Prognostic value of circulating tumor cells combined with neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma
Jia-Li Chen,Lu Guo,Zhen-Ying Wu,Kun He,Han Li,Chi Yang,Yun-Wei Han
Abstract BACKGROUND Circulating tumor cell (CTC) count and neutrophil-to-lymphocyte ratio (NLR) are both closely associated with the prognosis of hepatocellular carcinoma (HCC).AIM To investigate the prognostic value of combining these two indicators in HCC.METHODS Clinical data were collected from patients with advanced HCC who received immune therapy combined with targeted therapy at the Department of Oncology,the Affiliated Hospital of Southwest Medical University,Sichuan,China,from 2021 to 2023.The optimal cutoff values for CTC programmed death-ligand 1 (PDL1) (+) > 1 or CTC PD-L1 (+) ≤ 1 and NLR > 3.89 or NLR ≤ 3.89 were evaluated using X-Tile software.Patients were categorized into three groups based on CTC PD-L1 (+) counts and NLR: CTC-NLR (0),CTC-NLR (1),and CTC-NLR (2).The relationship between CTC-NLR and clinical variables as well as survival rates was assessed.RESULTS Patients with high CTC PD-L1 (+) expression or NLR at baseline had shorter median progression-free survival (m-PFS) and median overall survival (mOS) than those with low levels of CTC PD-L1 (+) or NLR (P < 0.001).Meanwhile,patients in the CTC-NLR (2) group showed a significant decrease in mPFS and mOS.Cox regression analysis revealed that alpha-fetoprotein (AFP),CTC PD-L1 (+),and CTC-NLR were independent predictors of OS.The time-dependent receiver operating characteristic curve showed that the area under the curve of CTC-NLR at 12 months (0.821) and 18 months (0.821) was superior to that of AFP and CTC PD-L1 (+).CONCLUSION HCC patients with high CTC PD-L1 (+) or NLR expression tend to exhibit poor prognosis,and a high baseline CTC-NLR score may indicate low survival.CTC-NLR may serve as an effective prognostic indicator for patients with advanced HCC receiving immunotherapy combined with targeted therapy.
Key Words: Circulating tumor cells;Neutrophil-lymphocyte ratio;Hepatocellular carcinoma;Prognosis;Survival;Marker
lNTRODUCTlON
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers worldwide and a major public health concern.The incidence rate of HCC has been soaring annually[1].HCC is projected to become the third leading cause of cancer-related deaths by 2030[2].Several treatment options are available for HCC,including liver transplantation,surgical resection,percutaneous ablation,radiotherapy,and arterial and systemic therapies[3].Clinical physicians need to comprehensively evaluate the patient’s condition and adjust individualized treatment plans.Immune checkpoint inhibitor (ICI) monotherapy has provided significant clinical benefits to 15%-20% of patients since ICIs became available in clinical practice.ICIs have brought hope to many patients with malignant tumors.However,effective biomarkers for identifying this population are currently lacking[4,5].Therefore,the identification of new predictive biomarkers to guide the selection of treatment plans is of great significance.
Currently,the diagnosis and treatment guidance for HCC patients primarily rely on imaging,tissue biopsy[6],and serum alpha-fetoprotein (AFP) levels[7,8].However,tissue biopsy procedures are complicated,imaging tests lack diagnostic sensitivity,and AFP specificity is only about 80%[9,10],all of which cannot accurately reflect tumor heterogeneity and dynamically monitor tumor progression.Therefore,identifying a novel minimally invasive or non-invasive diagnostic strategy is urgent to monitor the therapeutic effects of HCC and predict prognosis.
In recent years,“liquid biopsy” technology has sparked strong interest from researchers[11,12].Circulating tumor cells (CTCs) in liquid biopsies can provide information about abnormal protein expression,genomic mutations,and messenger RNA variations in solid tumors.They can also help understand the mechanisms underlying tumor occurrence,metastasis,and drug resistance from aspects such as cell morphology,migratory ability,and drug response[13,14].Multiple studies have shown promising prospects of CTCs in the diagnosis,treatment,and prognosis guidance of various malignant tumors[15-18].It has been demonstrated that programmed death-ligand 1 (PD-L1) expression is an essential biomarker for treatment decision-making in the era of immunotherapy.CTC PD-L1 expression can serve as an effective biomarker for clinical immunotherapy in various malignant tumors[19-21].Meanwhile,several studies have shown that neutrophil-to-lymphocyte ratio (NLR) is a potential marker of tumor prognosis in certain malignant tumors[22-24].In peripheral blood,CTCs can interact with inflammatory cells to induce systemic inflammation,promote metastasis,and worsen prognosis[25,26].Considering NLR based on CTCs can improve risk stratification and optimize management in cancer patients[27].It has been demonstrated that combining CTC with NLR can significantly improve the prognosis prediction of malignant gastrointestinal tumor patients[28].Therefore,the present study explored the prognostic value of CTC PD-L1 (+) in combination with NLR in HCC patients receiving immunotherapy.
MATERlALS AND METHODS
Patients
Clinical data were collected from patients with advanced HCC who received immunotherapy in combination with targeted therapy at the Department of Oncology,the Affiliated Hospital of Southwest Medical University,Luzhou,Sichuan,China,from 2021 to 2023.The inclusion criteria were as follows: (1) Pathologically diagnosed with HCC;(2) no previous anti-tumor treatment before admission;(3) Child-Pugh class A/B;(4) barcelona clinic liver cancer (BCLC) stage B/C;(5) received immunotherapy in combination with targeted therapy after admission.The exclusion criteria were as follows: (1) Patients with concomitant secondary primary malignancy;(2) intolerance to immune or targeted drugs during the tumor treatment process.
This study complied with the guidelines of the Helsinki Declaration.The research protocol was approved by the Clinical Trial Ethics Committee of the Affiliated Hospital of Southwest Medical University (approval number: KY2021063) and registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2100044198).All patients signed a written informed consent form before enrollment.
Detection of CTC PD-L1 (+)
Before treatment,4 mL of peripheral blood was collected from each HCC patient using an anticoagulant tube and processed within 6 h.PD-L1 (+) CTCs were captured using the CytoSorter®circulating tumor cell sorter (Watson Biotech) and CytoNanoChip by combining antiepithelial cell adhesion molecule (Anti-EpCMA) Ab and anti-cell-surface vimentin (Anti-CSV) Ab with the CytoNanoChip and counted under a fluorescence microscope.
Follow-up monitoring
All patients underwent comprehensive baseline examinations,including imaging and laboratory tests,before treatment.Progression-free survival (PFS) was defined as the time from the start of treatment to disease progression or death,while overall survival (OS) was defined as the time from the start of treatment to death or last follow-up.
Statistical analysis
SPSS 26.0 (IBM,Chicago,IL,United States) and R version 4.2.2 were utilized for statistical analysis.Descriptive statistics were used to analyze patient baseline characteristics.X-Tile statistical package (version 3.6.1,Yale University,New Haven,CT,United States) was employed to calculate optimal cutoff points for CTC PD-L1 (+) and NLR,and the significance of between-group differences was evaluated using the chi-square test.Kaplan-Meier survival curves were compared using the log-rank test (Mantel-Cox).Univariate and multivariate Cox regression analyses were applied to investigate the association between patient baseline characteristics and OS.The area under the curve (AUC) was obtained by plotting the receiver operating characteristic (ROC) curve.APvalue of < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
A total of 124 patients with advanced HCC were recruited.The baseline characteristics of enrolled patients are shown in Table 1.The majority of the patients were males (90.3%),with a median age of 55 years (range 27-78 years).Seventy patients (56.5%) had hepatitis B virus (+),46 (37.1%) had cirrhosis,49 (39.5%) had serum AFP levels ≥ 400 ng/mL,86 (69.4%) had multiple tumors,and 115 (92.7%) were in BCLC stage C.
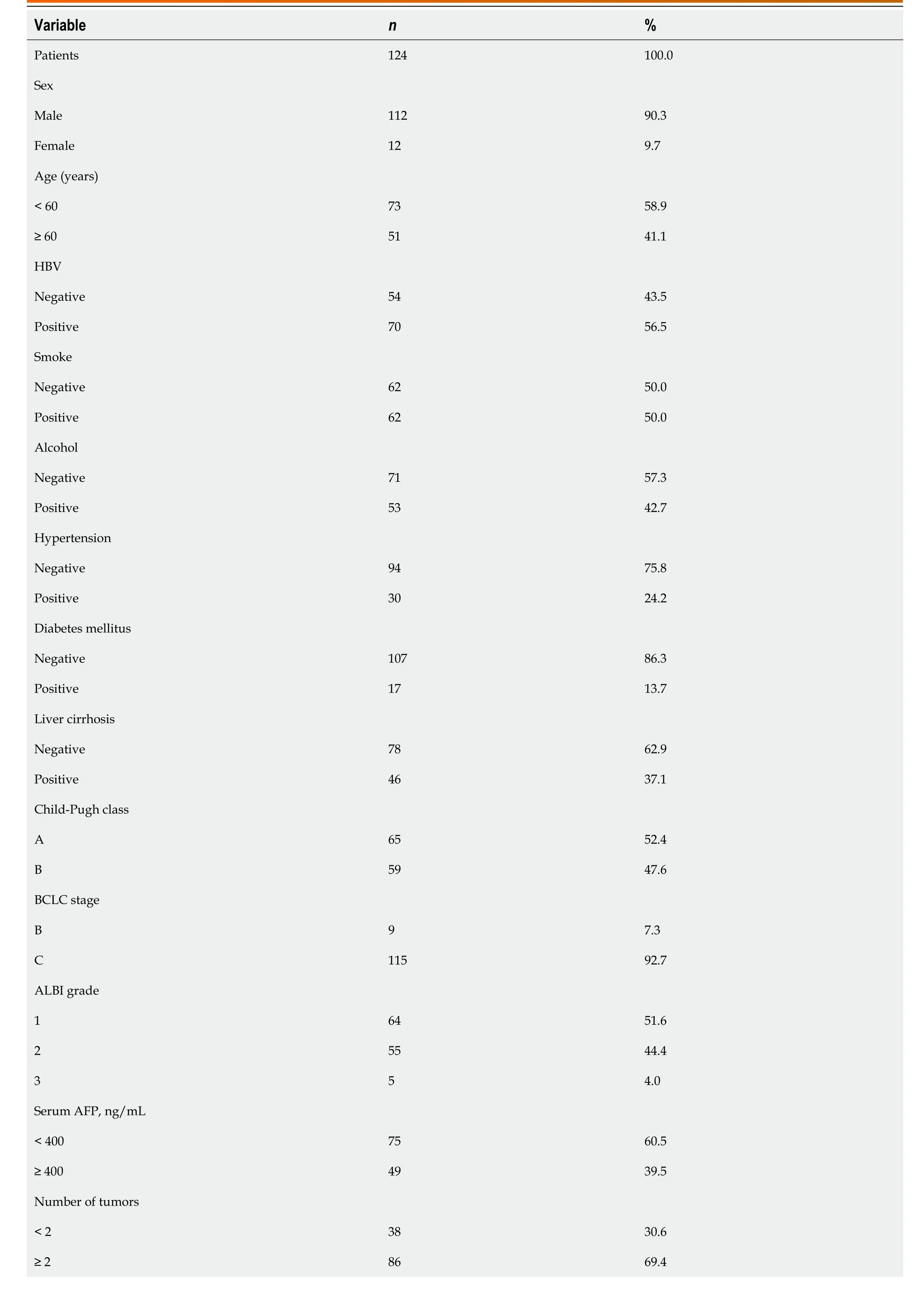
Table 1 Clinical characteristics of the hepatocellular carcinoma patients in the study
Correlation between CTC PD-L1 (+) and clinical variables and survival
According to X-Tile software,the optimal cutoff value for CTC PD-L1 (+) was 1,and patients were divided into two categories based on this threshold.Among them,68 patients (54.8%) had CTC PD-L1 (+) ≤ 1,while 56 patients (45.2%) had CTC PD-L1 (+) > 1.The relationship between CTC PD-L1 (+) and clinical variables is shown in Table 2.The results revealed that CTC PD-L1 (+) was associated with liver cirrhosis but not with other clinical variables.

Table 2 The correlation between circulating tumor cell PD-L1 (+) and neutrophil-lymphocyte ratio with clinical variables
Furthermore,the predictive significance of CTC PD-L1 (+) was examined between the two patient cohorts.It was found that patients with CTC PD-L1 (+) ≤ 1 had a longer median OS (mOS) [not reached (NR)vs6.0 months,hazard ratio (HR)=6.67,95% confidence interval (95%CI): 3.60-12.37,P< 0.001,Figure 1A) and median PFS (mPFS,7.8 monthsvs3.2 months,HR=3.59,95%CI: 2.27-5.69,P< 0.001,Figure 1B) compared with those with CTC PD-L1 (+) > 1.Meanwhile,Kaplan-Meier curve analysis revealed that the 2-year OS rates for patients with high and low expression of CTC PD-L1 (+) were 22.6% and 69.0%,respectively.
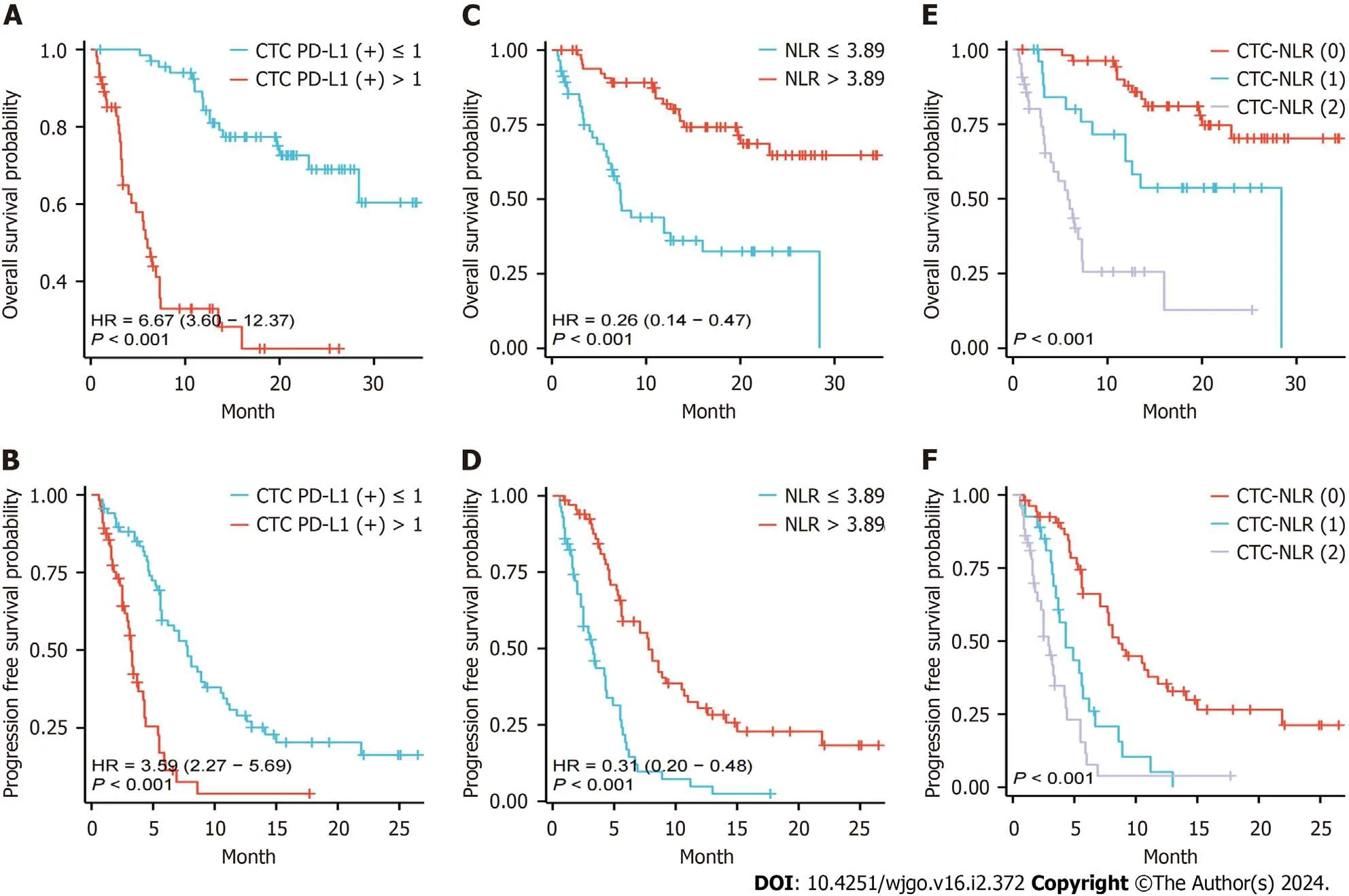
Figure 1 Survival rate comparison. A and B: Kaplan-Meier overall survival (OS) curves (A) and progression-free survival (PFS) curves (B) in different subgroups of hepatocellular carcinoma (HCC) patients with circulating tumor cell programmed death-ligand 1 (+);C and D:Kaplan-Meier OS curves (A) and PFS curves (B) in different subgroups of HCC patients with neutrophil-to-lymphocyte ratio;E and F: OS curves (A) as well as PFS curves (B) for different subgroups of HCC patients with circulating tumor cell-neutrophil-to-lymphocyte ratio.HR: Hazard ratio;CTC: Circulating tumor cell;PD-L1: Programmed death-ligand 1;NLR: Neutrophil-to-lymphocyte ratio.
Relationship between NLR and clinical parameters and survival
Based on the X-Tile software,the ideal cutoff value for NLR was 3.89.Patients were then classified into two groups based on this cutoff value,of which 67 (54.0%) patients had an NLR ≤ 3.89 and 57 (46.0%) patients had an NLR > 3.89.The relationship between NLR and clinical variables is displayed in Table 2.The results showed that NLR was significantly positively/negatively associated with cirrhosis,AFP,and PVTT but not with other clinical factors.
Additionally,the prognostic value of NLR was analyzed between the two groups and results revealed that patients with NLR ≤ 3.89 had longer mOS (NRvs7.3 months,HR=0.26,95%CI: 0.14-0.47,P< 0.001,Figure 1C) and mPFS (7.8 monthsvs3.3 months,HR=0.31,95%CI: 0.20-0.48,P<0.001,Figure 1D) compared with their counterparts.Moreover,Kaplan-Meier curve analysis showed that the 2-year OS rates of patients in the low and high NLR expression groups were 64.8% and 32.5%,respectively.
Relationship between CTC-NLR and survival
Based on the critical values of CTC PD-L1 (+) and NLR,the CTC-NLR score was calculated as follows: A score of 0 for CTC PD-L1 (+) ≤ 1 and NLR ≤ 3.89;a score of 1 for patients with CTC PD-L1 (+) > 1 or NLR > 3.89;and a score of 2 for CTC PD-L1 (+) > 1 and NLR > 3.89.
The NLR-CTC score was used to stratify patients for OS prediction.A total of 54 (43.5%) patients were categorized as CTC-NLR (0),27 (21.8%) as CTC-NLR (1),and 43 (34.7%) as CTC-NLR (2) (Table 3).The results showed that CTC-NLR scores at baseline were associated with sex,alcohol consumption,and cirrhosis,independent of other clinical variables.Kaplan-Meier curve analysis showed that patients in the CTC-NLR (0) group performed well in terms of OS and PFS (P< 0.001),while those in the CTC-NLR (2) group performed worse (Figure 1D and E).
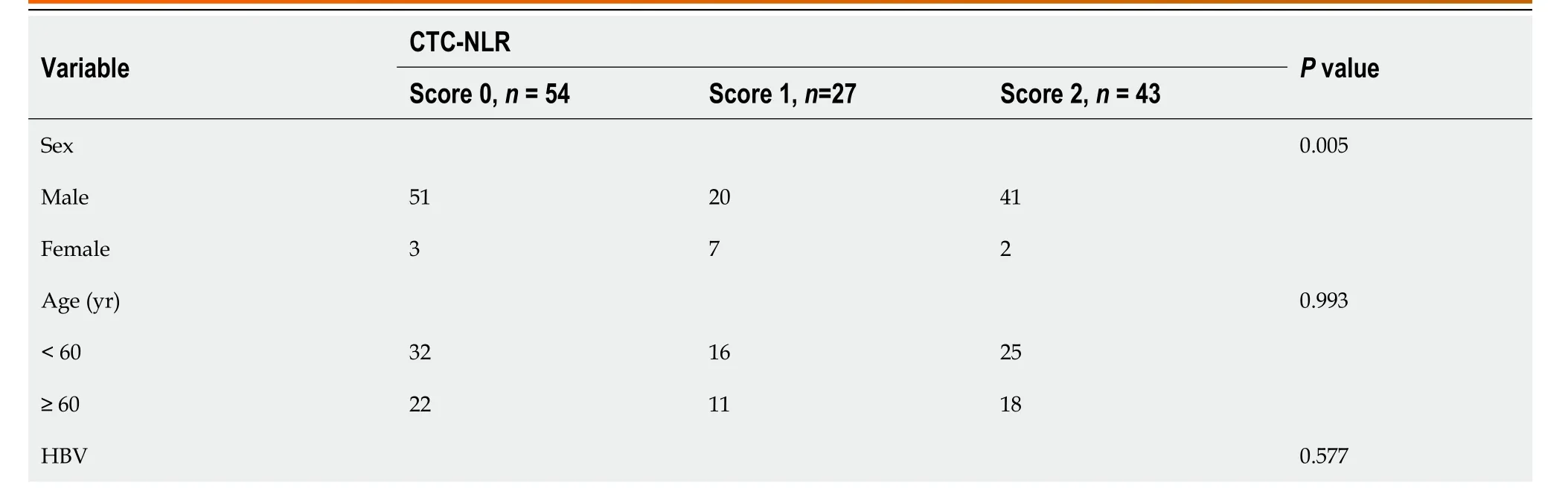
Table 3 The correlation between circulating tumor cell-neutrophil-lymphocyte ratio score and clinical baseline characteristics in hepatocellular carcinoma patients
Prognostic factors for OS
Univariate Cox regression analysis revealed that cirrhosis,albumin bilirubin grade,AFP,CTC PD-L1 (+),NLR,and CTCNLR were prognostic factors for OS (Table 4).These variables were then integrated into the multivariate Cox regression analysis.Because CTC PD-L1 (+),NLR,and CTC-NLR were highly correlated in this study,two independent multivariate models were constructed to eliminate multicollinearity between the three variables.Multivariate Cox regression analysis showed that CTC PD-L1 (+),CTC-NLR,and AFP were independent prognostic factors for OS (Table 5).
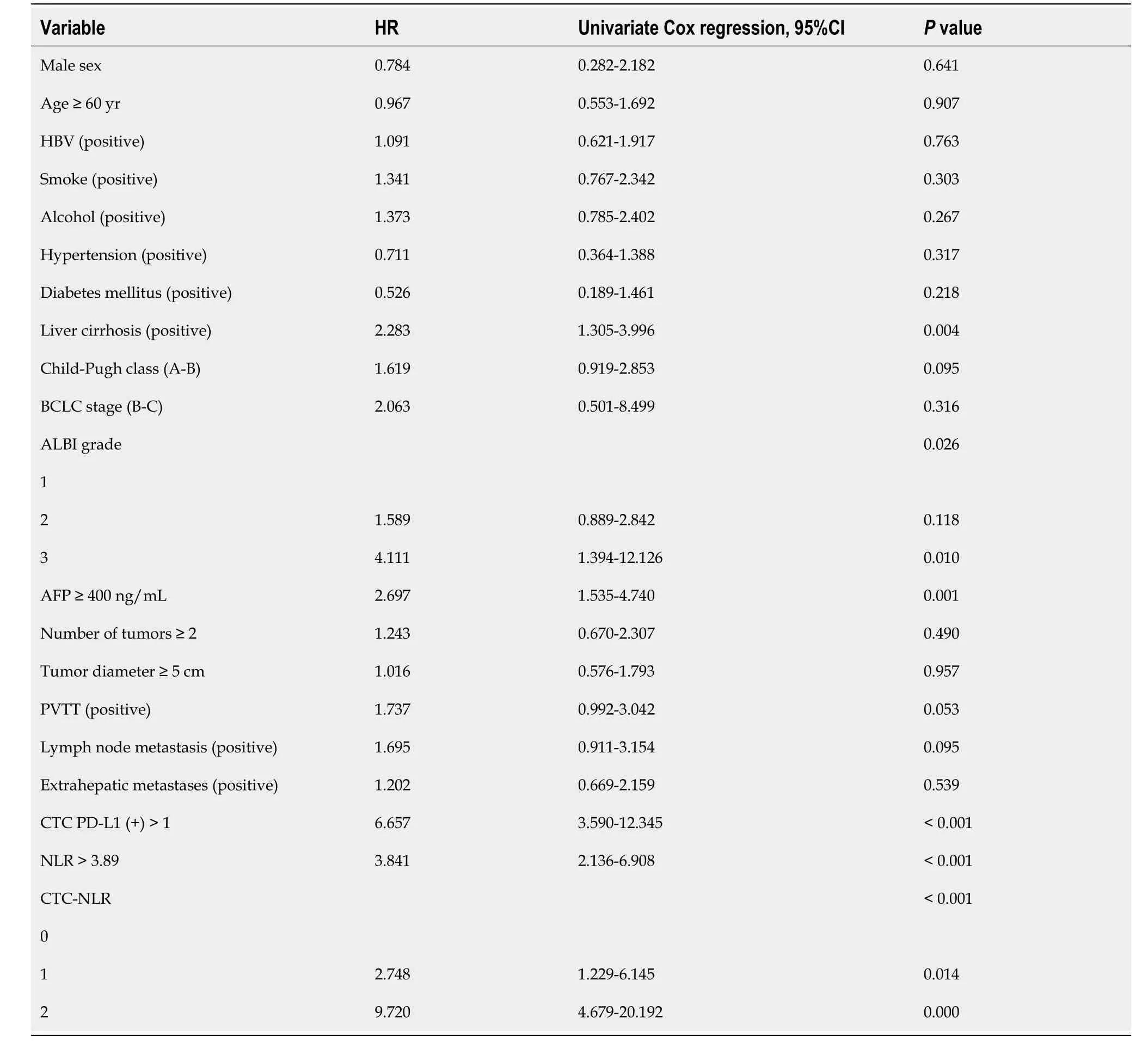
Table 4 Univariate Cox regression analysis based on overall survival
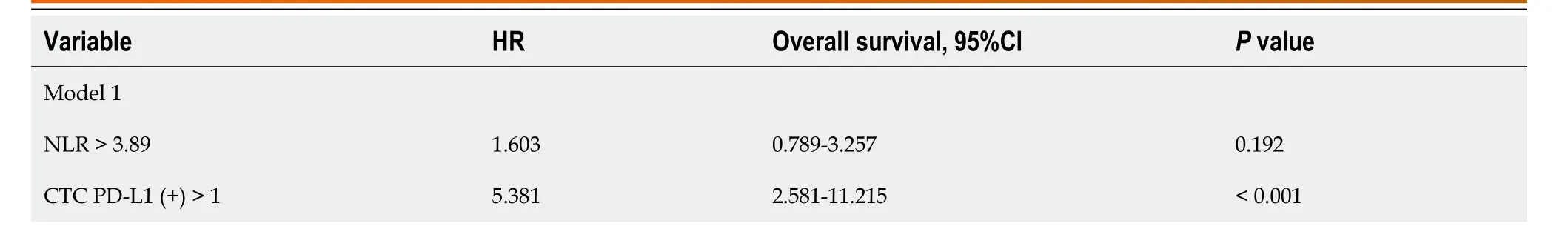
Table 5 Multivariate Cox regression analysis based on overall survival
Comparison of the predictive value of independent prognostic factors
The prognostic efficacy of AFP,CTC PD-L1 (+),and CTC-NLR was compared at 6-,12-,and 18-month OS using timedependent ROC curves.The results revealed that the prognostic efficacy of CTC-NLR (0.821) was significantly better than that of CTC PD-L1 (+) (0.789) and AFP (0.687) at 12-month OS (Figure 2).The prognostic efficacy of CTC-NLR (0.821) was also remarkably better than that of CTC PD-L1 (+) (0.794) and AFP (0.676) at 18-month OS (Figure 2).

Figure 2 Comparison of the area under the time-dependent receiver operating characteristic curve for overall survival prediction. A-C: Comparison of predictive efficacy of circulating tumor cell (CTC)-neutrophil-to-lymphocyte ratio (A),CTC programmed death-ligand 1 (+) (B),and alpha-fetoprotein (C) indicators at 6,12,and 18 months in hepatocellular carcinoma patients.CTC: Circulating tumor cell;PD-L1: Programmed death-ligand 1;NLR: Neutrophil-tolymphocyte ratio;AFP: Alpha-fetoprotein;AUC: Area under the curve.
DlSCUSSlON
As the first line of defense of the organism,neutrophils play an important role in natural immunity.With technological advances in recent years,the complexity and heterogeneity of neutrophils in physiological and pathological states have received extensive attention.Numerous clinical studies have shown that neutrophils can promote tumorigenesis and progression in multiple ways,which significantly correlate with the patient’s prognosis.Cancer immunosurveillance relies heavily on lymphocytes,which can kill tumor cells[29,30].NLR responds to a balanced relationship between tumorvalidated states and anti-tumor immune responses[31].Extensive studies have described NLR as a general prognostic factor for several cancer types[32-35].The current study found that a high NLR was associated with poorer PFS and OS but was not an independent factor affecting the prognosis,which may be due to the insufficiently long follow-up time and large sample size in our study.
CTC is considered a source of tumor metastasis and recurrence[36].The prognostic value of CTC has been demonstrated in breast,prostate,and colorectal cancer cancers,as well as small cell and non-small cell lung cancers[37-41].Meanwhile,some studies have shown that CTC is an independent risk factor for HCC,which is significantly correlated with the prognosis of patients[42-44],and the CTC PD-L1 expression assay has a broad application prospect for dynamic monitoring of disease changes and evaluation of treatment response during immunotherapy[45-47].These conclusions are consistent with our findings,in which high CTC expression of PD-L1 (+) at baseline was associated with poor prognosis in patients with HCC.
A combination of inflammatory index and CTC has been previously used to predict the outcome of malignant tumors[48,49].Considering that NLR is strongly associated with tumor progression and CTC can predict prognosis,we combine NLR with CTC to predict the outcome of HCC.We evaluated the combined metric CTC-NLR and categorized patients into three groups based on their scores and discovered that patients with lower CTC-NLR scores had a survival benefit from the treatment.It was finally established that the three metrics,CTC-NLR,CTC PD-L1 (+),and AFP,were independent prognostic indicators for OS in patients with HCC.Meanwhile,CTC-NLR exhibited the highest time-dependent AUC at 12 and 18 months (i.e.,0.821 for both).These findings demonstrated that a combination of CTC and NLR has a better prognostic value for HCC patients than CTC PD-L1 (+) or AFP alone.These data provide new avenues for risk stratification management of HCC.
Nonetheless,this study has several limitations.Firstly,this is a single center-based study.Secondly,because of the small sample size,we were unable to divide patients into training and validation cohorts.Finally,there was a relatively short follow-up period.Therefore,large-scale multicenter studies are warranted to validate and replicate our results.
CONCLUSlON
In summary,the current study suggests the combination of CTC and NLR scores as a new index for predicting survival in patients with HCC.HCC patients may benefit from pre-treatment assessment of their CTC-NLR scores for risk classification and clinical decision-making.
ARTlCLE HlGHLlGHTS
Research background
Patients with hepatocellular carcinoma (HCC) have a low benefit rate from immunotherapy.Clinical treatment lacks highly specific and sensitive prognostic biomarkers.
Research motivation
Investigation on whether the ratio of programmed death-ligand 1 (PD-L1) on circulating tumor cells (CTCs) combined with neutrophil-to-lymphocyte ratio (NLR) can serve as a biomarker for predicting the prognosis of immune combined targeted therapy in HCC.
Research objectives
Search for novel combined predictive biomarkers and apply them for risk stratification and clinical decision-making.
Research methods
This study adopts a method that combines clinical trials with data analysis.
Research results
HCC patients with high CTC PD-L1 (+) or NLR expression tend to exhibit poor prognosis,and a high baseline CTC-NLR score may indicate low survival.
Research conclusions
CTC-NLR may serve as an effective prognostic indicator for patients with advanced HCC receiving immunotherapy combined with targeted therapy.
Research perspectives
Future large-sample,multi-center studies are needed to further validate.
ACKNOWLEDGEMENTS
Thanks to Hangzhou Watson Biotech,Inc.(Hangzhou,China) for technical assistance.As well,we would like to thank the patients and their families for their contribution to the study.
FOOTNOTES
Co-first authors:Jia-Li Chen and Lu Guo.
Author contributions:Chen JL,Guo L,and Han YW conceived,designed and refined the study protocol;Wu ZY,Li H,and Yang C were involved in the data collection;He K analyzed the data;Chen JL,and Guo L drafted the manuscript;all authors were involved in the critical review of the results and have contributed to,read,and approved the final manuscript.Chen JL and Guo L contributed equally to this work.The reasons for designating Chen JL and Guo L as co-first authors are threefold.First,the research was performed as a collaborative effort,and the designation of co-first authorship accurately reflects the distribution of responsibilities and burdens associated with the time and effort required to complete the study and the resultant paper.This also ensures effective communication and management of post-submission matters,ultimately enhancing the paper's quality and reliability.Second,the overall research team encompassed authors with a variety of expertise and skills from different fields,and the designation of co-first authors best reflects this diversity.This also promotes the most comprehensive and in-depth examination of the research topic,ultimately enriching readers' understanding by offering various expert perspectives.Third,Chen JL and Guo L contributed efforts of equal substance throughout the research process.The choice of these researchers as co-first authors acknowledges and respects this equal contribution,while recognizing the spirit of teamwork and collaboration of this study.In summary,we believe that designating Chen JL and Guo L as co-first authors of is fitting for our manuscript as it accurately reflects our team's collaborative spirit,equal contributions,and diversity.
lnstitutional review board statement:This study complied with the guidelines of the Helsinki Declaration.The research protocol was approved by the Clinical Trial Ethics Committee of the Affiliated Hospital of Southwest Medical University (approval number: KY2021063) and registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2100044198).
lnformed consent statement:All study participants,or their legal guardian,provided informed written consent prior to study enrollment.
Conflict-of-interest statement:All authors have nothing to disclose.
Data sharing statement:The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
STROBE statement:The authors have read the STROBE Statement—checklist of items,and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Jia-Li Chen 0000-0002-2692-8179;Lu Guo 0000-0002-0938-9695;Zhen-Ying Wu 0000-0002-2269-8804;Kun He 0000-0002-1510-860X;Han Li 0000-0003-0846-3846;Chi Yang 0009-0007-6370-5541;Yun-Wei Han 0000-0002-0942-9095.
S-Editor:Lin C
L-Editor:A
P-Editor:Xu ZH
 World Journal of Gastrointestinal Oncology2024年2期
World Journal of Gastrointestinal Oncology2024年2期
- World Journal of Gastrointestinal Oncology的其它文章
- Does enhanced recovery after surgery programs improve clinical outcomes in liver cancer surgery?
- Cardiotoxicity induced by fluoropyrimidine drugs in the treatment of gastrointestinal tumors
- Effect of screening colonoscopy frequency on colorectal cancer mortality in patients with a family history of colorectal cancer
- Preoperative controlling nutritional status as an optimal prognostic nutritional index to predict the outcome for colorectal cancer
- Tumour response following preoperative chemotherapy is affected by body mass index in patients with colorectal liver metastases
- Expression of cyclin-dependent kinase 9 is positively correlated with the autophagy level in colon cancer
