high-performance liquid chromatograph (Shimadzu, Japan), equipped with a diode array detector (DAD; SPD-M20A), CBM-20A system controller and LC-solution 2.50 chromatographic workstation; column model: Shim-pack CLC-ODS (150×6.0 mL). The mobile phase was acetonitrile: methanol=11:5 (V/V) for the organic phase and 0.1% formic acid aqueous solution (V/V) for the aqueous phase with gradient elution. The mobile phases were solvent A (acetonitrile: methanol=11:5,V/V) and solvent B (0.1% formic acid,V/V). The gradient elution program was as follows: 0-6 min (6%-16% A), 6-28 min (16%-35% A), 28-35 min (35%-40% A), 35-45 min (40%-100% A), 45-55 min (100% A), 55-65 min (100%-6% A), and 65-75 min (6% A). The flow rates were 0-16 min (1.4 mL/min), 16-36 min (0.8 mL/min), and 36-75 min (1.2 mL/min). The detection wavelength was 345 nm and the column temperature was 40 ℃. The concentration of each phenolic was calculated based on the peak area in the HPLC chromatogram file of the phenolic compound, using the external standard method, and phenolic standard solutions from Sigma-Aldrich (St. Louis, MO, USA). Extracts from three replicates per treatment were tested.
The content of 20 volatile substances (α-pinene, β-pinene, β-caryophyll, limonene, borneol, camphene, cineole, γ-terpinene, β-myrcene, linalool, hexanol, cis-3-Hexen-1-ol, trans-2-Hexen-1-ol, decanol, decanal, trans-2-hexenal, 2-undecanone, 3-tetradecanone, cis-3-hexenyl-acetate, bornyl acetate) ofH.cordata. was determined using the method of Yang[22]by gas chromatography-mass spectrometry (GC-MS) with naphthalene as internal standard, and dichloromethane ultrasonic extraction method. The chromatographic conditions were the instrument model: GCMS-QP2010 gas chromatography-mass spectrometry (Shimadzu, Japan), equipped with GCMS-solution 2.10 chromatographic workstation; column: FactorFourTM: capillary column; VF-WAXms ( 30 m×0.25 mm×0.25 μm); program temperature rise: initial temperature of 40 ℃ for 3 min, warming up to 100 ℃ at 5.6 ℃/min, keeping 1 min, warming up to 125 ℃ at 3.1 ℃/min, warming up to 230 ℃ at 15 ℃/min for 5.44 min; inlet temperature: 250 ℃; carrier gas: high purity helium (99.999%); flow rate: 1.10 mL/min; no split; 1 μL injection volume. Mass spectrometry (MS) conditions: EI ion source; ionization voltage 70 ev; the temperature of the ion source was 200 ℃; the interface temperature was 260 ℃; the detector voltage was 1.2 KV; the solvent delay time was 3 min; and the selective ion scanning (SIM) was performed.
2.4 Statistical analysis of dataThe morphological parameters and secondary metabolites (phenols and volatiles) ofH.cordatawere statistically analyzed using Microsoft Office Excel 2010 software, and each treatment parameter was analyzed in three replicates. Ten biological replicates were set up for each experimental treatment ofH.cordata.
3 Results and analysis
3.1 Effect of different treatment groups on the growth status of seedlingsSignificant differences were observed in plant height, root length, fresh weight, and plant morphology ofH.cordata. The growth morphology can be seen from Table 1, the exogenous plant hormones IAA and GA3can stimulate the new shoot differentiation and expand the number of plants ofH.cordata. The treatment groups of all three exogenous plant hormones showed white fibrous root emergence at the nodes of the plant stems, and there were underground stems produced fromH.cordataseedlings when treated with 3 mg/L NAA, and the longest nodes of the plant stems were produced when the growth hormone was GA3when treated with different exogenous plant hormones inH.cordataseedlings as compared to control CK. As shown in Table 2, the plant height, root length, fresh weight and plant morphology ofH.cordata. also showed significant differences when different exogenous growth hormone concentrations were treated inH.cordataseedlings. When the exogenous plant hormone was NAA, the plants were in monoculture, and there was no significant difference in plant height in the 0.1 mg/L NAA treatment compared to CK, whereas the plant height was significantly greater than that of CK at concentrations of 2 and 3 mg/L, which were 5.82 and 6.91 cm, respectively, and the root length of NAA was significantly smaller than that of CK at concentrations of 1 and 2 mg/L, whereas that of NAA at 3 mg/L was significantly greater than that of CK, with an increase of 52.6%. In addition, all three concentrations of NAA resulted in a significantly higher fresh weight ofH.cordatathan CK. When the exogenous plant hormone was IAA, the single plant differentiated into two or three plants, and the fibrous roots at the stem nodes increased significantly; when the concentration of IAA was 2 mg/L, the plant height ofH.cordatawas 5.56 cm, which was the optimum growth concentration of IAA, where the fresh weight ofH.cordata.3.47 g at 3 mg/L IAA was significantly increased by 281.6% compared with that of CK. The exogenous plant hormone GA3resulted in significant bud differentiation and vigorous growth of plants; plant height, root length, and fresh weight were significantly enhanced compared with CK, in which the plant height ofH.cordata9.23 cm increased by 79.9% compared with that of CK in the treatment of 3 mg/L GA3, and the root length of 3.85 cm increased significantly by 41.5% compared with that of CK; GA3concentration of 1 mg/L significantly increased by 458.2% at a fresh weight of 5.47 g ofH.cordata., and the plants were differentiated into three plants with the longest stem nodes.

Table 1 Effect of different exogenous plant hormones on plant morphology of Houttuynia cordata seedlings
3.2 Effect of different exogenous plant hormones on six phenolics in seedlingsThere were differences in the four phenolics of theH.cordataseedlings from the different treatment groups. The content of chlorogenic acid was significantly greater than that of CK when exogenous plant hormone NAA was used to treatH.cordataseedlings, and the highest content of chlorogenic acid was 5.54 μg/g in 1 mg/L NAA treatment, which was a significant increase of 52.6%; however, the content of rutin, isoquercitrin, and quercitin was all significantly lower than that of CK (Fig.2-A). The content of chlorogenic acid, rutin, isoquercitrin, and quercitin was significantly higher than that of CK when exogenous plant hormone IAA was treated inH.cordataseedlings, with the strongest stimulation of chlorogenic acid, rutin, isoquercitrin, and quercitin by 1 mg/L IAA, which increased by 109.1%, 83.53 μg/g, 18.73 μg/g, 6.72 μg/g, respectively, and by 100.6%, 173.8%, and 198.7%, respectively (Fig.2-B). As can be seen from the figure, the content of the four phenolic compounds decreased with the increase in IAA concentration, and the content of isoquercitrin was significantly lower than that of CK in 3 mg/L IAA treatment. When the exogenous plant hormone was GA3, the content of chlorogenic acid, rutin, isoquercitrin and quercitin ofH.cordatawas significantly higher than that of CK in 3 mg/L GA3treatment, the content of 6.00, 85.33, 16.35, and 6.58 μg/g significantly increased by 65.3%, 104.9%, 139.0%, and 191.2%, respectively, and in 1 mg/L GA3treatment, the content of all four phenolic compounds was significantly less than that of CK (Fig.2-C). As can be seen from the figure, the higher the concentration of GA3, the higher the phenolic content ofH.cordata. The kaempferol-3-oglucorhamnoside and quercetin were not detected in all treatment groups. Overall, both NAA and IAA promoted the accumulation of chlorogenic acid and NAA inhibited the accumulation of rutin, isoquercitrin, and quercitin inH.cordata, while both low concentrations of IAA and high concentrations of GA3promoted the accumulation of chlorogenic acid, rutin, isoquercitrin and quercetin inH.cordata.
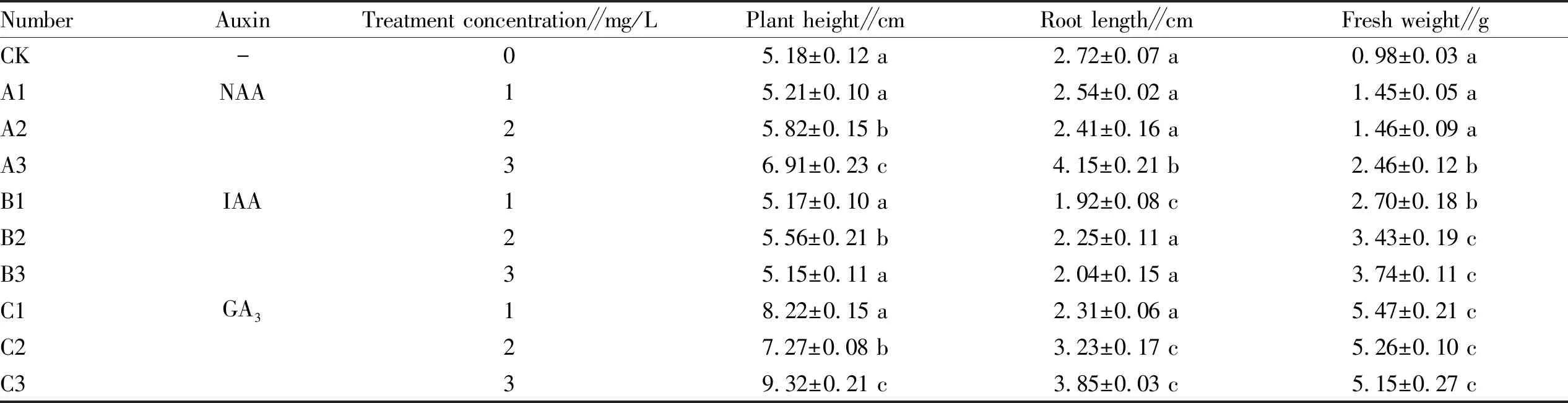
Table 2 Effect of different exogenous plant hormones on growth morphology of Houttuynia cordata seedlings
3.3 Effect of different exogenous plant hormones on volatile compounds of seedlingsAs shown in Fig.3, the accumulation of volatile substances inH.cordataseedlings treated with different exogenous plant hormones was not the same. The accumulation of α-Pinene was significantly greater than that of CK (0.24 μg/g) in all treatment groups ofH.cordataseedlings, and the highest α-Pinene content was found in 2 mg/L NNAA and 2 mg/L IAA treatments, amounting to 8.3 and 8.6 μg/g, respectively (Fig.3A). The accumulation of β-Pinene was significantly greater than that of CK (0.18 μg/g) in all other treatment groups ofH.cordataseedlings, with content ranging from 0.59 to 5.89 μg/g, except for the 2 mg/L GA3treatment group (Fig.3A). The accumulation of β-Myrcene and Limonene was significantly greater than that of CK (1.30 μg/g) and CK (0.12 μg/g) inH.cordataseedlings from the other treatment groups, with content ranging from 2.55 to 31.20 μg/g and from 0.21 to 2.63 μg/g, respectively, except for the 2 mg/L GA3treatment group (Fig.3A and B). The accumulation of Camphorate was significantly greater than that of CK (0.08 μg/g) in all treatment groups ofH.cordataseedlings, with content ranging from 0.19 to 2.75 μg/g, except for the 2 mg/L GA3treatment group (Fig.3B). Accumulation of Cineole in 1 mg/L IAA, 1 mg/L GA3, and 3 mg/L GA3-treatedH.cordatawas significantly greater than that in CK (0.04 μg/g), and their content was 0.12-1.22 μg/g, whereas none of the other treatment groups were detected (Fig.3B). The accumulation of γ-Tepinene was significantly greater than that of CK (0.19 μg/g) inH.cordatain all treatment groups except for the 3 mg/L IAA and 2 mg/L GA3treatment groups (Fig.3C). The Hexanol content ofH.cordatawas significantly greater than that of CK (0.04 μg/g) under the treatments of 1 mg/L NAA, 1 mg/L IAA, 2 mg/L IAA, 3 mg/L GA3(Fig.3D). The accumulation of cis-3-hexen-1-ol by 1 mg/L IAA,and 3 mg/L GA3-treatedH.cordatawas significantly greater than that of CK, whereas none of the other treatment groups were detected (Fig.3D). The accumulation of Trans-2-hexen-1-ol was significantly greater than that of CK (0.42 μg/g) in all other treatment groups ofH.cordata, with content ranging from 0.65 to 17.49 μg/g, except for the 3 mg/L NAA treatment group (Fig.3D). Under the three exogenous plant hormones treatments, the accumulation of Decanal and Linalool inH.cordatawas significantly greater than that in CK (104.39 μg/g), with content ranging from 180.13 to 1 535.27 μg/g and from 4.92 to 44.68 μg/g, respectively (Fig.3E and C). Except for the 3 mg/L NAA and 2 mg/L GA3treatment groups, the accumulation of Borneol was significantly greater than that of the control group (0.42 μg/g) (Fig.3A), and the content of lobelia ranged from 0.65 to 17.49 μg/g in theH.cordataseedlings (Fig.3C). The accumulation of Decanal was significantly greater than that of CK inH.cordataseedlings when treated with exogenous plant hormones IAA and GA3, ranging from 5.70 to 44.51 μg/g (Fig.3D). The accumulation of Trans-2-hexenal was significantly greater than that of CK in all treatment groups ofH.cordata, with content ranging from 0.37 to 2.87 μg/g (Fig.3E). The accumulation of 3-Tetradecanone was significantly greater than that of CK inH.cordatain all treatment groups, ranging from 11.84 to 219.02 μg/g (Fig.3F). The accumulation of 2-Undecanone was significantly greater than that of CK (0.83 μg/g) in fritillary seedlings in all treatment groups, with content ranging from 1.26 to 13.62 μg/g, except for the 2 mg/L GA3treatment group (Fig.3F). The accumulation of cis-3-Hexenyl-acetate ester was significantly greater than that of CK (0.05 μg/g) in all treatment groups ofH.cordataseedlings, with content ranging from 0.18 to 7.36 μg/g (Fig.3G). The accumulation of Bornyl acetate was significantly less than that of CK (0.22 μg/g) in 2 mg/L GA3treatments, and the accumulation of Bornyl acetate was significantly greater than that of CK in all other treatment groups ofH.cordataseedlings (Fig.3G).

Note: A-NAA, B-IAA, C-GA.
4 Discussion
4.1 Effect of different exogenous plant hormones on the growth of seedlingsIn this study, we reported the effects of NAA, IAA, and GA3as exogenous plant hormones on the growth and morphology ofH.cordataexogenous plant hormone administration promoted the growth and development ofH.cordata. In a recent study, it was predicted that plant hormones alter plant body sugar metabolism, which is responsible for the regulation of sugar metabolism involved in plant growth and promotes the growth and development of plants to show different plant forms[23-24]. The effects of different exogenous plant hormones in plants on the plant height, root length, fresh weight, and plant morphology ofH.cordataseedlings differed. The experiments showed that a high concentration of NAA promoted the underground stems ofH.cordata, implying that the addition of a high concentration of NAA could promote the induction of underground stem generation ofH.cordatain the process of tissue culture. In addition, it has been shown that IAA can increase endogenous GA accumulation and the two plant hormones synergistically regulate plant growth and development[14]. In our study, we found that IAA increased leaf surface area and number of tufted shoots, and GA3promoted plant height, root length, and branching inH.cordata, which shows that IAA and GA3are effectively involved in vascular cell division and differentiation. In Glycin max, IAA has been reported to stimulate plant height, number and area of leaves, number of branches, and seeds per plant[9]. The concentration of exogenous plant hormones also affected the growth and morphological performance of the plants. With the addition of 3 mL/L of NAA, the plant height, root length, and fresh weight ofH.cordataseedlings were optimized; with the addition of 2 mL/L of IAA, the plant height, root length, fresh weight, and morphology were better; whereas the higher the concentration of GA3, the better the whole physiological, and morphological performance ofH.cordata. Our study shows that to achieve the optimal conditions of tissue culture, we can choose the growth hormone species reasonably and increase or decrease the concentration of growth hormone appropriately according to the different needs of the plants to effectively stimulate the growth of the plants to improve the medicinal value, which provides a basis for further research on tissue culture ofH.cordatavulgaris and provides a theoretical basis for the development and utilization of medicinal plants.
4.2 Effect of different exogenous plant hormones on secondary metabolites of seedlingsExogenous plant hormones can not only regulate physiological processes such as development, metabolism, and senescence by affecting nucleic acids, proteins, and enzymes in plants, but also regulate the synthesis of secondary metabolites such as flavonoids, terpenoids, and alkaloids in the plant body[25-26]. NAA, IAA, and GA3are plant hormones that can be synthesized in the plant or stimulated in the form of an additive growth hormone to regulate certain biochemical reactions in the plant, which in turn leads to the regulation of changes in secondary metabolite content[27]. The experimental results of this study showed that NAA promoted the accumulation of chlorogenic acid and inhibited the accumulation of rutin and quercetin inH.cordata; IAA promoted the accumulation of chlorogenic acid inH.cordata; and both high concentrations of NA and GA3, and low concentrations of IAA promoted the accumulation of quercetin. It is implied that we can purposefully apply a certain amount of plant hormones to enhance the medicinal value components ofH.cordatawhen we are culturing them. Yang[28]etal.demonstrated thatArtemisiaabsinthiumstimulated the biosynthesis of a variety of secondary metabolites, including phenylpropanoids, flavonoids, terpenoids, and alkaloids in leaves under the treatment of three exogenous hormones (MeJA, SA, and ABA). This result provides a new perspective to study the effects of exogenous hormones on the growth ofArtemisiaabsinthiumleaves.
The exogenous plant hormones NAA, IAA, and GA3regulated monoterpenes, sesquiterpenes, aldehydes, ketones, alcohols, and esters to different degrees, but the overall results were similar, and all of them resulted in a significant up-regulation of the content of volatile substances inH.cordata, of which the accumulation of the 20 types of volatile substances inH.cordataby 1 mg/L IAA was extremely significant (Fig.3). This is consistent with Dong’s findings that the addition of the exogenous plant hormone methyl jasmonate (MeJA) favors the biosynthesis of terpenoids in lavender over a range of concentrations[29]. Both exogenous ethylene (ETY) and abscisic acid (ABA) plant hormones induced steroid and triterpenoid biosynthesis pathways in the hairy roots ofCalendulaofficinalis[30]. From this, we inferred that the addition of exogenous plant hormones to stimulate secondary metabolism in medicinal plants provides a feasible method to improve the medicinal constituents ofH.cordata, and also lays the foundation for the study of the process of exogenous plant hormone regulation and the mechanism of influence on the secondary metabolites of plants.
5 Conclusion
Our experiments confirmed that exogenous plant hormones not only had a regulatory effect on the growth and development ofH.cordata, but also had an important influence on the four phenolic substances and 20 volatile substances ofH.cordata, which provides a theoretical basis for increasing the content of secondary metabolites ofH.cordata, improving the medicinal efficacy ofH.cordata, and developing and utilizing other functions; at the same time, it provides a new research idea and direction for the regulatory mechanism of exogenous plant hormones on the growth and development and secondary metabolites of medicinal plants. However, the research on how exogenous plant hormones regulate plant morphology and thus affect secondary metabolites is still lacking, which will be the focus and hotspot of future research.
- Medicinal Plant的其它文章
- Research Progress in the Treatment of New Bone Formation of Ankylosing Spondylitis
- Current Status of Mongolian Medicine Treatment for Breast Hyperplasia
- Anti-Tumor and Anti-Diabetic Effects of Sarsasapogenin
- Research Overview of Zhuang Medicine Fumigation Lotions
- Antioxidant and Hypoglycemic Ability of Ardisia gigantifolia Stapf Parts
- Incidence and Risk Factors of Sub-syndromal Delirium in Patients after Cardiac Surgery

