Decade Milestone Advancement of Defect-Engineered g-C3N4 for Solar Catalytic Applications
Shaoqi Hou, Xiaochun Gao, Xingyue Lv, Yilin Zhao, Xitao Yin, Ying Liu, Juan Fang, Xingxing Yu, Xiaoguang Ma, Tianyi Ma, Dawei Su✉
ABSTRACT Over the past decade, graphitic carbon nitride (g-C3N4) has emerged as a universal photocatalyst toward various sustainable carbo-neutral technologies.Despite solar applications discrepancy, g-C3N4 is still confronted with a general fatal issue of insufficient supply of thermodynamically active photocarriers due to its inferior solar harvesting ability and sluggish charge transfer dynamics.Fortunately, this could be significantly alleviated by the “all-in-one” defect engineering strategy, which enables a simultaneous amelioration of both textural uniqueness and intrinsic electronic band structures.To this end, we have summarized an unprecedently comprehensive discussion on defect controls including the vacancy/non-metallic dopant creation with optimized electronic band structure and electronic density, metallic doping with ultraactive coordinated environment (M–Nx, M–C2N2, M–O bonding), functional group grafting with optimized band structure, and promoted crystallinity with extended conjugation π system with weakened interlayered van der Waals interaction.Among them, the defect states induced by various defect types such as N vacancy, P/S/halogen dopants, and cyano group in boosting solar harvesting and accelerating photocarrier transfer have also been emphasized.More importantly, the shallow defect traps identified by femtosecond transient absorption spectra (fs-TAS) have also been highlighted.It is believed that this review would pave the way for future readers with a unique insight into a more precise defective g-C3N4 “customization”, motivating more profound thinking and flourishing research outputs on g-C3N4-based photocatalysis.
KEYWORDS Defect engineering; g-C3N4; Electronic band structures; Photocarrier transfer kinetics; Defect states
1 Introduction
Solar-to-chemicals/electricity oriented by photocatalysts has been regarded as a promising supplement for existing energy types [1–13].Nowadays, the emerging graphitic carbon nitrides (g-C3N4) have attracted numerous research attention [14, 15], outperforming the traditional TiO2materials, particularly in the research fields of solar-driven H2evolution reaction (HER) [16–19], CO2reduction reaction (CRR) [20–26], N2reduction reaction (NRR) [27–32], photocathodic protection (PCP) [33–37], pollutant removal [38–41], and oxygen evolution reaction (OER) [42–45].Despite the application discrepancy, they all share similarities until the electrons are involved in redox reactions in an aqueous solution [5, 46].Specifically, this progress in g-C3N4materials can be classified into 5 steps (Fig.1): (1) When the irradiation energy is larger than the bandgap (typically around 2.7 eV) [7, 47], the electrons and holes in g-C3N4can be excited; (2) once irradiation, the electrons in valance band maximal (VBM: 1.57 V vs.standard hydrogen electrode (SHE)) would be excited into conductive band maximal (CBM: - 1.13 V vs.SHE), leaving the VB occupied with holes (oxidizing ability) and CB with electrons (reducing ability), respectively.(3) Afterward, the electrons would transfer from bulk to surface and finally reach the active interfacial sites to participate in the redox reaction.It is worth mentioning that the CB position in g-C3N4must be more negative than the desired reduction potential so that the reductive reactions (HER, CRR, NRR, pollutant removal, PCP) can take place.Similarly, the VB position should be more positive than the required oxidation potential to satisfy the oxidation reactions such as OER and ·OH generation.However, the separation and transport of photocarriers (electrons and holes) in both bulk-phase and surface of g-C3N4are not smooth as there are mainly two recombination pathways: (4) The excited electrons in CB are very active and prone to recombine with holes in VB, mainly releasing energy with the radiative fluorescence; (5) The electrons migrated from CB to surface are also susceptible to be trapped by the defect-associated surface states and then recombine with holes, releasing energy in a nonradiative way with heat.
2 Challenges
Since the pioneered work on the discovery of g-C3N4for photocatalytic H2evolution by Wang et Al.[7], g-C3N4has emerged as a hot metal-free photocatalyst with environmental benignity that attracts numerous research attention.Despite the various photocatalytic applications, g-C3N4is still confronted by the above-mentioned five fundamental steps, of which the initial photoexcitation followed by photocarrier transfer processes are quite complex.Specifically, we summarize the most intractable challenges that impede the large-scale applications of g-C3N4.On the charge excitation side, the challenge is:
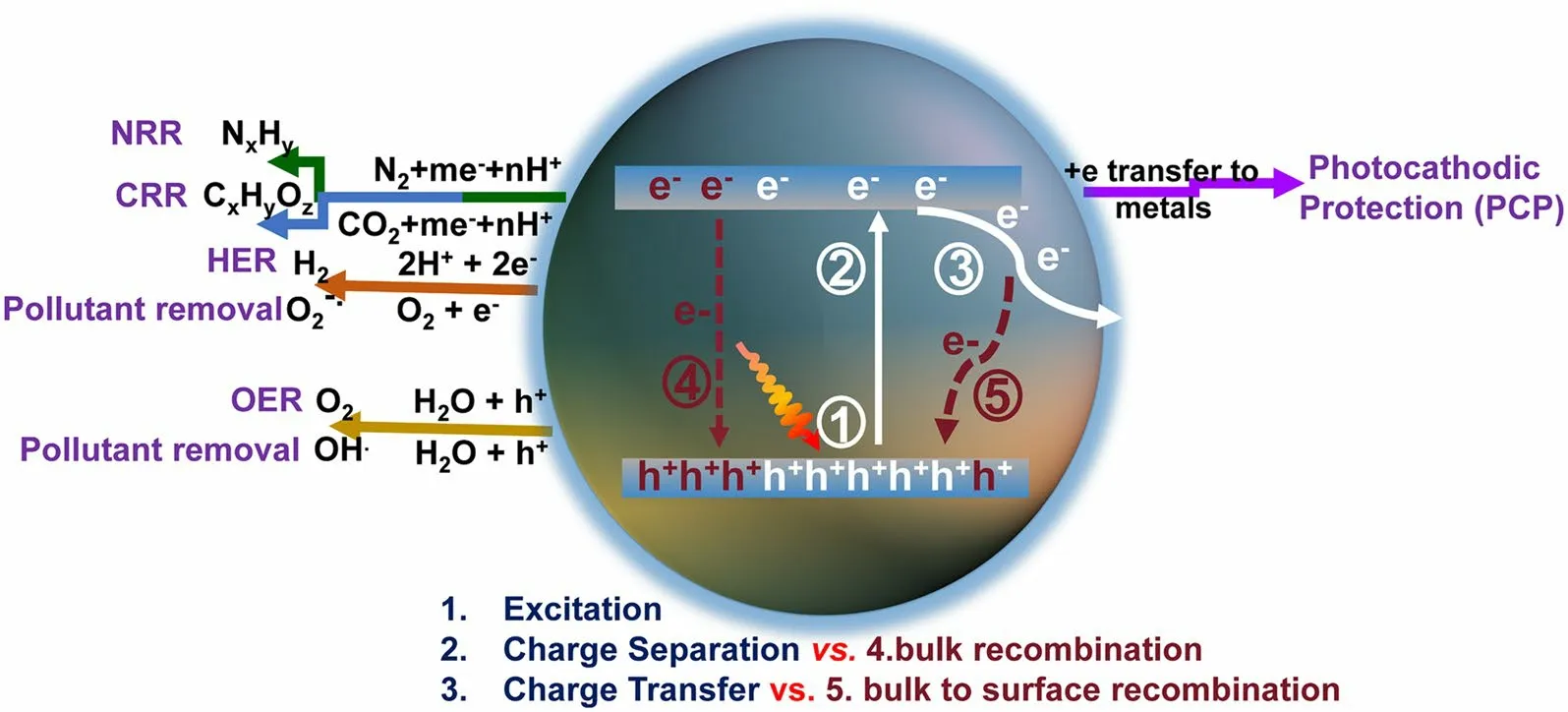
Fig.1 Schematic photoexcitation, charge transport, and solar applications for g-C3N4
(1) Insufficient solar light absorption Photoexcitation acts as the primary and fundamental step for solar applications of g-C3N4, of which if there are more excited photocarriers, there would be more efficient photocarriers involved in the final redox reaction.So far, enormous efforts to create a more abundant specific surface area with porous nanostructures to enhance the multiple solar scattering, diffraction, and absorption have been demonstrated to be efficient in boosting the corresponding photocatalytic activity of g-C3N4[48–54].However, the bandgap of bulk g-C3N4is around 2.7 eV which means the hole/electron pairs can only be excited under light wavelength shorter than 460 nm, which occupies only around 16.5% of the solar spectrum.The low absorption of longer visible light longer than 460 nm and even near-infrared light leads to a limited amount of photoexcited electrons and holes, which would dramatically lower the solar activity of g-C3N4.While on the charge transport side, the challenges are more complicated, which include:
(2) Sluggish photocarrier transfer kinetics For the lowest unoccupied molecular orbital (LUMO) of pristine g-C3N4, no electrons appeared around the bridging N atoms, which indicates the electron in g-C3N4would only be excited and transferred within one C6N7unit, thus hindering the electron transfer along the in-plane direction and increasing the photocarrier possibility of being recombination [55].To this end, the intrinsic localizedπconjugated network of g-C3N4leads to slow photocarrier mobility with low electronic conductivity and sluggish photocarrier transfer kinetics in the horizontal direction.In addition, the insufficient polymerization degree of g-C3N4also generates edged amino groups which could act as charge traps, further hindering the photocarrier transfer in the vertical direction.Therefore, both situations can lead to a sluggish charge transfer process, thus fewer electrons or holes presenting in the interfacial surface of g-C3N4.
(3) Severe photocarrier recombination in bulk-phase The excited active electrons in CB are not in thermodynamical equilibrium and thus prone to return to the ground state, releasing energy via a non-radiative transition.This is particularly true for bulk g-C3N4as the severe electron localization has largely restrained the photocarrier transfer rate, leaving a longer time for photocarrier bleaching.Additionally, there are no additional energy levels between CBM and VBM, lacking the temporary photocarrier “reservoir” to buffer the photoexcited electrons or holes from CBM and VBM, respectively.Therefore, the photogenerated electrons and holes cannot be separated efficiently, resulting in insufficient photocarrier for redox reactions, which needs to be urgently improved.
(4) Severe photocarrier recombination in the surface As we know, the intensive pyrolysis of melamine or other precursors would lead to the NH3gas, and the active H* during the heat treatment can induce a considerable portion of edge amino groups, which lowers the polymerization degree.As g-C3N4is a N-containing material, the presence of amino groups would inevitably induce a relatively strong interlayered van der Waals interaction, which is prone to become the surface traps to bleach the photocarriers.Furthermore, if the experimental condition contains impurities, there also might be a bigger possibility to induce more surface traps.The surface recombination would happen in a less-easy detected manner, releasing the recombination energy in a nonradiative way of heat.However, this point has less been emphasized in comparison with the former bulk-phase recombination, which needs to be alleviated in the next studies on photocarrier transfer dynamics and g-C3N4-based photocatalytic activities.
3 Solution: Defect Engineering
Defect engineering refers to the introduction of impurities to the matrix or regulation of atom periodicity of semiconductors, which has been successfully proven to be an efficient strategy in tailoring the electronic band structures, optical properties, and conductivity of photocatalysts [56–62].Intriguingly, apart from the intrinsic merits changes, the extrinsic morphology of g-C3N4can also be optimized in terms of precursor types, reaction templates, and annealing conditions (pyrolysis atmosphere, heating rate, annealing time, and pressure).As a result, the defective g-C3N4samples normally enable significant improvements in extended solar harvesting ability, efficient photocarrier transfer process, as well as higher surface area with more abundant active sites, thus leading to a comprehensive activity increase for various photocatalytic applications.To this end, we believe defect engineering could be regarded as an “all-in-one” strategy to boost the solar utilization of g-C3N4as it takes the most important factors, namely the electronic band structure and nanostructure into consideration toward various photocatalytic applications.
Despite great achievements have been made in boosting the solar activity of g-C3N4via morphology modification [48–54] and hybrid construction [63–74], the electronic band structure and photocarriers transfer in bare g-C3N4should be emphasized as they are the basement for further performance enhancement.Fortunately, these drawbacks of g-C3N4have been demonstrated to be significantly ameliorated via a defect engineering strategy.Defect engineering refers to the introduction of impurities to the matrix or regulation of atom periodicity of semiconductors, which has been successfully proven to be an efficient strategy in tailoring the electronic band structures, optical properties, and conductivity of photocatalysts [56–62].Intriguingly, apart from the intrinsic merits changes, the extrinsic morphology of g-C3N4can also be optimized in terms of precursor types, reaction templates, and annealing conditions (pyrolysis atmosphere, heating rate, annealing time, and pressure).As a result, the defective g-C3N4samples normally enable significant improvements in extended solar harvesting ability, efficient photocarrier transfer process, as well as higher surface area with more abundant active sites, thus leading to a comprehensive activity increase for various photocatalytic applications.To this end, we believe defect engineering could be regarded as an “all-in-one” strategy to boost the solar utilization of g-C3N4as it takes the most important factors, namely the electronic band structure and nanostructure into consideration toward various photocatalytic applications.
Throughout the research history of self-modified defective g-C3N4[7, 75–99], there exists various defect types including the C/N vacancies [100, 101], heteroatom dopants [102], metallic dopants [103], grafted functional groups [89] as well as crystallinity improvement [83] toward the solar-driven HER, CRR, NRR, OER, PCP, and pollutant removal applications as reflected by the surging publications and citations since 2012 (Fig.2).Generally speaking, with these defect modification strategies, the bandgaps of g-C3N4can be dramatically reduced, rendering an enhanced solar harvesting ability even to almost 600 nm [102].While for the N vacancies [101] or heteroatomic doping with higher electronegativity atoms such as P/S/F [102], there might be new energy levels (defect states) lying in the forbidden bands or strong electronic polarization effect, respectively.For instance, the O-/S/F-doped g-C3N4would induce an electron redistribution and electronic polarization [104], even with the electrons accumulating around the bridging N sites in the HOMO and LOMO, leading to a faster charge transfer kinetics [55].Additionally, Gao et al.proposed an N vacant and S-doped g-C3N4with shallow defect states, which enabled a higher photocatalytic HER rate of 4,219.9 μmol g-1h-1, which was 29.1-fold higher than unmodified g-C3N4[91].The shallow defect states could act as a temporary electron reservoir to accommodate the electrons from CBM, suppressing the bulk-phase photocarrier recombination.The Co single atoms (SAs) were successfully doped into the g-C3N4matrix forming the Co–N bonding via a microwave method to promote the CO yield achieved the highest value of 1.056 μmol mg-1[105].In addition, the crystalline g-C3N4with cyano groups also exhibited a high photocatalytic HER of 64 μmol h-1as its enhanced charge transfer rate and optimized photocarrier separation [106].
The topic of our review is unique as it focuses on the self-defect engineering of g-C3N4, limiting the range out of morphology control, heterostructures, and coupling compounds [107, 108].Firstly, we introduce the challenges confronted by bulk g-C3N4, mainly including insufficient solar light absorption (particularly the longer wavelength than 450 nm) and the inferior photocarrier separation efficiency in both bulk-phase and surface.Compared with other reviews on one or several defect types [62], this review is a more comprehensive view as it includes all kinds of defect controls including vacancy creation, non-metal/metallic doping, functional groups grafting, particularly crystallinity enhancement, and defect traps, which have been discussed as the solutions of defect engineering.Importantly, theoretical guidance in understanding defect roles and redox mechanism, emphasis on the defect states, and probing of photocarrier kinetics by the introduction of femtosecond transient spectrum have also been throughout reviewed.Last but not least, the limits and outlook of defective g-C3N4have been proposed to bring more comprehensive insights for the ultimate goal of defect “customization” for future readers.
3.1 Design Principles of Defect Engineering

Fig.2 a Research history outline of defective g-C3N4 photocatalysts for solar applications [7, 55, 81, 87, 89, 91, 100, 101, 103, 104, 109–113]; Number of annual publications and citations b using “g-C3N4” as the title from 2012 to 2022 and c using “g-C3N4” plus “hydrogen evolution”, or “CO2 reduction”, or “nitrogen reduction”, or “oxygen evolution”, or “pollutant removal”, or “photocathodic protection” as topics in 2022.Adapted from ISI Web of Science, dated 8th June 2023
In general, the defect engineering on g-C3N4should obey three important principles, namely the basic creation of abundant active sites, enhanced solar harvesting ability, and efficient transport (Fig.3).(i) For the former abundant active sites, the synthetic strategy mainly focuses on the precursor modification along with the thermal etching at desired gas atmospheres.The scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscopy (AFM), and Brunauer–Emmett–Teller (BET) techniques have been used to characterize the corresponding porous structures.(ii) For the enhanced solar harvesting requirement, the bandgap calculated via UV–visible diffuse reflectance spectra (UV/Vis DRS) should be optimized with experimental feedback.Importantly, density functional theory (DFT) calculations are a good guidance tool to learn the defect merits.(iii) As for the latter efficient photocarrier transport, time-resolved fluorescence spectroscopy (TRPL), photocurrent, and electrochemical impedance spectroscopy (EIS) are powerful tools to evaluate the extent of photocarrier separation efficiency by getting the lifetimes, photocurrent, and trapping resistance results.It can also be optimized by those approaches of solar harvesting.To achieve the ultimate goal of defective g-C3N4with the best performance, more research work needs to be carried out in the near future, including the precise control of crystallinity, defect states with shallow positions or even optimized surface states.To achieve the defect customization, more advanced in situ probing technologies are also required such as the in situ diffuse reflectance infrared Fourier transform spectrums (DRIFTS), and in situ Femtosecond transient spectrums.
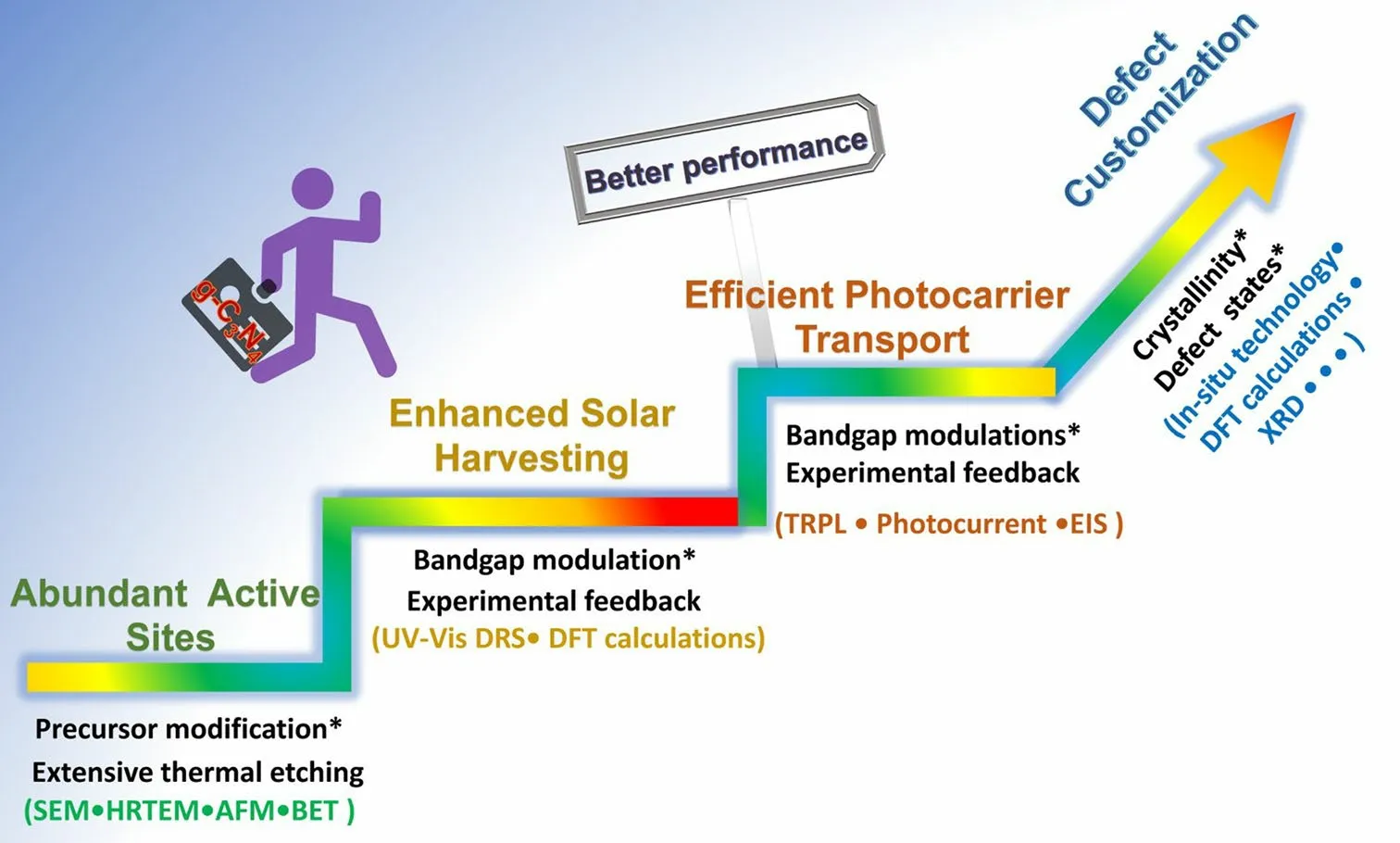
Fig.3 Design principles of defective g-C3N4 toward better photocatalytic performance
3.2 Vacancies with Optimized Band Structures and Electronic Density
By changing the experimental conditions for g-C3N4synthesis, either C vacancies or N vacancies can be obtained, of which the vacancy type and position can be identified by the electron paramagnetic resonance (EPR) signal and resolved X-ray photoelectron spectroscopy (XPS) peak area ratios of C or N species.Generally speaking, both C and N vacancies could impart g-C3N4with an optimized electronic structure, including a narrower bandgap, enhanced solar light absorption, and more favorable charge separation and transport, thus rendering an improvement of solar utilization (Fig.4a).Based on the geometrical configuration, the C vacancies only occur in the three-coordinated sites with the edge and inner sites to be C3Nand C3N’, respectively.While for N vacancies, the vacant positions can be classified into two-coordinated N sites (N2c), inner threecoordinated N sites (N3c’), and outer three-coordinated N sites (N3c), respectively.With the growing knowledge of theoretical calculations, the formation of defect states and the reaction mechanism for vacant g-C3N4have also been discussed in depth.It is worth mentioning that the C vacancies preferred to induce a delocalizedπbonding at the bridging N3Csites [104], thus boosting the electrons transferring between different C6N8units.In this way, the photocarrier transport would be more efficient, giving rise to an overall photocatalytic performance enhancement.While the N vacancies might work in a different way additional energy levels (C3+, defect states) would be induced in the forbidden band [114].These defect states could play a positive role in that, on one hand, the band excitation energy can be reduced with enhanced solar harvesting ability.On the other hand, these states could accommodate the migrated electrons from CBM, suppressing the photocarrier recombination process.Benefiting from the above-mentioned factors, the N vacant g-C3N4generally delivers a substantially promoted photocatalytic activity [91].Therefore, this section would start with C vacancies, then N vacancies, and finally both C vacancies and N vacancies in one g-C3N4material.
3.2.1 C Vacancies with Enhanced Electronic Polarization

Fig.4 Defect control of C3N vacancies.a Possible C vacant and N vacant positions in g-C3N4; b high-resolution C 1 s and N 1 s XPS spectra; c DRS spectra and Tauc plot [115].Copyright 2015, Wiley–VCH.d Electron paramagnetic resonance (EPR) spectra; e ELF diagrams (left: T 500; right: TN500); f adsorption energy and charge density difference between T 500/TN 500 and NO [116].Copyright 2020, Elsevier
An initial work on C vacant g-C3N4was reported by Yang’s group [115].Specifically, the porous holy C vacant g-C3N4nanosheets (HGCN) were obtained by the thermal exfoliation of bulk g-C3N4(BGCN) under NH3atmosphere.Compared to pristine BGCN, HGCN owned plentiful in-plane pores that were more accessible to aqueous solution and reduced van der Waals interaction, which could significantly enhance the mass transport and photocarrier separation for water splitting.However, the author claimed that the C vacancies might originate from the loss of graphitic C species according to the increased peak-area ratio of N=C–N to C=C from 0.13 to 0.14 for BGCN and HGCN, respectively (Fig.4b).This explanation could be fuzzy because the C=C peak for both samples was not obvious, and this weak signal might also come from the equipment or sample contamination.Different from our expectation, HGCN showed an enlarged bandgap of 2.90 eV that was 0.31 eV higher than BGCN (Fig.4c), which was supposed to be the quantum confinement effect owing to the small grain size and ultra-thin merits of HGCN nanosheets.Additionally, the authors also insisted that, owing to the C vacancies, the enhancement of light absorption in HGCN in the nearinfrared region could also be witnessed.Benefiting from the above-mentioned factors, this C vacant g-C3N4exhibited a prolonged charge lifetime and enhanced HER rate, which was 1.7 and 20-fold higher than bulk g-C3N4.
Different from the above study, Li et al.fabricated the tubular g-C3N4with C vacancies presented in the edge C3Nsite via the pyrolysis of urea and melamine mixture under an inert N2atmosphere, and the corresponding products were labeled as TN-x(xpresents annealing temperature) [116].The authors claimed that the N2atmosphere was critical for the g-C3N4morphology and defects generation.Especially, g-C3N4obtained without N2(T 500) displayed a tubular length of 20 μm and diameter of 1–2 μm and bulky nanoplates inside.In contrast, TN 500 was observed with thin nanosheets inside, which further demonstrated the exfoliation process induced by the N2atmosphere.In addition, the Lorentzian line for TN 500 was considerably attenuated, confirming the appearance of C vacancies (Fig.4d).According to the DFT calculations, both samples exhibited a strong covalent interaction with NO due to the high electronic location function (ELF) value of around 0.81 eV (Fig.4e), indicating the stronger electronic polarization effect due to the absence of C vacancies.Furthermore, the NO molecules can be more easily activated by TN 500 as reflected by its larger adsorption energy and carried total charge (Δq) of - 3.25 eV and 0.16 e, which are 1.79 eV and 0.13 e higher than those of T 500, respectively (Fig.4f).This demonstrated that the NO molecules were extremely easy to be absorbed and activated over TN 500 than T 500, which would facilitate the redox kinetics of NO photo-oxidation activity.Therefore, TN 500 showed the highest removal rate of NO at 47.7%, implying the superior role of surface C vacancy in accelerating the NO removal rate.

Fig.5 Defect control of C3N vacancies.a Possible formation mechanism of carbon vacancy; b ESR spectra of g-C3N4 and Cv-g-C3N4; c C vacancy could significantly inhibit the recombination of photogenerated carriers; d Calculated band structure of g-C3N4; e calculated electron density of g-C3N4; f Calculated electron density of Cv-g-C3N4; g Calculated band structure of g-C3N4 [100].Copyright 2016, Elsevier.h UV–vis DRS and optical photographs; i CH3OH yields of g-C3N4, g-C3N4-C, g-C3N4-D, g-C3N4-T, CM/g-C3N4, and 3DM C/g-C3N4, where C, D, T, and CM present the precursors of cyanamide, dicyandiamide, thiourea, and a mixture of cyanuric acid and melamine; j Schematic diagram illustrating the band structures of g-C3N4, 3DM C/g-C3N4 and the probable photocatalytic process [117].Copyright 2021, Elsevier
Giving a simple simulated model of g-C3N4with a triazine unit, Wang’s group suggested that the vacant C only appeared in the three-coordinated sites (Fig.5a).In short, the vacancies could be obtained after a facile annealing process of bulk g-C3N4under hot Ar flow at 520 °C for 1 h [100].The Ar molecules were very active with high energy doing the irregular motion and thus had a bigger chance to hit the C atom surface than the N atom due to the smaller carbon molecular weight.Consequently, the C atoms were sputtered from the triazine framework to form the C vacant g-C3N4(Cv-g-C3N4).This was in good agreement with the smaller peak area ratio of C–N3/C–C for Cv-g-C3N4(1.2) than bulk g-C3N4(2.7).Therefore, it was reasonable to see the weaker EPR signal of a Lorentzian line centered at about 3512 G, suggesting the decreased unpaired electron density around C vacancies (Fig.5b).Interestingly, the authors claimed that these C vacancies could induce unsaturated N atoms with paramagnetic centers to attract more electrons from CB and break the symmetry of Cv-g-C3N4with electron delocalization, further suppressing the photocarrier recombination (Fig.5c).As for the calculated electronic band structures, Cv-g-C3N4displayed a narrower bandgap and higher energy level density of VB than pristine g-C3N4(Fig.5d-g), revealing its enhanced solar light harvesting ability and more excitable electrons due to the electron delocalized effect caused by C vacancies.Benefiting from the above-mentioned factors, Cv-g-C3N4changed the H2O2formation pathway from a two-step single electron indirect reduction into a one-step two-electron direct reduction way, delivering a 14-times higher H2O2formation than bulk g-C3N4.Delivering the same C vacant position, Wang and co-workers synthesized the 3D macropore g-C3N4with C vacancy (3DM C/g-C3N4) via the calcination of polymethylmethacrylate (PMMA) spheres with cyanuric acid and melamine at 500 °C for 2 h [117].The authors claimed that 3DM C/g-C3N4had abundant macropores due to the in situ thermal removal of PMMA spheres, arousing an increased BET surface area with plentiful reactive sites for better capture and utilization of visible light.According to the UV–visible diffuse reflectance spectra (DRS, Fig.5h), due to the introduction of C vacancies, the 3DM C/g-C3N4showed a much wider solar light absorption range from 400 to 800 nm, which was significantly stronger than its counterparts of g-C3N4and CM/g-C3N4with the corresponding limited absorption edges of 460 and 435 nm, respectively.Furthermore, the photoluminescence (PL) spectra illustrated a much lower 3DM C/g-C3N4intensity compared to those of CM/g-C3N4and original g-C3N4.This implied the presence of C vacancies in 3DM C/g-C3N4can not only enhance light absorption but also suppress photocarrier recombination, which finally boosted the highest CH3OH formation rate up to 7.5 μmol g-1h-1(Fig.5i).Most importantly, the electronic band structure showed that, due to C vacancies, the 3DM C/g-C3N4displayed a more negative CB position than bulk g-C3N4by 0.52 eV, further indicating its stronger driving force toward CO2reduction (Fig.5j).
3.2.2 N Vacancies with Defect States
Similar to the O vacancy-induced Ti3+in the TiO2system with additional defect states [118], Niu et al.claimed that the N vacancies in g-C3N4could also arouse C3+states with new energy levels [114], which has also been demonstrated by the following work on N2Cvacant g-C3N4[101].In detail, the target defective samples were prepared by the annealing of bulk g-C3N4(BCN) from 475 to 550 °C under an H2atmosphere, labeling as CN-x(xwas heating temperature).Their N2Cvacant position was confirmed by the decreasing XPS peak area ratio of C–N=C/N–C3at 3.82 and the increasing EPR signal of CN-xin comparison with those of BCN.It is interesting to see, with the increasing heating temperature, the color of CN-xgradually turned to brown, and the Urbach tail became wider with enhanced solar harvesting ability (Fig.6a).This was ascribed to the defect states (also called midgap states), which had also been verified by the additional energy levels around Femi levels near CBM of defective g-C3N4via DFT calculations results (Fig.6b).It is worth to mention that the N vacancy induced defect states were deeper as the increased heating temperature (Fig.6c), which was good for solar harvesting but detrimental for photocarrier separation.As these deep defect states could act as photocarrier recombination centers to lower the photoactivity of defective g-C3N4.As a result, we observed CN-550 exhibited an inferior photocatalytic H2evolution rate of 55.64 μmol h-1, which was 13.5% lower than CN-525.This point is of critical importance for future defect design.
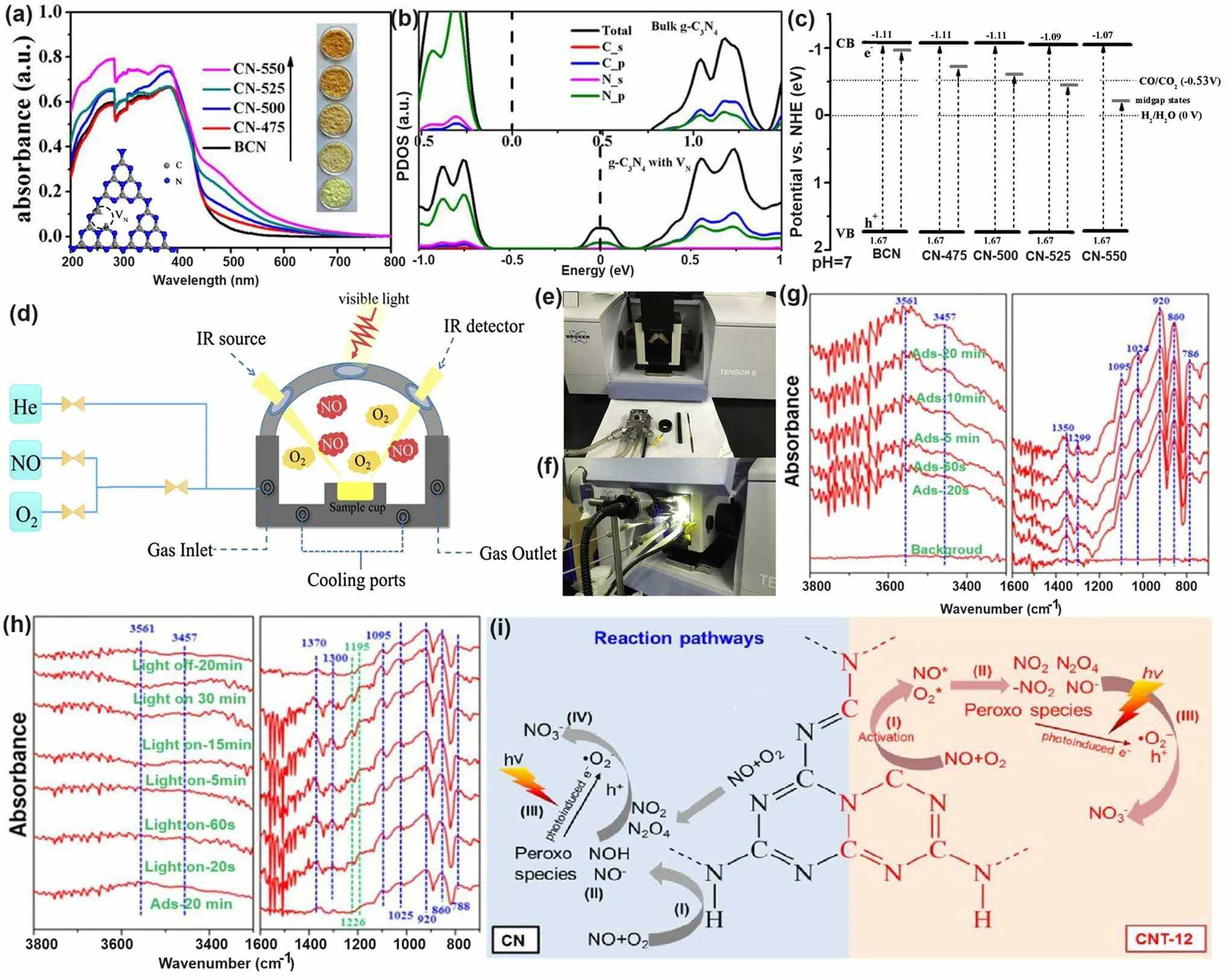
Fig.6 Defect control of N2C vacancies.a UV–vis DRS of BCN and CN-x; b calculated PDOS of BCN and N vacant g-C3N4; c schematic illustration of the electronic structure of BCN and CN-x [101].Copyright 2017, American Chemical Society.d Schematic diagram of in situ FT-IR reaction cell; digital photos of e a Tensor II FT-IR spectrometer and loading parts and f in situ FT-IR measurement working condition [113].Copyright 2018, Elsevier.In situ DRIFTS images for g adsorption of NO/O2 and h photocatalytic reactions of Nv-CN; i proposed reaction pathways for adsorption and the photocatalytic oxidation of NO over pristine (left) and N-deficient (right) g-C3N4 [39].Copyright 2019, American Chemical Society
The N vacancies have been demonstrated to be efficient in boosting the g-C3N4-based NO removal.For instance, Dong et al.synthesized the N vacant g-C3N4via the heat treatment of urea (CN-U) with a considerably enhanced EPR signal [113].Impressively, they employed an in situ FT-IR setup to monitor the active species change and reveal the redox mechanism.As reflected in Fig.6d–f, the instrument was composed of an FT-IR spectrometer, a diffuse reflectance cell with IR and solar irradiation windows, a high-temperature reaction chamber, a gas line, and a cooling system.The gas inlet and outlet enabled the chamber purification to obtain the clean NO and O2feeding gas.The diffusion testing mode could identify the real-time active species by identifying the typical functional groups of NO oxidization intermediates.As a result, CN-U was observed with much stronger NO absorption and activation performance during the redox reaction.In a following-up work, the in situ DRIFTS observation and in-deep calculations were carried out to the inner mechanism by employing the prepared N2Cvacant g-C3N4(Nv-CN) as photocatalyst [39].Taking the best Nv-CN sample for example, its in situ DRIFTS confirmed that the new peaks around 1350/1299 cm-1and 1024 cm-1were nitro compounds (-NO2) and bidentatestate, respectively (Fig.6g).Other peak intensities around 3604, 1095, and 786 cm-1were much higher than pristine g-C3N4, further demonstrating the efficient absorption and activation of NO on the Nv-CN surface.However, its NOH peak intensity was much weaker, suggesting NO and O2were rapidly absorbed on Nv-CN, rather than generating the less active terminal N–H bonds.Furthermore, once irradiation, Nv-CN also showed new peaks at around 1500–1600, 1226, and 1192 cm-1, assigning to the monodentate nitrate bidentate nitrate, and bidentate nitrite, respectively (Fig.6h) [119].Additionally, peaks assigned to other types of nitrates and peroxo species of Nv-CN were much stronger than those of unmodified g-C3N4, implying the boosted photocatalytic activity owing to the presence of N2Cvacancies.Based on the above DRIFTS analysis, one can conclude that the different reaction pathways (Fig.6i): (1) For pristine g-C3N4, it showed a poorer absorption and activation ability of NO and O2, delivering a primary and less-active pathway of NH →NOH →OOH →NO-and surface peroxo species.(2) For Nv-CN, it showed a more efficient redox pathway of directly generating bidentate states into –NO2, then to NO3-in the presence of ·O2-.This promoted NO removal activity of Nv-CN was ascribed to its significantly enhanced adsorption energy toward O2(- 5.99 eV) and NO (- 5.91 eV) with spontaneously bond breaking than pristine g-C3N4(0.48 and 0.29 eV) according to DFT calculations.Additionally, the authors also claimed the N vacancy concentration was critical to boost the best photocatalytic NO removal efficiency.Otherwise, these would become photocarrier traps, leading to severe photocarrier recombination.This meaningful work has paved researchers with new insight to redox mechanism along with more precise controls are needed for future vacancy study.
Taking the best Nv-CN sample for example, its in situ DRIFTS confirmed that the new peaks around 1350/1299 cm-1and 1024 cm-1were nitro compounds (- NO2) and bidentate-state, respectively (Fig.6g).Other peak intensities around 3604, 1095, and 786 cm-1were much higher than pristine g-C3N4, further demonstrating the effi-cient absorption and activation of NO on the Nv-CN surface.However, its NOH peak intensity was much weaker, suggesting NO and O2were rapidly absorbed on Nv-CN, rather than generating the less active terminal N–H bonds.Furthermore, once irradiation, Nv-CN also showed new peaks at around 1500–1600, 1226, and 1192 cm-1, assigning to the monodentate nitrate bidentate nitrate, and bidentate nitrite, respectively (Fig.6h) [119].Additionally, peaks assigned to other types of nitrates and peroxo species of Nv-CN were much stronger than those of unmodified g-C3N4, implying the boosted photocatalytic activity owing to the presence of N2Cvacancies.Based on the above DRIFTS analysis, one can conclude that the different reaction pathways (Fig.6i): (1) For pristine g-C3N4, it showed a poorer absorption and activation ability of NO and O2, delivering a primary and less-active pathway of NH →NOH →OOH →NO-and surface peroxo species.(2) For Nv-CN, it showed a more efficient redox pathway of directly generating bidentate states into -NO2, then to NO3-in the presence of ·O2-.
Chen’s group has compared the N2Cand N3Cvacancies in affecting the electronic band structures of g-C3N4by DFT calculations using the simple triazine-based framework as calculated models [120].As the unstable nature of N vacancies, they also employed the H atoms to statured with these N defects in theoretical analysis and used the H2atmosphere to get the N vacant g-C3N4with amino group in experiment (Fig.7a).Regarding the pure N3Cvacant g-C3N4, it showed larger bandgap values than the N2Cone, indicating its inferior role in enhancing the solar light harvesting ability (Fig.7b).So as the H statured N-deficient g-C3N4.However, we observed the latter had a much lower bandgap than the former, which was attributed to the band-like defect states below the CBM.This explained why the abovementioned CN-xhad new defect states under the same H2atmosphere (Figs.7c–e and 6c).As a result, the experimental N-vacant g-C3N4with edge H atoms prepared under H2(g-C3N4(H2)) exhibited the highest HER rate, which was 4.8 times higher than pristine g-C3N4.Recently, Li et al.prepared the N3Cvacant AC-CNx through the calcination of melamine/azodicarbonamide (AC), where x is the mass of AC while the melamine mass was kept at 10 g [121].Compared to pristine g-C3N4, AC-CN4 showed a reduced XPS intensity of –C2N and –C3N, and increased –N2C/–N3C peak-area ratio by 0.867 than those of bulk g-C3N4(CN), further indicating the N vacancies were located at –N3C sites (Fig.7f).Interestingly, during the NO removal activity, these N vacancies played a critical role in boosting the concentration of singlet O2(1O2), which was verified to be the active species as reflected by the TEMP spin trapping EPR spectra (Fig.7g) and active species confirmation experiment (Fig.7h).According to the theoretical calculations, in contrast with CN, AC-CN4 exhibited an enhanced NO and1O2adsorption energy by 1.12 and 2.3 eV, respectively (Fig.7i).This was ascribed to the strong electronic polarization effect, which contained electron-rich and electron-poor areas, giving a polar chemical interaction with other gas.Thus, the redox kinetics were accelerated.In addition, the incorporated N vacancies as the reactive sites in AC-CN4 also quenched the adsorption of intermediates (NO2)/final products (NO2–and NO3–).All these factors rendered AC-CN4 with a significantly improved NO removal rate of 40.3%, which was 2.28-fold higher than CN, reflecting the efficient role of N vacancies (Fig.7j).
Similarly, Tian and co-workers synthesized the N3Cvacant g-C3N4via the polymerization of urea (10 g) and ammonium acetate (0.1–0.5 g), of which the product was named g-C3N4-N3C-X(X: mass of ammonium acetate) [122].It is worth mentioning that the decomposition of ammonium acetate would generate CO2and NH3, which was critical to etch the N3Clattices, leaving the g-C3N4with gas bubbles during the pyrolysis process, and thus produced a porous nanosheets structure.In comparison with pure g-C3N4, g-C3N4-N3C-Xdisplayed a reduced peak area ratio of N3C/N2Cfrom 0.42 to 0.31, further evidencing the formation of N3Cvacancies.Remarkably, when the ammonium acetate mass reached 0.3 g, the N vacant g-C3N4achieved the highest H2O2and N2fix rates of 1098 and 1086 μmol g-1h-1, which was 11.1 and 15.5 times higher than pure g-C3N4.The authors further employed DFT calculations to reveal the reaction mechanism of H2O2and N2reduction, respectively.As shown in Fig.8a, we observed a much smoother reaction pathway of O2reduction on g–C3N4–N3C-0.3 with the highest Gibbs free energy change (ΔG) of 1.1 eV from *OOH to *H2O2step, which was 0.17 eV smaller than that of pure g-C3N4to form *OO specie.Regarding to the NRR reaction, the situation was much more complex as the reaction mechanisms can be classified into the distal pathway and alternating pathway.In detail, for the former pathway, one can see the rate-determining step of pure g-C3N4was between *NNH2to *N with a ΔGof 1.01 eV, which was 0.42 eV higher than that of g-C3N4-N3C-0.3 for the conversion from *NH2to *NH3(Fig.8b–c).For the latter pathway, a similar result was also reflected by the 0.29 eV lower ΔGfor g-C3N4-N3C-0.3.Additionally, O2preferred to be spontaneously absorbed onto the g-C3N4-N3C-0.3 surface, and N2exhibited a dramatically reduced absorption and active barrier on its surface than pure g-C3N4due to the much lower or even negative ΔGup to - 1.91 eV, strongly suggesting the ultra-active sites of the N3Cvacancy.
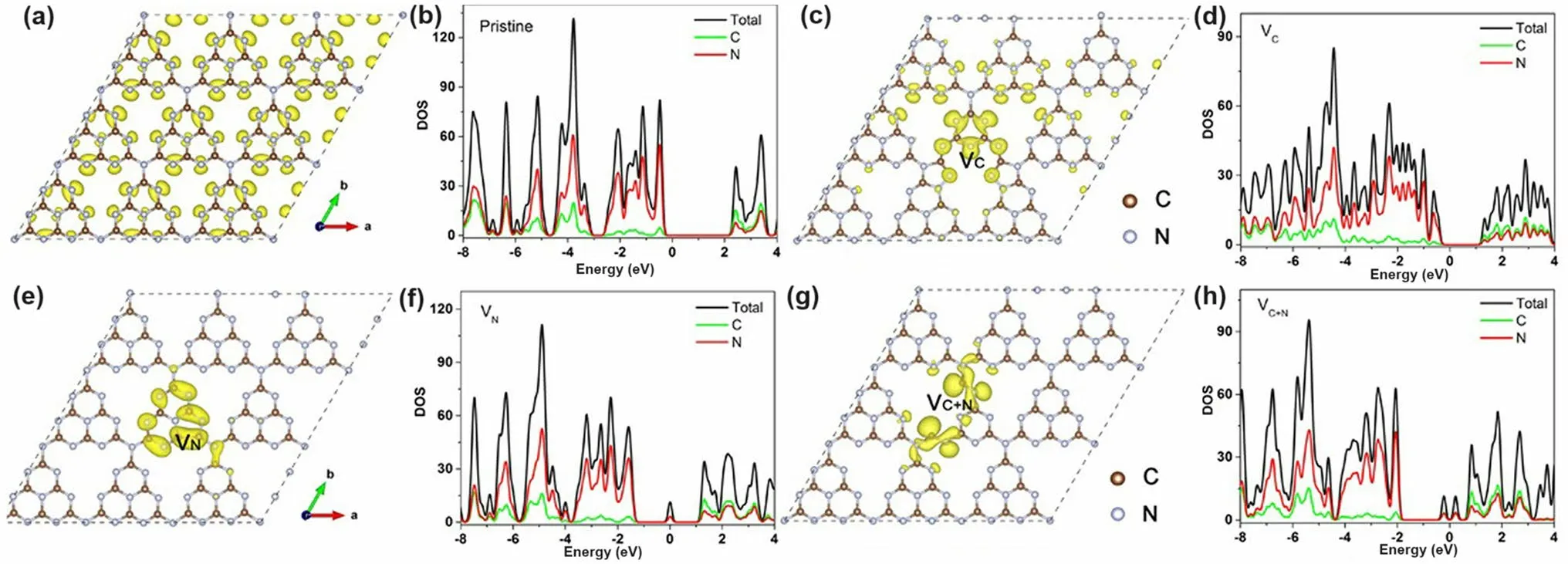
Fig.9 Defect control of C3N’ and N2C vacancies.Optimized atomic structures and DOSs diagrams of a, b pure g-C3N4; c, d C vacant g-C3N4; e, f N vacant g-C3N4; g, h C vacant and N vacant g-C3N4.The Fermi levels are located at 0 eV [123].Copyright 2019, Wiley–VCH
Apart from the single C vacancies and single N vacancies in the g-C3N4matrix, researchers started to explore the synergistic effect of both vacancies on photocatalytic performance.A typical synthesis and theoretical work were carried out by Ren’s group [123].They used a very ingenious He+ion irradiation method to avoid the impurities from extra chemicals.The irradiation ions with a certain energy E0would hit the atoms and gradually lose energy after a series of collisions with target atoms.If the hitting energy was larger than the displacement energy, the C/N atoms would be sputtered out.In this case, the input energy was high enough to hit both C and N atoms out, and this non-chemical selectivity made both vacancies exist simultaneously.By controlling the hitting parameters with total energy fluence from 0 to 86.25 × 1013ions cm-2, the C/N vacancies gradually increased, of which the N defect concentration was much higher than C according to the experimental XPS analysis and theoretical Stopping and Range of Ions in Matter (SRIM) simulations.Despite the experimental failure to obtain single C or single N vacant g-C3N4, their DFT calculations explained the influence of single vacancy and C/N vacancies on the electronic band structures (Fig.9).Compared to the bulk g-C3N4, one can see that the C vacant g-C3N4had a dramatically reduced bandgap by 0.97 eV, only 1.48 eV, extending the optical absorption (Fig.9a–d).For the V vacant g-C3N4case, its bandgap was slightly reduced by 0.07 eV and formed the C–C bond into a five-ring unit to keep the structure stable.Interestingly, defect states were lying below the CBM of V vacant g-C3N4(Fig.9e), which was also consistent with the obvious tail absorption in the DRS result.The calculated electronic density results indicated the electrons preferred to localize around the N vacancies (Fig.9f–h).Notably, the presence of both C and N vacancies enabled g-C3N4with slight bandgap narrowing and more defect states localized around the vacancies.The authors also claimed that this was good for electron-trapping to enhance the photocarrier separation.In summary, the C vacancy was more effective in narrowing the bandgap while the N vacancy was more useful in creating defect states.Taking together, we can observe both optimized solar harvesting ability and photocarrier transfer.As a result, the C/N vacancy co-modified g-C3N4exhibited a significant HER rate of 1271 μmol g-1h-1, which was 19 times higher than the bulk g-C3N4.
Therefore, based on the above review, we can come to a summary of this vacancy section.The C vacancies and N vacancies realized by annealing bulk g-C3N4or modified precursors under different atmospheres or physical treatments such as plasma environments are both beneficial to optimize the electronic band structure with enhanced solar light absorption and photocarrier transport.Additionally, recent reports on defective g-C3N4with vacancies at different positions toward various solar applications are listed and comparable in Table 1.We believed this would help readers to find clues for more precise control of vacancy creation.Despite significant progress has been made, therealso remain some ambiguities that need to be resolved in the near future.Firstly, a single vacancy and both vacancies cannot be obtained with precise concentration control via one synthetic strategy.Their deep relationships are hard to distinguish, such as to which extent can the synergistic effect reach the optimized state.Secondly, the study on vacancyassociated defect states (midgap states) is still in the beginning, and defect control on tuning their position needs to be specified.Thirdly, the photocarrier transfer dynamics in the bulk-phase and surface are different, which urgently needs to be discussed in depth.Since the vacancies might exist in both bulk and surface, a more advanced time-resolved spectrum should be paid into this section to reveal the detailed photocarrier separation and transfer progress.

Table 1 Comparison of vacant g-C3N4 toward various solar applications
3.3 Non-metal Dopants with Optimized Band Structures and Electronic Density
The metal-free merit of g-C3N4can also be maintained by non-metal doping with heteroatoms such as C, P, S, O, B, and F [158].Similarly, similar to C and N vacancies, these non-metal dopants also enable g-C3N4with optimized electronic structure, enhanced visible-light harvesting, and high charge separation efficiency.
3.3.1 C Dopants with Electronic Delocalization
The C dopants, replacing the bridging N atoms in the g-C3N4matrix, have been demonstrated to improve the bulk electronic conductivity due to the presence of delocalized bigπbonds between the hexatomic rings and substituted C [104].In a typical work, Zhao and coworkers fabricated the C-doped g-C3N4using melamine and melamine-based resin foam as precursors [159].After the thermal decomposition, C atoms were in situ doped into the g-C3N4framework.The enhanced conductivity was verified by the reduced charge transfer resistance (Rct) according to the EIS measurement.Additionally, the C-doped g-C3N4also shows extended solar absorption from visible light to near-infrared (800 nm).As a result, this defective C-doped g-C3N4exhibits an excellent NO photodegradation constant of 0.95 min-1.A similar study was also reported by Zhang and colleagues, which employed a hydrothermal method to obtain the C-doped g-C3N4with glucose and melamine as precursors [160].
To further boost solar light absorption and suppress photocarrier recombination, the C-rich g-C3N4with both N vacancies and porous structure was designed [161].Different from previously isolated C dopants, these C dopants existed in the form of C rings, which were realized by the additive of conjugated methyl-cyclodextrin.The g-C3N4photocatalyst consisted of three layers with the pure carbon nitrides in the core, the carbon dopant layer in the middle, and the carbon layer in the outermost layer.The unique structure of gradual C-doped g-C3N4endowed itself with not only enhanced electronic conductivity but also a narrower bandgap and stronger solar light absorption.More importantly, the C dopants and N vacancies induced the formation of mid-gap states, which could further lower the photoexcitation energy which is smaller than the bandgap.In addition, the mid-gap states can act as a temporary reservoir to accept the migrated electrons from CB, and thus the recombination process of electrons and holes was suppressed.Therefore, the C defective g-C3N4displayed an exceptional solar-driven HER rate of 125.1 μmol h-1g-1, which was over 21-fold as high as the pristine g-C3N4.
3.3.2 N Dopants with Defect States
Recently, N-doped g-C3N4has been proposed via the annealing of melamine cyanurate supermolecules via the hydrothermal reaction of melamine and aminourea hydrochloride [162].Doping at an edge three-coordinated C site, the doped N atoms induced defect states in the electronic band structure near CBM, thus extending the solar harvesting ability to almost 550 nm.Additionally, the N-deficient g-C3N4exhibited an improved TC removal rate of 93.3% within 60 min.Despite the different N-doping sites of the inner three-coordinated C atom, Umare et al.explained the reason for enhanced photocatalytic HER activity of N-rich g-C3N4in depth using the DFT calculations [163].Before this, in the experiment, they successfully synthesized the polymerized g-C3N4(PCN) by directly annealing melamine in air.Those prepared with lower N-doping levels employing aminoguanidine hydrochloride/urea as the precursor and higher N-doping levels with urea/aminoguanidine hydrochloride/melamine as the precursor were named APCN and NPCN.In increasing order of PCN < APCN < NPCN, we saw a gradually enhanced photocatalytic HER rate of 5.81, 6.97, and 40.32 mmol g-1, respectively.The authors then gave three calculated models to simulate g-C3N4with different N-doping concentrations in terms of band structures, charge density distribution, as well as ΔGchange (Fig.10).Compared to PCN, NPCN displayed new energy levels that were also called defect states/midgap states in the forbidden band, mainly due to the existence of new N dopants (Fig.10a–d).This was also true for APCN.The authors claimed that this might be more advantageous for photocarrier separation from these defect states and CBM.The electronic density pictures also revealed there were more electrons transferred from N dopants to H atoms, further accelerating the photocarrier transfer kinetics (Fig.10e).The H2evolution pathway also confirmed a smoother H* adsorption/desorption process due to the slight ΔGchange of - 0.18 eV, which was 0.31 and 0.03 eV smaller than those of PCN and APCN, respectively (Fig.10f).Therefore, we can conclude that N dopant is also beneficial to the photocatalytic ability optimization of g-C3N4, which was even similar to the work-principle of N vacancies as above mentioning [123].

Fig.10 Defect control of N dopants.a, c Optimized electronic structure and b, d DOS of PCN and NPCN; e three-dimensional charge density distribution (blue color denotes electron accumulation, whereas green color to depletion region); f calculated hydrogen adsorption energy on the PCN, APCN, and NPCN catalyst [163].Copyright 2023, Elsevier
3.3.3 P Dopants with Defect States
Similar to the above-mentioned gradual C-doped g-C3N4, Ran and colleagues demonstrated that P atoms could also induce the formation of mid-gap states in g-C3N4with an extended solar light absorption to 557 nm and high photocarrier separation efficiency both theoretically and experimentally (Fig.11a) [110].In addition, due to the more extensive thermal etching of protonated precursor, the porous P-doped g-C3N4nanosheets (PCN-S) also presented a quantum size effect with an enlarged bandgap by 0.23 eV higher than the bulky P-doped g-C3N4(PCN-B, Fig.11b).Intriguingly, a greater reductive driving force and promoted mass-transfer process for PCN-S was achieved owing to the more negative CBM and the macroporous structure, respectively.Therefore, this PCN-S exhibited an outstanding HER rate of 1596 μmol h-1g-1and an apparent quantum efficiency of 3.56% at 420 nm.Researchers also found an interesting result that the phosphorous precursors played an important role in the P-doping sites [158].For instance, the P atoms were prone to replace the bay or corner C sites in the tri-s-triazine units to form the P–N bonding when using 1-butyl-3-methylimi-dazolium hexafluorophosphate (BmimPF6) as P source [99].The P atoms were found to be doped into the g-C3N4lattices to form a P–N bond when using (NH4)2HPO4as a P precursor [164].Despite the different P doping sites, both situations can achieve excellent solar performance due to the narrowed bandgap and accelerated photocarrier transfer kinetics.
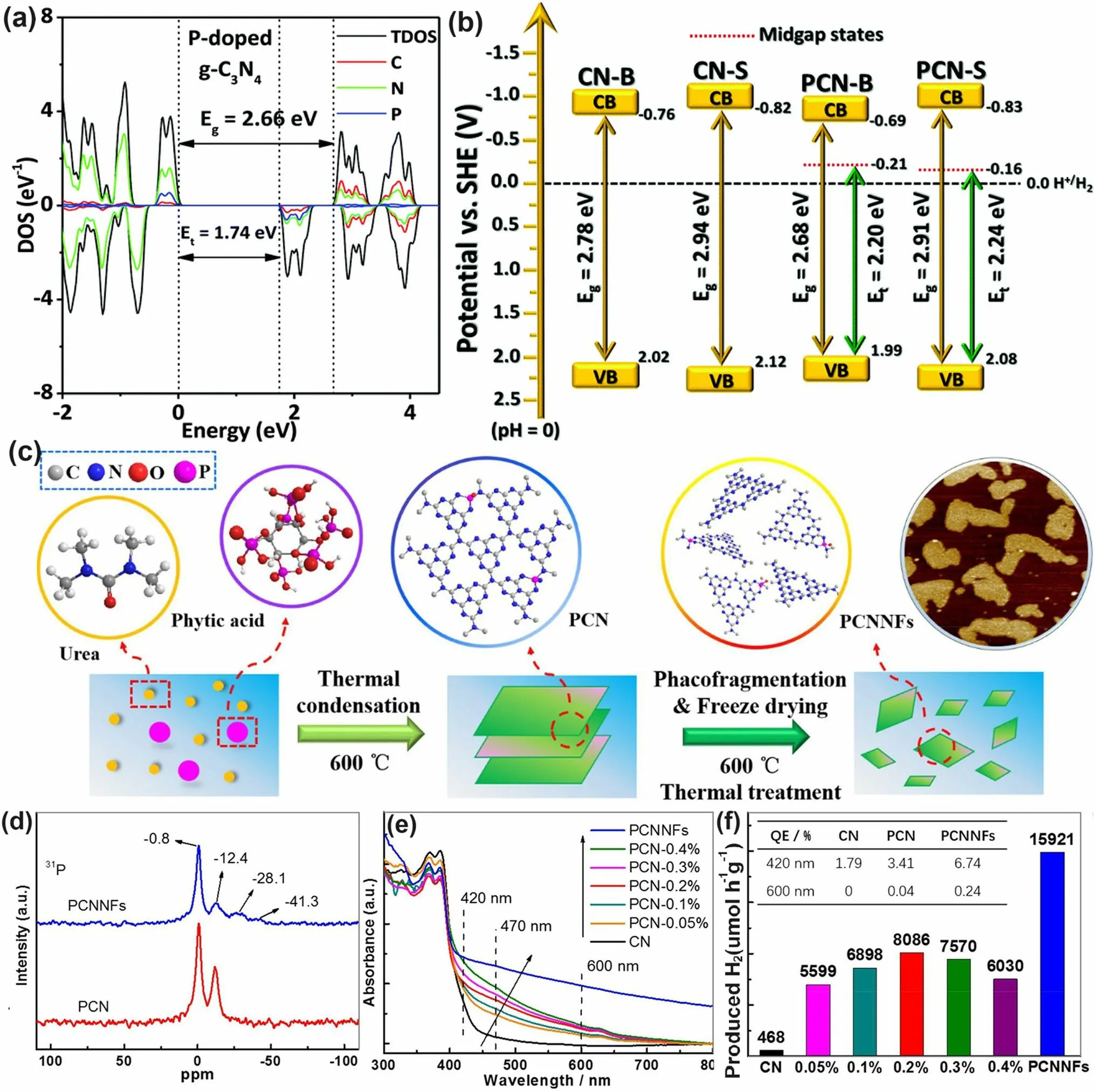
Fig.11 Defect control of P dopants.a Density of states (DOSs) of P-doped g-C3N4; b Electronic band structure of P-doped g-C3N4, CN-B, and CN-S [110].Copyright 2018, Elsevier.c Illustration of the preparation process of PCNNFs; d 31P MAS NMR spectra of PCN and PCNNFs; e UV–vis DRS and f photocatalytic HER rates under visible-light irradiation (λ > 420 nm, inset: QE comparison at 420 and 600 nm) for CN, PCNx, and PCNNFs [102].Copyright 2015, Royal Society of Chemistry
In follow-up work, Fang’s group synthesized a variety of P-doped g-C3N4nanoflakes (PCNNFs) by a former thermal condensation of phytic acid and urea (product: PCN-x,xis the mass ratio of phytic acid/urea), followed by a nanostructure tailoring of phaco-fragmentation and freeze-drying (Fig.11c) [102].The porous PCNNFs exhibited fragmentized nanoflakes with significantly improved BET surface area of 223.2 m2g-1, which shortened the interfacial diffusion path of active species and thus accelerated the transfer and separation of photocarriers.The P substituted site was proved to be the corner C connected to the tertiary amine according to the two distinctive peaks around - 0.8 and - 12.4 ppm as shown in the31P solid-state magic angle spinning nuclear magnetic resonance (MAS NMR) spectra (Fig.11d).Moreover, the excellent visible-light absorption of PCNNFs was substantially extended to 800 nm, superior to those of bulk g-C3N4and PCN-x, indicating the effi-ciency of P-doping and advanced nanostructure (Fig.11e).It is worth mentioning that the quantum efficiency at 600 nm of PCCNFs was about 0.24%, far more exceeding its counterparts.This was also due to the narrowed sub-bandgap from VB to midgap states induced by the P dopants.As a result, irradiated with visible light, the photocatalytic HER rate of PCNNFs was up to 15,921 μmol g-1h-1, which was 34-folder higher than that of bulk g-C3N4(Fig.11f).In another typical work, Wu et al.used urea and butyl phosphate as precursors to prepare P-doped g-C3N4(PxC3N4, wherex= 1, 2, 3 corresponds to the butyl phosphate volume of 2, 5, and 8 mL) [165].Due to the introduction of the P element, the edge of P3C3N4nanosheets was smoother and more regular compared with that of bulk g-C3N4.It displayed the narrowest bandgap of 2.49 eV, which was dramatically reduced than bulk g-C3N4(2.7 eV).Due to the P-doping, the solar absorption reached 470 nm, and thus an increased photocatalytic UO22+removal rate of 84% within 20 min was witnessed.
3.3.4 S Dopants with Improved Redox Driving Force
Early theoretical research work in 2012 revealed that the S atoms prefer to replace the two-coordination N sites in the aromatic ring and induce an impurity energy level just below the CB, which is beneficial to cause a red shift of solar light absorption threshold and improve the electronic conductivity of g-C3N4[166].Afterward, Chen et al.proposed an exceptional work on S-doped g-C3N4employing the H2S atmosphere as the S feeding source to achieve a homogenous S-doping at the atomic level with the pyrolyzed product labeled as C3N4-xSx[167].Its homogenous doping was confirmed by the almost same and stable XPS signal of S 2pspectra during Ar+sputtering from 20 to 420 s.The authors claimed that this was of vital importance to achieving: (i) the localized states induced by S dopants and (ii) the elevation of VBM through the mixing of S 3pstates with N 2pstates (Fig.12a–c).The extended VB width was believed to accelerate the mobility of holes, boost charge transfer kinetics, and thus give rise to a better photo-oxidation effi-cacy (Fig.12d).Furthermore, due to the unique synthetic strategy, the grain size of C3N4-xSxwas also dramatically reduced, inducing a remarkable quantum confinement effect (Fig.12d).This could render it a higher driving force for redox reaction due to the more positive VBM and more negative CBM positions.Therefore, C3N4-xSxshowed an overwhelming phenol removal activity under irradiation withλ> 400 nm.Other precursors such as thiourea [168–170] and urea/benzyl disulfide [171, 172] have also been reported to work as the S source, which was much “green” than the toxic and corrosive H2S gas.
In another typical work, the S-doped g-C3N4has demonstrated its superior role in boosting the photocatalytic CRR activity by altering the rate-determining step and reducing the Gibbs free energy from 1.43 to 1.15 eV [55].Detailed theoretical calculations have been carried out using the pristine g-C3N4and S-doped g-C3N4molecules containing four C6N7units as models.According to the HOMO and LUMO diagrams from Fig.12e-f, one can see the electrons in HOMO were distributed on N atoms only.While for LUMO, electrons were localized in both C atoms and N atoms.However, no electrons appeared around the bridging N atoms, which indicated the electron in g-C3N4would only be excited and transferred within one C6N7unit, thus increasing the photocarrier possibility of being recombination (Fig.12e).In contrast, the electrons in HOMO and LUMO were distributed on the undoped units and S-doped units, respectively (Fig.12f).This implied the electrons in S-doped g-C3N4can migrate within the surrounding C6N7units and the photocarrier separation efficiency could be significantly enhanced.Furthermore, the accelerated thermodynamics was verified by the optimized CRR pathway.For g-C3N4, the rate-determining steps were the conversion of CO2to COOH* and HCHO to CH3O* with ΔGvalues of 1.41 and 1.43 eV, respectively (Fig.12g).In contrast, the determining step for S-doped g-C3N4is the formation of COOH* only with a reduced ΔGof 1.15 eV (Fig.12h), suggesting the more favorable CRR progress which was also in good accordance with previous reports [168].
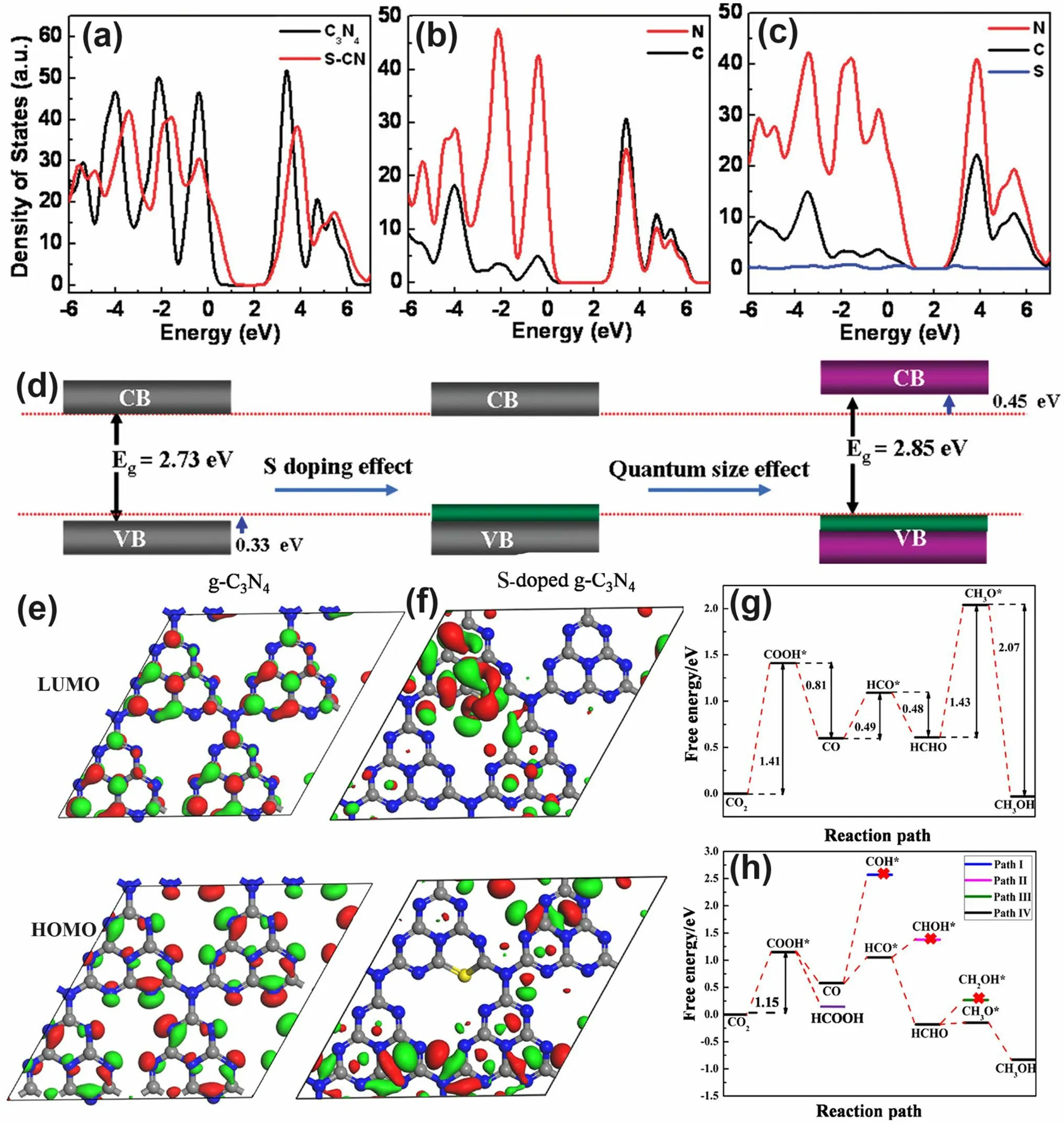
Fig.12 Defect control of S dopants.a Total DOSs of pristine C3N4 and C3N4-xSx; partial DOSs of b C3N4 and c C3N4-xSx; d graphic illustration of band structure change of C3N4 by S-doping and quantum confinement effect (QCE) [167].Copyright 2010, American Chemical Society.LUMOs and HOMOs of e g-C3N4 and f S-doped g-C3N4; calculated free energy diagram to the reaction paths followed by CRR on g g-C3N4 and h S-doped g-C3N4 [55].Copyright 2018, American Chemical Society
Ke et al.employed urea and benzyl disulfide as precursors to obtain the S-doped g-C3N4(SC3N4-X,X= 1, 2, 3 presenting the annealing temperature of 560, 600, and 650 °C) [171].The S dopants have been found to overcome the stronger planar hydrogen bond between the tri-s-triazine unit and NH/NH2group, favoring the layered g-C3N4exfoliation into nanosheets.Thus, SC3N4-3 exhibited the highest BET surface area up to 298.2 m2g-1, providing abundant sites for redox reactions.Consisting with previous studies, we observed that the S dopants also rendered SC3N4-3 with a reduced bandgap of 2.10 eV, which was 0.64 eV smaller than bulk g-C3N4with an extended visible-light absorption from 458 to 530 nm.As a result, the photodegradation constant and UO22+removal efficiency of SC3N4-3 achieved 0.16 min-1and 92%, which was 1.78 and 1.58-folder better than those of other g-C3N4materials.Cao and co-workers further synthesized the porous S-doped g-C3N4nanosheets with C vacancies (SCNNSS) by facile pyrolysis of thiourea [32].The thickness of SCNNSSwas only 2.5 nm, revealing a significantly improved BET surface area of 75.24 m2g-1.Although the presence of C vacancies broadened the bandgap of SCNNSS, its CB position was lifted from - 0.95 to - 1.04 eV, endowing it with a promoted driving force toward the photocatalytic NRR activity.Therefore, the nitrogen fixation rate of SCNNSSreached 5.99 mM h-1gcat-1, which was 2.8-fold the amount of bulk g-C3N4(2.13 mM h-1gcat-1), confirming the critical role of S doping.
3.3.5 O Dopants with Electronic Polarization
Chen and co-workers first reported that O atoms were prone to substitute the two-coordinated N atoms next to sp2-hybridized C atoms, forming the N–C–O and C–O bond [173].These O dopants could significantly optimize the electronic band structure of g-C3N4with a reduced bandgap of 0.21 eV in comparison with that of the bulk one.However, their VBM remained the same, indicating the VBM of the O-doped g-C3N4primarily depended on the N 2porbitals.Owing to the electronegativity discrepancy between N and O, more charge density would be presented near O atoms.Therefore, this would cause additional defect-related surface energy levels below the CBM, accelerating the photocarrier transfer and separation in O-doped g-C3N4.As a result, both excellent MB (methyl blue) photodegradation and H2evolution rate were achieved for the defective g-C3N4.Another work presented by Zhang’s group reveals that O dopants can shorten the C–N/C = N bonds due to the more negative electronegativity of O, which shortens the charge diffusion pathway from bulk to surface and boosts the charge transfer rate [75].Additionally, the theoretical differential charge density diagram clearly showed the electronic polarization effect aroused by O atoms, similar to the “innerbuilt electric field”, which gave the electron an extra transfer driving force.Thus, the HER rate was significantly improved.
Fu and colleagues prepared the hierarchical porous O-doped nanotubes (OCN-Tubes) by the successive high-temperature etching and curling-condensation of bulk g-C3N4[174].Due to the defect regulation, OCN-Tubes were not only doped by O atoms but exhibited a porous nanotube structure with an enhanced specific surface area of 36 m2g-1(Fig.13a, b).Furthermore, it also displayed an enhanced visible-light absorption as the PL peak increased from 450 to 475 nm.Also, the suppressed photocarrier recombination of OCN-Tubes was shown according to the dramatically reduced PL intensity (Fig.13c).The CH3OH yield of OCN-Tube in CRR activity is 0.88 μmol g-1h-1, far more exceeding that of bulk g-C3N4(0.17 μmol g-1h-1).Lu et al.prepared O-doped g-C3N4(O-CNx,x= 1, 2, 3 representing the molar ratio of ammonium acetate/melamine of 5, 10, and 30) via a direct thermal polymerization of melamine and ammonium acetate [175].In good accordance with the above-mentioned studies, the O-CN2 showed a dramatically reduced bandgap by 0.52 eV in comparison with the bulk one.This was also verified by the extended visible-light absorption edge in Fig.13d, which was inferred to boost the photocatalytic performance.As expected, the as-prepared O-CN2 displayed a 10-time-higher HER of 1062.4 μmol g-1h-1than that of bulk g-C3N4(Fig.13e).In addition, good cycling stability of O-CN2 for photocatalytic H2production is also observed in Fig.13f.
3.3.6 B Dopants with Narrowed Bandgap
Wang’s group synthesized the B-doped g-C3N4nanotubes (BCNT) via thermal pyrolysis of H3BO3and melamine (Fig.13g) [176].The unique BCNT structure with 0.3 μm thickness of the tube wall further improved its BET surface area from 17.8 to 27.9 m2g-1.Compared with bulk CN, BCNT showed a similar VB position at around 1.53 V vs.NHE but a more negative CB position by 0.17 eV (Fig.13h), indicating a narrowed bandgap that would allow more electrons to be generated under the same circumstances, and thus there were more ·O2-radicals for NO removal.Due to the B-doping, the photocatalytic NO degradation rate of BCNT was, therefore, the best value of 30.4% within 30 min when irradiated by visible light, which was 10% larger than that of bulk g-C3N4(20.8%, Fig.13i).

Fig.13 Defect control of O/B dopants.a Typical FESEM of OCN-Tube; b XPS survey spectra and c PL spectra of g-C3N4 and OCN-Tube [174]; Copyright 2017, Wiley–VCH.d UV–vis DRS and e HER rates of g-C3N4 and O-CNx samples; f stability test of O-CN2 under visiblelight irradiation (λ > 420 nm) [175]; Copyright 2018, Elsevier.g Schematic synthetic diagram of tubular BCNT; h band structure of CN and BCNT; i photocatalytic NO removal activities of CN, CNT, and BCNT under visible-light irradiation [176].Copyright 2018, Elsevier
3.3.7 Halogen Dopants with Narrowed Bandgap and Electronic Polarization
Halogen doping (F, Cl, I, Br) has been the research hotspot since the first pioneering work on F-doped g-C3N4by Wang’s group in 2010 [177].They speculated the F atoms preferred to bind with C atoms due to the electronegativity difference, and this would lead to the partial conversion of C-sp2to C-sp3followed by a decreased in-planar order.Their DFT calculations demonstrated that the F dopants in the bay C sites extended both the HOMO and LUMO to higher positions.While the corner C sites made the LUMO to higher energy levels, the HOMO lower energy levels.The experimental results showed the F-doped g-C3N4boosted the photocatalytic oxidization of benzene to phenol in the presence of visible light.These results indicated that F dopants were critical to changing the electronic band structure of g-C3N4and provided the basement for further redox modification.This work was also consistent with the published work by Ding and co-workers, who also investigated the F-doped g-C3N4had a larger bandgap of 2.81 eV than bulk g-C3N4of 2.68 eV [178].Moreover, the authors also claimed that the B/F co-doped g-C3N4not only met the demand of non-induced recombination centers plus enhanced solar light absorption but satisfied the requirement of overall water splitting with overpotentials.

Fig.14 Defect control of halogen dopants.a Calculated F-doped g-C3N4 models in interstitial space; calculated band structures and DOSs of b F-C3N4; c Cl-C3N4; d Br-C3N4; e I-C3N4; work functions of f F-C3N4; g I-C3N4 [109].Copyright 2017, Elsevier.h PL intensity; i ATZ removal rate comparison of various halogen atom-doped g-C3N4 [179].Copyright 2022, Elsevier
Yu et al.found different halogen-doping positions in the g-C3N4monolayer using the first principle investigation [109].Specifically, they found F and Cl atoms preferred to be presented in the interstitial space due to the smallest formation energy of 1.15 and 3.52 eV, which was particularly dramatically lower than the N3/C2 sites, respectively (Fig.14a).Actually, this was not strictly truth which they were not impossible to doping into N2 sites owing to the slightly higher formation energy (1.53 and 3.77 eV) compared to those of the interstitial space.Other halogen atoms of Br and I have a 1.6–2.6 times larger atomic radius than F and Cl, making them very unstable if directly displacing C and N atoms.Therefore, all halogen atoms were the most thermally stable in the interstitial space.Additionally, the electronic bandgaps were, in a rating order of 0.64, 0.95, 1.13, and 1.14 eV for F, I, Br, Cl-doped g-C3N4, which suggested a promoted solar harvesting ability than bulk g-C3N4(Fig.14b–e).Furthermore, with the bigger atomic number and higher electronegativity, the work function (Ф, calculated using the equation of Φ=EVac-EF(whereEvacand EF are positions of vacuum level and Fermi level) became smaller from 4.15 to 3.30 eV (Fig.14f–g), implying the easier for electrons to escape.As a result, these halogen atoms doped g-C3N4exhibited an extended light absorption even to 1000 nm.
Recently, a deep and systematic study on the halogen-doped g-C3N4has also been reported on photocatalytic ozonation (PCO) to remove the atrazine (ATZ) [179].An increasing ATZ removal order for CN < Br–CN < Cl–CN < I-CN < F–CN was witnessed, which was also basically consistent with the bandgap order from the above-mentioned calculations by Yu’s group [109].The reasons were ascribed to (1) All halogen-doped g-C3N4, particularly the F-CN, showed a narrowed bandgap at around 2.61 eV, enhancing its visiblelight absorption; (2) DFT calculations revealed that the uneven distribution of electrons on halogen-doped g-C3N4benefited the gas absorption.Among them, F-CN showed the highest O3and O2absorption energies of - 5.53 and - 4.55 eV, which further boosted the redox reaction kinetics; (3) the F-CN also displayed the largest water contact angle of 54.8°, which implied the optimized hydrophobicity merit that was more favorable for O3absorption; (4) the photocarrier recombination of these doped g-C3N4was significantly suppressed as reflected by the reduced PL intensity when compared to bulk g-C3N4(Fig.14h).These four factors combined to render the halogen-doped g-C3N4with an effective ATZ removal rate of up to 66.5% under visible light (Fig.14i).Other works reported in recent years agreed well with this discovery, revealing the promising applications of halogen doping on g-C3N4for improving photocatalytic performance [177, 180–182].Table 2 summarizes recent reports on non-metal doped g-C3N4toward various solar applications.
3.3.8 Co-doping with Synergistic Effects
Single-element doping enabled the optimization of the electronic band structure and photocarrier transfer progress of g-C3N4.Intriguingly, the heteroatomic co-doping that could combine the merits of these single dopants is also efficient in boosting its photocatalytic activity [158, 183, 184].For instance, Ma et al.prepared P and O co-doped g-C3N4that exhibited enhanced RhB photocatalytic degradation effi-ciency [185].The B/F co-modified g-C3N4also showed promoted HER performance[186].A typical C/O-doped g-C3N4synthesized from the calcination of protonated melamine has also attracted extensive research attention as its detailed information on both experimental results and calculations including the doping sites, bond length, and changed charge density distribution [104].Taking the doping position firstly for example, Gao et al.found O dopants might be more favorable than C dopants at the first doping progress due to the unstable C-doped g-C3N4with positive formation energy from 0.75 to 2.21 eV (Fig.15a).As for first O-doping and then C-doping, the values for defective g-C3N4could reach the least values of - 0.97 and - 1.2 eV, indicating a more spontaneous doping process.After comparing the electron density around the defects for both bulk g-C3N4and C/O-doped g-C3N4, one can see more electrons were accumulating on the N4 sites and fewer electrons around C2 sites, indicating an enhanced electronic polarization effect (Fig.15b–d).This would act as an “inner-built electric field” that can accelerate electron transfer with a driving force like Coulombic force (Fig.15e).In addition, the bond length around these O and C dopants were all shortened by 0.02 Å, which was more beneficial for mass diffusion and charge transfer (Fig.15f).More importantly, due to the C-doping into the N3Csites, a delocalizationπbond was formed, which enabled the excited electrons transfer among the tri-s-triazine units with better electronic conductivity (Fig.15g).As a result, we observed a boosted photocurrent density and HER rate of 320 μA cm-2and 830.1 μmol g-1h-1, which were 60 and 7 times higher than those of bulk g-C3N4.
3.3.9 Dopants and Vacancies with Synergistic Effects
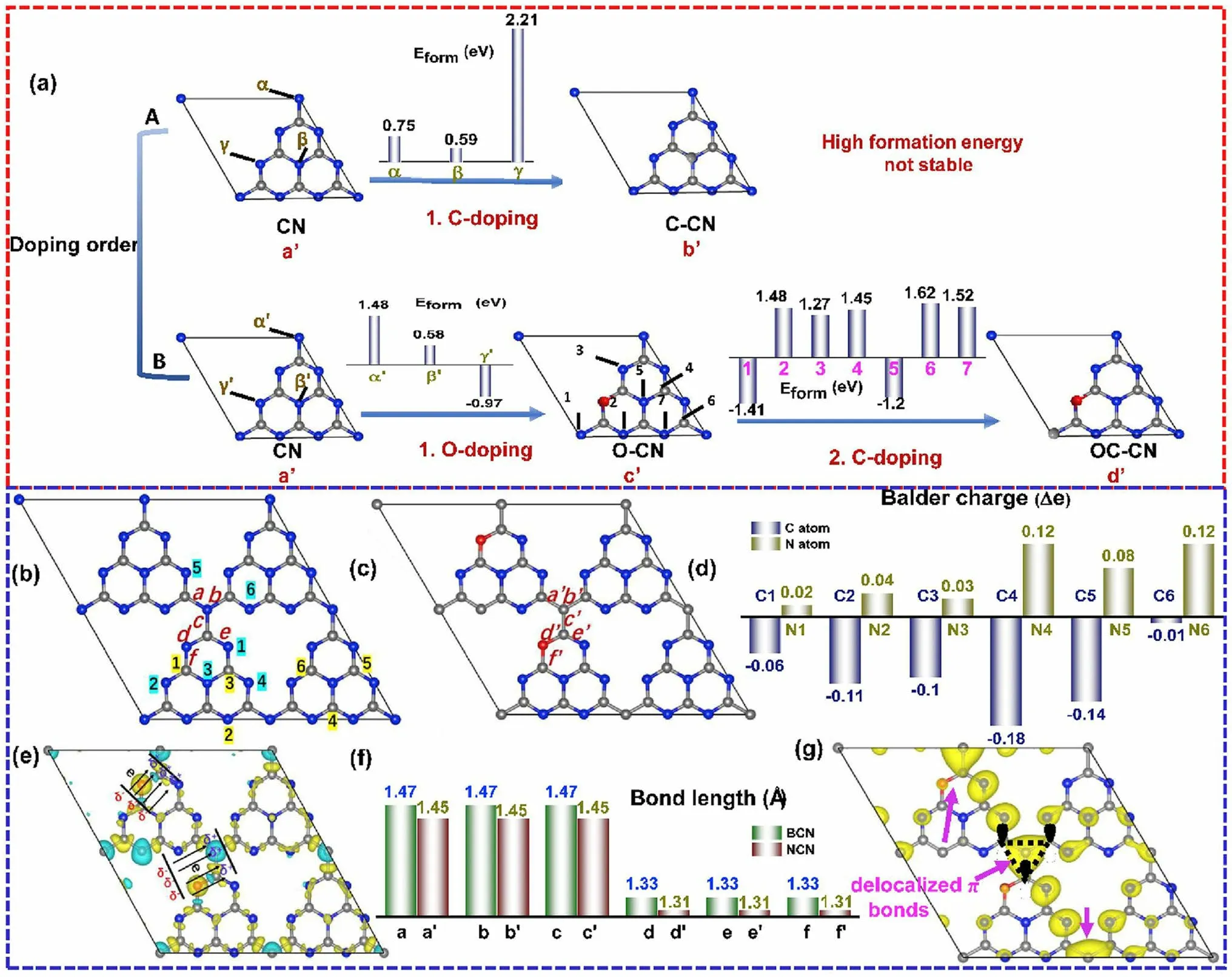
Fig.15 Defect control of co-doping.a Illustration of various C(O)-doped g-C3N4 with different doping order: (a’) CN; (b’) C–CN; (c’) O-CN and (d’) OC-CN; b configuration of original non-doped BCN; c optimized configuration of NCN; d Bader charge change; e differential charge density between BCN and NCN; f bond lengths of BCN and NCN; g π orbital distribution (VBM-5) of NCN (C, N and O atoms are shown in grey, blue and red.Olive and cyan illustrate the increase and decrease of electron distributions) [104].Copyright 2019, Elsevier
Inspired by the research work combining different vacancies and dopants, our group also proposed a novel defective g-C3N4(DCN) with both N vacancies and S dopants via a dual-solvent-assisted synthetic strategy [91].Employing the protonated melamine obtained in the presence of glycol via a solvothermal reaction as the precursor followed by a subsequent annealing process with molten sulfur at 550 °C under N2atmosphere, we determined DCN with an S-doping level of 0.5% and a moderate N vacancy concentration.With this defect control, DCN also exhibited a porous prisms nanostructure of 500 nm and an enhanced BET surface area of 169.10 m2g-1, boosting the active sites for photocatalytic HER activity.Furthermore, the glycol and molten sulfur solvents were both critical to inducing N vacancies and S dopants and induced both shallow defect states and optimized surface states.The former could be revealed by the experimental defective energy levels which were 0.49 eV to the CBM of DCN.More importantly, in theoretical calculations, bulk g-C3N4(BCN, Fig.16a), g-C3N4unit with one N vacancy (DCN-NV, Fig.16b), and g-C3N4unit with one S dopant (DCN-S, Fig.16c), were proposed to unveil the different roles of N vacancy and S dopant.According to the ELF results (Fig.16a–c), DCN-S showed the densest electron density toward the C3N4unit cell void, corresponding to one of the lone pair electrons of S.This indicated DCN-S was more favorable to boost the electron polarization effect that could enable an accelerated photocarrier transport.As for the DOSs, we can see both DCN-Nv and DCN-S displayed new defect states around the Fermi level (Fig.16d–f).However, the N vacancies in DCN pushed these additional energy levels closer to the VBM in comparison with the S dopants, indicating too much N vacancy concentration would worsen the electron band structure with deep localized states to severely recombine the photocarriers.This was also in good line with the XPS result that DCN displayed a moderate peak area ratio of –C3N/C=N–C at around 0.287.Furthermore, this S-doped and N vacant g-C3N4also reveal optimized surface states with the highest surface trapping resistance (Rtrapping) of 9.56 × 103Ω cm2and the slowest decay kinetics of surface carriers (0.057 s-1), which guaranteed the smooth surface charge transfer rather than being the recombination sites.As a result, it exhibited a superior H2evolution rate of 4219.9 µmol g-1h-1, which was 29.1-fold higher than unmodified g-C3N4.
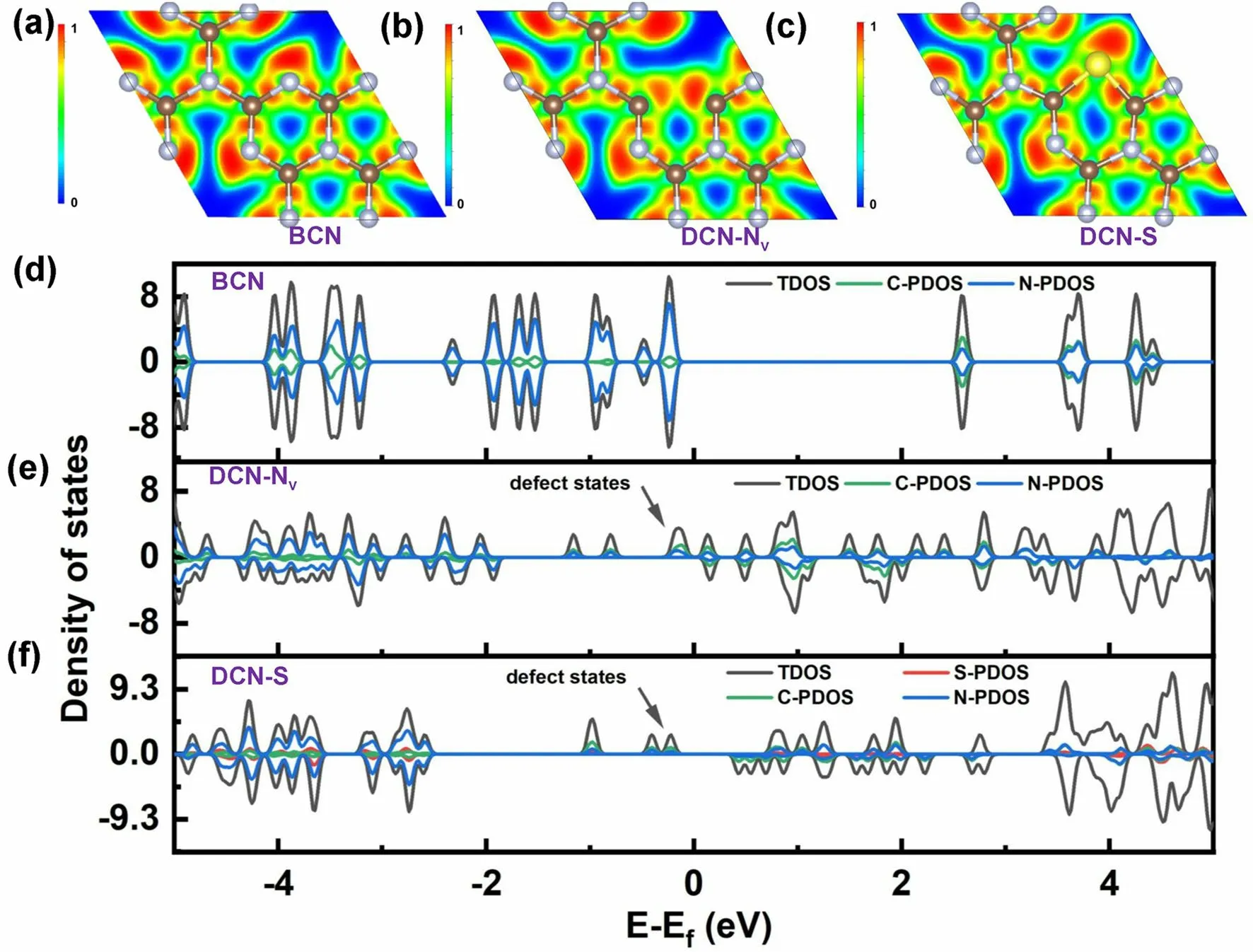
Fig.16 Defect control of dopant and vacancy.ELF plots of a BCN; b DCN-Nv; c DCN-S; total density of states (DOS) and partial density of states (PDOS) of d BCN; e DCN-Nv, and f DCN-S [91].Copyright 2023, Wiley–VCH
In another typical work, multiple defects-modified g-C3N4catalysts with B-F or B-S co-doping combined with N vacancies (donated as B–F–Nv, B–S–Nv) have also shown great potential for the optimization of electronic band structure and enhancement of photocatalytic CRR performance [187].As shown in Fig.17a, there were two C and three N doping positions assigned to C1, C2, N1, N2, and N3 which could be doped or vacant for B/F/Nv.According to the formation energy, B and F preferred to be presented in the C1 site and connected with the N2 site.However, after introducing the Nv at N3 site, the F atoms would transfer from N2 to B side due to its strong electronegativity, leaving the rest C–N becoming into sp hybridization (Fig.17b).However, as for B–S–Nv, B and S atoms preferred to be at the C1 and N2 sites while Nv presented at new N2 site next to this unit, forming a five-ring unit to keep the structure stable (Fig.17c).According to the DOSs diagrams, the bandgap of B/F co-doped g-C3N4was found to be 3.06 eV, significantly higher than 2.77 eV of g-C3N4.However, due to the presence of Nv, the bandgap of B–F–Nvmaterial reduced to 2.67 eV, suggesting the N vacancy’s role in narrowing the bandgap with extended solar absorption (Fig.17d).Interestingly, the S dopant was also significant in further reducing the bandgap of B–S–Nvto 1.16 eV than F dopant of B–F–Nv(Fig.17g).Additionally, the HOMO and LUMO of B–F–Nvand B–S–Nvhave little overlap (Fig.17e, f, h, i), which can effectively facilitate the separation of photogenerated electrons and holes.Furthermore, new electron distribution of HOMO and LUMO on bridging N atoms could promote the migration of photogenerated charge carriers, thereby enhancing solar utilization efficiency.The photocatalytic CO2reduction reaction is a complex process that usually generates multiple products.Figure 17j shows the specific reaction pathway of the photocatalytic CO2reduction reaction:

Fig.17 Defect control of multiple defects type in g-C3N4.a Top view of pristine g-C3N4 geometries; Optimized geometries of b B–F–Nv and c B–S–Nv; calculated band structure of d B–F–Nv; e LOMO for B–F–Nv; f HOMO for B–F–Nv; g calculated band structure of B–S–Nv; h LOMO for B–S–Nv; i HOMO for B–S–Nv; j Gibbs free energy diagrams of photocatalytic CO2 reduction; k representative geometries of the stable points in the specific reduction process for CO2 on g-C3N4 [187].Copyright 2022, Multidisciplinary Digital Publishing Institute
*CO2→ *COOH → *CO → *HCHO → *OCH3→ *CH3OH → *CH3→ *CH4.Based on this, it can be concludedthat the products generated in the photocatalytic CO2reduction reaction were CO, HCHO, CH3OH, and CH4.Figure 17k provides the Gibbs free energies required for different catalysts at different stages of the photocatalytic CO2reduction reaction.According to ΔGchange, we can infer the main product of B–F–Nvfor photocatalytic CO2reduction reaction was CH3OH due to the following uphill energy (0.16 eV) for the conversion of *CH3OH to ·CH3.On the other hand, B–S–Nvhad a lower ΔGchange for catalyzing the CO2reduction reaction, facilitating the reaction to proceed to the final step and generate CH4.This would inspire future researchers to tune the defect types to enhance product selectivity.

Table 2 Comparison of non-metal doped g-C3N4 toward various solar applications
4 Metallic doping with Active Coordinate Environment
Generally speaking, the metallic dopants in g-C3N4normally induce enhanced solar light absorption, fast electron transfer, and high photocarrier separation efficiency [158].Metal doping is usually realized by the thermal pyrolysis of the mixture of g-C3N4precursors and a soluble metal salt.In the early stage of metallic doping, researchers have not specified the metal existence form of either in nanoclusters aggregation or atomic distribution as the limits of ordinary TEM and XPS technologies.This situation has changed since the employment of the special aberration-corrected transmission electron microscope (AC-TEM) and K-edge X-ray absorption fine structure (EXAFS) that can distinguish the metal morphology at the atomic level with very high resolution and identify the metal coordination environment with both interaction and bonding species [209].For the former, one can clearly see whether metal is in small aggregation or atomic well-dispersion.For the latter, researchers need to analyze the spectrums to figure out the only metal interaction such as metal-N peaks without any other peaks such as metal–metal peak, metal-oxide peak, and so on.Based on the above knowledge, the development of metallic-doped g-C3N4toward various photocatalytic applications was extended as follows:
4.1 Alkali Metallic doping with M–Nx Bonding
The typical alkali metals, such as K and Na dopants, were found to exhibit different roles in regulating the electronic band structure and optical properties of g-C3N4[210–212].In a detailed theoretical study, Xiong et al.found both K and Na atoms can narrow the bandgap and strengthen the solar light absorption of g-C3N4[210].Additionally, K atoms preferred to be presented in the interlayer space and thus provided electrons with better vertical transfer pathways.In contrast, the Na atoms preferred to chemically bond with the in-planar N atoms via ion bond due to the easy escaping of Na 3 s electrons.Based on their experimental results, the K-doped g-C3N4reflected a better photocatalytic NO removal activity than the Na-doped one, suggesting the prominent role of K-doping over Na-doping.
Other alkali metals, such as Ba and Rb, have also been verified to be efficient in boosting the solar activity of g-C3N4[89, 213].For instance, Hu et al.employed a facile salt-assisted method to synthesize the Ba-doped g-C3N4(BaCN-C3N4) [89].The successful Ba-doping was revealed by the obvious Ba 3dsignal according to the full-scan XPS spectrum.Compared to the original g-C3N4, BaCN-C3N4displayed an extended optical absorption edge by 10 nm and its bandgap was slightly narrowed by 0.05 eV, showing the improved visible-light absorption due to Ba dopants.The DFT calculations revealed that due to the insert of Ba dopants into the g-C3N4heptazine ring cavity, its bandgap can be dramatically reduced from 1.84 to 0.89 eV (Fig.18a-f).It is worth mentioning that the presence of BaCl2during the pyrolysis process could induce the formation of cyano groups, which further lowered the BaCN-C3N4to 0.2 eV (Fig.18g-i).However, the CBM and VBM of these samples were all contributed by C 2pand N 2porbits, and Ba was not involved in the construction of the band edge structure (Fig.18c, f, i).The authors also claimed that this narrowed bandgap of BaCNC3N4might be aroused by the reduced corrugation amplitude due to Ba incorporation.Combined with the electronic polarization around Ba dopants and cyano groups, the charge transfer dynamics can be further boosted.As a result, the 7%-BaCN-C3N4achieved an excellent tetracycline (TC) degradation rate and HER rate of 63.6% and 10,316 μmol g-1h-1, significantly, exceeding the bulk g-C3N4.Another piece of systematic work proposed by Zhang et al.indicated that Rb atoms are the best alkali dopants in promoting the photocatalytic activity of g-C3N4[213].It can be seen from Fig.18j that Rb-doped g-C3N4exhibited the most extended solar harvesting ability with the lowest bandgap of 2.0 eV, which was 0.65 eV smaller than pristine g-C3N4, implying the substantially improved visible-light absorption.Moreover, the Rb-doped g-C3N4also reflected the best conductivity in the solar range from 300 to 1100 nm, demonstrating the fastest charge transfer kinetics, which might favor the redox reaction (Fig.18k).Along with its merit of the lowest electron transport barrier, Rb-doped g-C3N4displayed the highest CO yield of 12.1 μmol g-1(Fig.18l).Therefore, based on the above experimental results and DFT calculations, we can conclude that the alkali metals, particularly Rb-doped g-C3N4,hold great prospect for g-C3N4-based solar applications.
4.2 Transition Metallic doping with M–Nx, M-C2N2, M–O Bonding
Apart from alkali metals, transition metals such as Co, Cu, Fe, Ce, and Bi have also been verified to promote the photocatalytic activity of g-C3N4[158, 183].For example, Deng et al.used nickel formate and urea as raw materials to prepare the Ni-doped g-C3N4samples (NCN-X) [214].For NCN-1, it presented the porous nanosheet structure with folded and rolling edges with a thickness of 10 nm.The Ni dopants were well distributed on the g-C3N4surface as reflected by the energy dispersive spectroscopy (EDS).With the increasing Ni content, NCN-X displayed not only an enhanced harvesting ability of solar light but also a longer absorption edge with decreasing bandgaps of 2.73, 2.68, 2.61, and 2.45 eV for CN, NCN-1, NCN-2, and NCN-3, respectively.Combined with the Mott–Schottky results, the VB potentials of NCN-2 reached the maximum value of 1.59 eV, which was conducive to the transfer of photoexcited carriers and charge separation progress.Therefore, we can observe NCN-2 displayed the highest MO degradation rate of 97.3% within 90 min and the highest HER rate up to 155.71 μmol g-1h-1among all the g-C3N4-based photocatalysts.
Although significant advances have been made in the research fields of metallic-doped g-C3N4toward various solar applications[215, 216], the precise active sites between metallic dopants and g-C3N4, and the existing form of metals were still not clear.Recently, metal single atoms (M-SAs) have exhibited a promising prospect with stable stability in electrochemical catalysis such as water splitting [217, 218], CO2reduction[219, 220], and so on [221, 222].Since then, more research attention has been devoted to the M-SAs doped g-C3N4systems, including identifying the metallicnitrogen (M–N) interaction [223], metallic-oxygen interaction [224], forming the dual-atom catalysts [225] or even SAs@metal clusters catalysts (Fig.19a) [226].
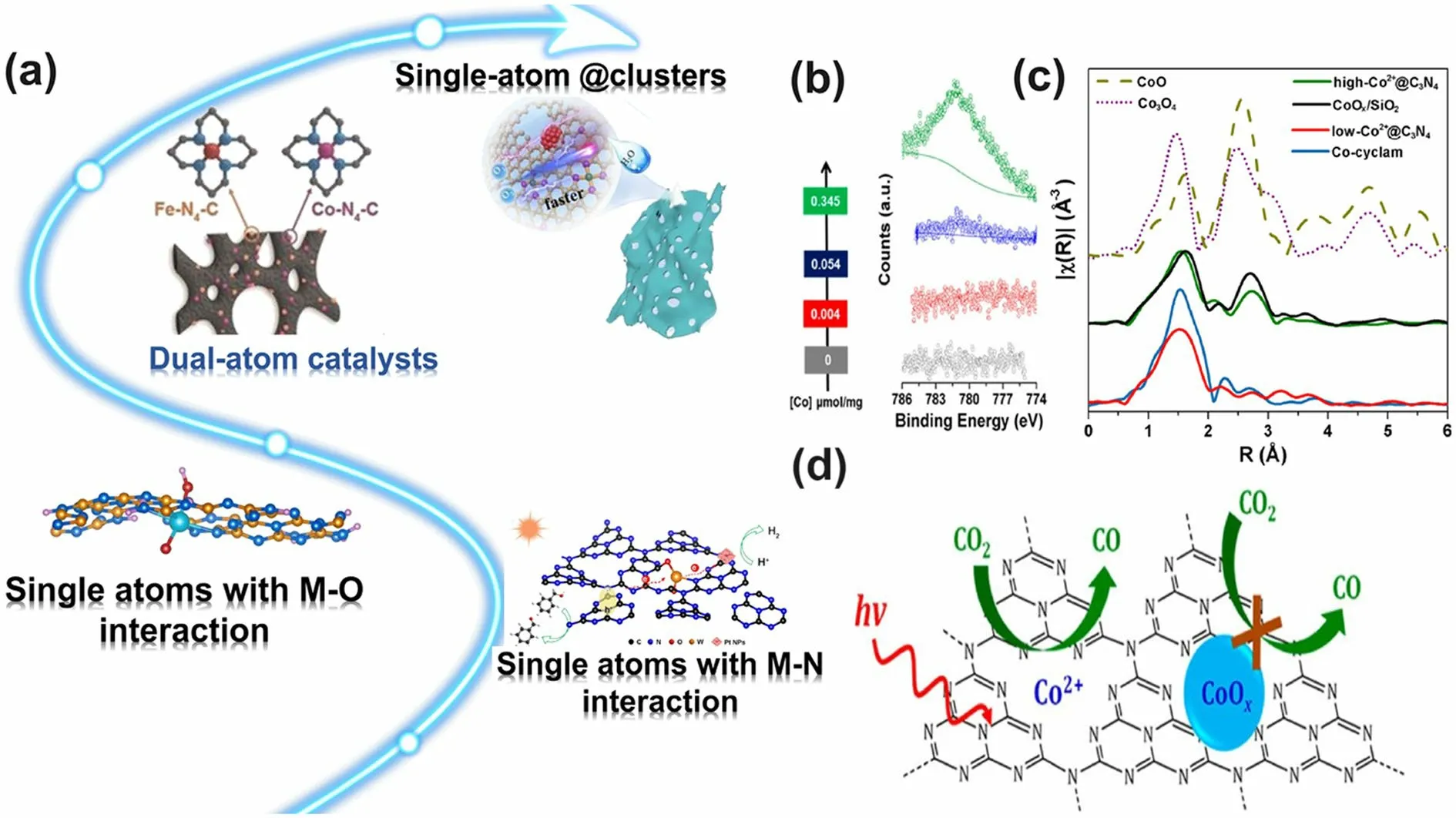
Fig.19 Defect control of SAs-doped g-C3N4.a Research evolution of single atoms-doped g-C3N4 [223–226]; b XPS of bare C3N4 and Co2+@C3N4; c Fourier transform magnitude of k2- weighted Co K-edge EXAFS spectra; d schematic diagram of photocatalytic CO2 reduction mediated by a single CO2+ site on C3N4 [227].Copyright 2018, American Chemical Society
Taking the M–N interaction first, Co single atoms (SAs) were successfully doped into the g-C3N4matrix forming the Co–N bonding via a microwave method in the presence of triethanolamine (TEA), and the resultant sample was labeled as Co2+@C3N4[227].The XPS confirmed the presence of Co2+with an obvious peak assigning to Co 2p3/2transition at 781 eV when the Co-doping level increased from 0.004 to 0.345 μmol per 1 mg g-C3N4(Fig.19b).Interestingly, the Co SAs were detected only in low-Co2+@C3N4as reflected by the Co–N interaction at 1.55 Å along with the absence of Co–Co at 2.7 Å according to the Fourier transform magnitude Co K-edge X-ray absorption fine structure (EXAFS) spectrum (Fig.19c).While the high-Co2+@C3N4samples showed similar peaks with CoOx/SiO2, implying the Co SAs were prone to aggregate into nanoparticles at high precursor concentrations.Furthermore, the g-C3N4substrate was inferred to be the C-doped type rather than the O-doped type as reflected by the superior CRR performance of C-doped g-C3N4, which also coincided with the previous study [105].Additionally, when the Co content reached 0.128 μmol mg-1, the CO yield achieved the highest value of 1.056 μmol mg-1.However, excessive Co content resulted in the formation of CoOx, endowing a lower CRR activity with low CO selectivity, demonstrating the superior redox selectivity of the single atomic Co2+sites (Fig.19e).
In another typical M-SAs work, the N vacant g-C3N4synthesized at different temperatures were chosen as the metal deposition substrates as the N vacancies could stabilize Pt single atoms (PtSA) [228].The as-prepared samples were named PtSA-CNX, where X was the annealing temperature.It is not surprising to see the resulting PtSA-CN620 exhibited a uniform PtSA coverage density of 0.35 mg m-2without any obvious aggregates from the HAADF-STEM image (Fig.20a), which was much better than those obtained in a lower temperature at 400 and 560 °C.According to the Fourier transform K3weighted (EXAFS) spectrum, the wavelet transform (WT) maximum at 5.61 Å-1of PtSACN620, PtSA-CN560, and PtSA-CN400 revealed that the existence of PtSA in the form of Pt-C, Pt–N, and Pt-O (Fig.20b).Furthermore, the calculated H2desorption energies on Pt nanoparticles (PtNP), and PtSA next to the twocoordination N (N2C) and C (C2C) sites were 1.33, 1.10 and 0.18 eV, respectively (Fig.20c–e).The authors ascribed this to the high proton-reduce degree and shorted H distance on the C2Csites, which was beneficial to the H2evolution kinetics.For the optical property, the solar absorption of this PtSA-modified g-C3N4was enhanced as the annealing temperature increased.Among them, PtSA-CN620 also displayed the most extended absorption edge to 640 nm with the smallest bandgap of 2.17 eV and the fastest photocarrier transfer dynamics with the lowest average photocarrier time of 3.40 ns, further demonstrating the superior role of PtSA.Thus, we observed the highest HER rate of 174.5 mmol g-1h-1per PtSA for PtSA-CN-620.
Fig.20 Defect control of single atoms doping.a High-angle annular dark-field scanning transmission electron microscope (HAADF-STEM) of PtSA-CN620; b k3-weighted FT spectra at k space of Pt foil and various PtSA-CN samples; H2 desorption on PtSA at c C2C and d N2C sites and e PtNP-C3N4 [228].Copyright 2019, Elsevier.f FT of Pd-CV-CN, Pd foil, and PdO at Pd K boundary; g DOSs diagram of g-C3N4 and Pd-Cv-CN; h photocatalytic NO removal activities of various g-C3N4-based samples [97].Copyright 2021, Elsevier.Reaction pathways for CO2 reduction to i HCOOH and CH3OH on Pd/g-C3N4 and j HCOOH, CH3OH, and CH4 on Pt/g-C3N4 catalyst (color code: Pd, pine green; C, gray; O, red; H, white) [229].Copyright 2016, American Chemical Society
Inspired by this, the C-vacancy-rich g-C3N4obtained at 600, 550, and 500 °C was also fabricated as the matrix for Pd SAs with the corresponding defective samples abbreviated as CV-CN, CV-CN-1, and CV-CN-2, respectively [97].Owing to the large specific surface area of Cv-CN at around 92.0 m2g-1, a high Pd SAs coverage density can be achieved.A deeper discussion on the chemical interaction was identified to be the Pd–N bond located near 2.03 Å according to the XAFS spectrums (Fig.20f).This was due to the stabilization effect of C vacancies with Pd SAs, which also agreed well with the previous report [105].The DOSs were analyzed to interpret how the Pt SAs affect the electronic band structure of Cv-CN.Compared to g-C3N4, Pd-Cv-CN exhibited more negative peaks with the changed peak shape on the negative side but similar peaks on the positive side (Fig.20g).Therefore, Pd-Cv-CN showed lifted energy levels with a narrower bandgap.In addition, the impurity energy levels were ascribed to the regulation of Pd 4dorbit, which was supposed to boost solar utilization.Specifically, an inhibited PL intensity was shown for Pd-Cv-CN in contrast with Cv-CN, suggesting improved photocarrier separation.As a result, Pd-Cv-CN showed the highest NO removal efficiency of 56.3% within 30 min, which was 10.3% higher than the Pd nanoparticles-modified one (Fig.20h), validating the critical role of Pd SAs toward photocatalysis.
Gao et al.compared the possible reaction pathways of photocatalytic CRR on the Pd SAs- and Pt SAs-modified g-C3N4via the DFT calculations [229].For Pd SAs-g-C3N4(Fig.20i): (1) The rate-determining step was the hydrogeneration of HCOOH* with a reaction barrier of 1.46 eV, which was believed to happen thermodynamically according to previous reports [230].(2) Compared to the following high barrier (1.46 eV) for CH3OH generation, HCOOH+* was more likely to leave from Pd SAs-g-C3N4due to its dramatically reduced desorption barrier of 0.46 eV.Therefore, the resulting final product for Pd SAs-g-C3N4was HCOOH.However, regarding the Pt SAs-g-C3N4(Fig.20j): (1) The barrier of HCOOH* hydrogeneration was about 0.27 eV lower than that of Pd SAs-g-C3N4, indicating a more favorable reaction on Pt SAs surface.(2) The higher HCOOH+* desorption energy of 1.06 eV might imply the following hydrogeneration pathways were more thermodynamically favorable for both CH3OH+* and then CH4+* generation.Thus, the final CRR product on Pt SAs-g-C3N4was suspected to be CH4.This intriguing work suggested that different types of M-SAs might have different roles in tailoring the redox selectivity, which was critical for future photocatalyst design.
Apart from the widely investigated M–N interaction between SAs and the g-C3N4matrix [231], researchers have found that M–N2C2coordination for Ag SAs was more favorable to boosting the photocatalytic HER activity [111].Specifically, the Ag–N2C2-modified g-C3N4(Ag–N2C2/CN) was prepared via a novel annealing process with the selfassembled melamine cyanurate and Ag-containing salt.As reflected by the Ag K-edge XANES (Fig.21a), the absorption line of Ag–N2C2/CN lied between Ag foil and Ag2O, indicating the oxidation state of Ag was between 0 to + 1 due to the strong interaction between Ag and tri-s-triazine units.According to the k3-weighted EXAFS result, there were only two main peaks at 1.55 and 2.41 Å, corresponding to the first coordination shell of Ag–N and the second coordination shell of Ag-C, respectively (Fig.21b).Further EXAFS fitting result evidenced the coordination numbers of Ag–N and Ag-C were 2.3 and 1.8, demonstrating the Ag SAs generation to be coordinated with N2C2interaction (Fig.21c–d).This interaction significantly contributed to the electronic polarization effect around the Ag–N2C2sites which was stronger than Ag–N and Ag nanocluster sites, indicating its superior role in accelerating the photocarrier transfer and separation.The Gibbs free energy required for each step in the reaction process of H2O → HO-H → H*→ H2was also calculated.It was found that Ag–N2C2/CN had the lowest energy requirement for each step in the photocatalytic water splitting process (Fig.21e), thus leading to an enhanced photocatalytic performance of 1.8 mmol g-1h-1, much higher than Ag–N/CN.
Very recently, Yu et al.discovered that the single-atom metal–oxygen bonding was favorable for the photocatalytic OER activity, which paved a promising resolution to future efficient water splitting [224].The single atom metal–oxygen doped polymerized g-C3N4(MO-PCN-x, where M can be Mo and W, X was annealing temperature) was mainly obtained by the annealing of protonated melon absorbed MOxn-ions powder, and its possible calculated structure is also given in Fig.21f.According to the EPR result, we can see that, in comparison with PCN, the MO-PCN exhibited enhanced signals both in dark and under irradiation, indicating the increased delocalized electronic density and enhanced photoexcitation process, respectively (Fig.21g).The authors claimed it was the higher Mo–O content than W–O in PCN that induced the stronger EPR signal of Mo-PCN550 than that of W-PCN600.Furthermore, the calculated bandgap of Mo-PCN550 was much smaller than that of PCN (2.3 eV), measuring only 0.54 eV (Fig.21h-i), indicating the promoted solar harvesting ability of Mo-PCN550, which was also consistent with experimental results.Benefiting from the reduced bandgap and promoted photocarrier transfer and separation of M–O bonding, W-PCN600 exhibited the highest overall water splitting rates of H2/O2production rate of 76.9/3.4 μmol h-1m-2, significantly surpassing PCN600.
As for the bimetallic single-atom doping, Choi’s group successfully synthesized the single-atom catalyst with dualatom-sites featuring neighboring Sn(II) and Cu(I) centers embedded in C3N4framework (DAS-Snx-Cu100-x/C3N4) by annealing of mixture of urea and Sn– and Cu-acetylacetonate (x was the mass ratio of Sn-acetylacetonate in the mixture of Sn– and Cu-acetylacetonate) [103].Through the STEM and XANES characterization, the best DAS-Sn75-Cu25/C3N4sample has demonstrated its dual-atom-sites with the coordination environment of M–Nxbonding without the presence of any M–O or M-M bonding.Importantly, they also employed the in situ transmission FTIR spectroscopy for monitoring the photocatalytic CRR processes, which was similar to that of Fig.6d-f.Specifically, the reaction cell was sealed with CaF2windows and a spacer, and located in the FT-IR instrument with a mercury cadmium telluride (MCT) as detector (Fig.22a).The transparent window with a thin layer of g-C3N4samples and a solution of 10 vol% TEA in CO2-saturated 0.1 m KHCO3well mimicked the CRR process.Interestingly, the in situ FT-IR results indicated the C = O stretching mode at around 1712 cm-1assigned to *HCOOH was found for both DAS-Snx-Cu100-x/C3N4and SA-Sn/C3N4,not for SA-Sn/C3N4(Fig.22b-d).Additionally, the most significant peak at 1637 cm-1assigned to the C = O stretch of *HCHO intermediate was only found for DASSnx-Cu100-x/C3N4, indicating its main product of HCHO, not HCOOH.Furthermore, the NMR test demonstrated the carbon source of HCHO originated from outer CO2rather than g-C3N4, implying the stability of DAS-Snx-Cu100-x/C3N4.Using the triethylamine (TEA) as a proton donor, DAS-Snx-Cu100-x/C3N4first combined with CO2and electron to form *OCHO, then the *HCOOH leaving H2O to form *CHO, and finally with proton to become HCHO with the highest produced rate of 259.1 µmol g-1and a selectivity of 61% after 24 h irradiation, far more exceeding its counterparts of single-atom based photocatalysts.

Fig.22 Defect control of bimetallic single atoms doped g-C3N4.a Schematic illustration of setup for in situ photochemical transmission infrared absorption spectroscopy; b in situ FTIR spectra of SA-Sn/C3N4, SA-Cu/C3N4, and DAS-Sn75–Cu25/C3N4 photocatalytic systems at 5 and 30 min in CO2-saturated 0.1 m KHCO3 and 10 vol% TEA in D2O solution.Each spectrum at 0 min was used as a baseline; deconvoluted spectra in the range of 1500–1800 cm-1 of c SA-Cu/C3N4 and d DAS-Sn75–Cu25/C3N4 at 30 min; e proposed mechanism of CO2 conversion to HCHO over DAS-Sn75–Cu25/C3N4 photocatalyst [103].Copyright 2022, Wiley–VCH
In another typical bimetallic single-atom work, Ning and colleagues proposed a Co and Mn SAs co-doped g-C3N4(Mn1Co1/CN) via the atom confinement and supramolecular self-assembly strategy [232].To determine the forming existence of Co and Mn elements, researchers studied the Mn K-edge XANES spectra and k3-weighted Fourier-transform Mn K-edge EXAFS spectra of the samples.As shown in Fig.23a, the Mn1Co1/CN peaks located between Mn foil and Mn2O3, suggesting the valance state of the Mn species lied between 0 and + 3.Figure 23b clearly shows Mn1Co1/CN had only one main peak located at 1.63 Å without the Mn–Mn bonding, indicating the Mn–N coordination and Mn existed in the form of SAs rather than nanoparticles.This was also true for Co SAs as there was only one Co–N interaction signal located at around 1.73 Å (Fig.23c).Due to the synergistic effect of Mn and Co SAs, the CO produced rate of Mn1Co1/CN reached 47 μmol g-1h-1(Fig.23d).To identify the roles of single atoms in photocatalytic CRR activity, the O2evolution and CO production tests were further performed.It was observed that Mn SAs were beneficial to O2generation (Fig.23e) leading to the final oxidization of H2O2while Co SAs for CO formation (Fig.23f).According to the DFT calculations, it was evident that CO2molecules were more easily adsorbed near Co atoms in Co1/CN as reflected by the bigger bond bending angle and longer bond length in comparison with that of Mn1/CN (Fig.23g-h).This verified that Co SAs were the CO2absorption and activation sites.For the H2O oxidization reaction, this was the opposite that Mn SAs were the active sites.According to the in situ DRIFTS measurement, multiple intermediate products such as CO2-, HCOO-, and HCO3-were generated, making it easy to form byproducts such as HCOOH and HCO3H (Fig.23i).The combined effect of Co and Mn greatly reduced the production of these byproducts and improved the CRR selectivity.To confirm that the generated CO coming from the reduction of CO2rather than other carbon-containing reactants, the researchers used an isotope of C,13C, to label the C atom (Fig.23j).The mass spectrometry signal of the obtained product has an m/z value of 29, corresponding to13CO, thus proving that CO was derived from CO2feeding gas.The synergistic effect of Mn and Co SAs indicated the advantage of bimetallic single atoms doping over single-atom doping for photocatalytic CRR performance.In addition, we summarize recent advances in the metallic doped g-C3N4for various solar activities in Table 3.

Fig.23 Defect control of bimetallic single atoms doped g-C3N4.a Mn K-edge XANES spectra of the Mn foil, Mn2O3, MnO and Mn1Co1/CN; b k3-weighted Fourier-transform Mn K-edge EXAFS spectra of the samples; c k3-weighted Fourier transform Co K-edge EXAFS spectra of the samples; Photocatalytic activities of samples with d double single-atom active sites, e single-atom oxidation sites and f single-atom reduction site in CO2 reduction; Structure of h Co1/CNCO2 and Mn1/CNCO2; i in situ DRIFTS result of Mn1Co1/CN for CO2 reduction; j Mass spectra of 13CO driven for Mn1Co1/CN during light irradiation under 13CO2 atmosphere [232].Copyright 2022, Wiley–VCH
5 Grafted Functional Groups with Optimized Band Structure
Grafting the organic functional groups onto the g-C3N4has also been verified as one of the most promising defect controls for tuning its physicochemical properties with optimized band structure, enhanced solar absorption as well as fast photocarrier transport [257].Previously reported functional groups included the -C≡N (cyano group), O = C–NH2(urea-like group), -COOH, -C = O, -OH (O-containinggroups), and various aromatic rings, of which -C≡N is the most widely investigated one (Table 4).
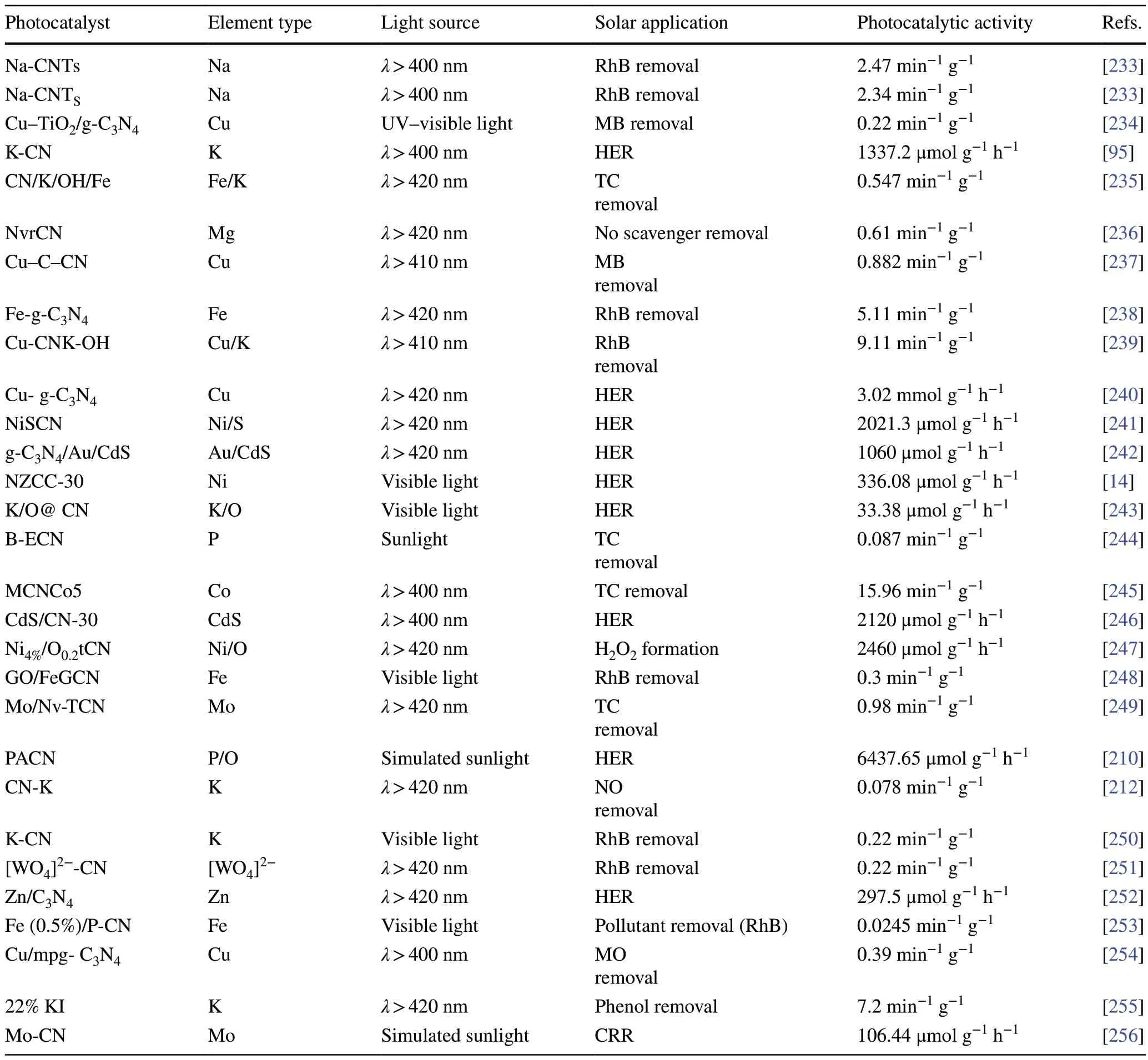
Table 3 Recent studies on the metallic-doped g-C3N4 for photocatalytic performance
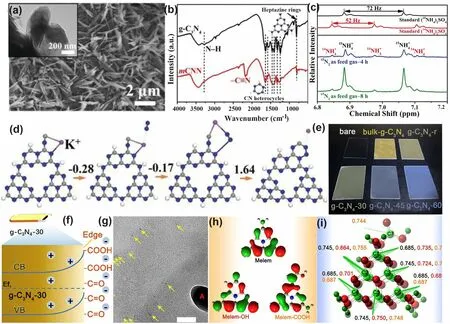
Fig.24 Defect control of cyano groups grafting.a SEM and TEM (inset) images of mCNN; b FT-IR spectra and c 1H NMR spectra of photocatalytic reaction mediums with 15N2 as feed gas for different reaction times and standard (14NH4)2SO4 and (15NH4)2SO4 samples; d K+-assisted –C/N regeneration process.Blue: N, grey: C, white: H, purple: K+ [89].Copyright 2019, Wiley–VCH.e Optical images of bulk g-C3N4, oxidized g-C3N4 samples (g-C3N4-x, x is the oxidized time), and g-C3N4-r (reducing g-C3N4-60 with NH2NH2·H2O); f Schematic diagram of possible depletion layer and the band-bending effects near the edge of g-C3N4-30; g TEM image of g-C3N4-30 after Pt deposition; h HOMO of melem+, melem-OH+, and melem-COOH+; i Mulliken charge distribution of different carbon atoms in melem+ (black), melem-OH+ (red), and melem-COOH+ (orange) [87].Copyright 2019, Elsevier
5.1 Cyano Groups (-C≡N) with Defect States
For example, Zhang and co-workers first proposed a universal alkali hydroxide-assisted preparation of defective g-C3N4with abundant -C≡N groups and N vacancies using urea as a precursor [258].With the increasing content of alkali hydroxide, the resulting g-C3N4showed a progressively narrowed bandgap to 2.36 eV with promoted visiblelight absorption to 525 nm.A similar phenomenon was also observed for other g-C3N4precursors such as melamine, thiourea, and urea.The DFT calculations also confirmed the narrowed bandgap induced by -C≡N modification, while the coexistence of -C≡N and N vacancies would further lead to additional defect energy levels.Besides, a high photocarrier separation efficiency was also achieved by the -C≡Nmodified g-C3N4, rending it with enhanced photocatalytic HER rate up to 6.9 mmol g-1h-1.
Subsequently, the -C≡N grafted g-C3N4nanoribbon (mCNN) was developed via the annealing of dicyandiamide and KOH followed by an ultrasonication treatment for photocatalytic NRR (Fig.24a) [89].The -C≡N bonding inmCNN was confirmed by the typical absorption peak around 2150 cm-1according to the FT-IR spectra (Fig.24b) and XANES spectra of N K-edge with the broad peak at around 406.3 eV, assigning to the electron transition from N 1sto C–N σ*orbital.Compared to pristine g-C3N4,mCNN showed an extended solar light absorption with apparent shoulder peaks at around 450 nm as evidenced by UV–vis DRS.Moreover, the absorption tail ofmCNN was ascribed to the -C≡N induced subgap states, which extended its solar light absorption range to 700 nm, implying the significantly boosted solar utilization.As a result, themCNN exhibited a promoted NH3yield than pure g-C3N4under both N2and Ar atmosphere.A deeper mechanism investigation of the NRR pathway was further explored using an isotope labeling method identified with the1H NMR technique and theoretical calculations.As depicted in Fig.24c, both14NH4+and15NH4+were detected during themCNN-based NRR activity, reflecting the14N atoms in g-C3N4were involved in the redox reaction.It’s worth mentioning that, with the increasing reaction time, the ratio of the collective integral area of15NH4+after 4 and 8 h was 1.65, indicating the continuous generation of15NH4+originating from outer15N2feeding gas.However, for14NH4+, the ratio reduced to 1.07, suggesting the replaced N frommCNN was exhausted after a certain time.Considering the active sites of N defects in other systems [113], the authors proposed a possible NRR reaction mechanism that the -C≡N groups that acted as the part of C2N4rings to gradually evaluated into NH3with continued regeneration from the outer15N source.Further DFT calculations on NH3generation ofmCNN confirmed the doped K+was also critical for the stabilization of unsaturated C atoms to form C2N4rings and the fixation of N2(Fig.24d).
5.2 O-containing Groups with Optimized Electron Flow
The O-containing functional groups, such as the -COOH, -C = O, and -OH, have also endowed g-C3N4with enhanced solar utilization [259–261].Wang and colleagues prepared the edge functional g-C3N4with -COOH and -C = O groups via an acid oxidization method which was similar to Hummers’ method for graphene exfoliation [87, 262].With an increasing oxidization etching time, the color of g-C3N4turned from yellow to blue, which indicated more O-bearing group species and enlarged bandgaps (Fig.24e) [87].Namely, a short time within 30 min rendered g-C3N4with -COOH and -C = O groups, which were beneficial to build an optimized electron flow of which a thicker charge depletion layer and band bending were presented when compared to the pristine g-C3N4and reduced g-C3N4-30 sample (Fig.24f, g).The HOMO diagrams of melem, melem-OH, and melem-COOH imply that the electrons were prone to accumulate in the O atoms with less electron density probability around the neighboring C atoms (Fig.24h).Consistently, the amount of positive charge on the edge of C atoms from O-containing groups-modified g-C3N4was much higher than those away from the edge or those from pristine g-C3N4, further demonstrating the electrons tend to aggregate on the edge of g-C3N4-30 nanosheet (Fig.24i).The authors believed this could accelerate the charge separation and narrow the bandgap, facilitating the generation of H2O2for bacteria removal.In contrast, a long oxidization time of 60 min would induce the -OH groups, which was suspected of hindering the in-planar charge transfer and lowering the photocatalytic activity.However, the -OH has been demonstrated to be in good favor of other solar applications according to a previous report [263], which might suggest the complex situation of -OH-modified g-C3N4due to the different synthetic methods or different functional groups combination.
5.3 Aromatic Ring Groups with Enhanced Redox Driving Force

Fig.25 Defect control of aromatic ring groups grafting.a FT-IR of CN and CTHP30; b solid-state 13C MAS NMR spectra, c UV–vis DRS with Tauc plots (inset), d electronic band structure of the pristine CN, CHP, THP, and CTHP30 [264].Copyright 2020, Wiley–VCH.e Schematic diagram of the possible photocatalytic reaction mechanism on quinoline ring and naphthalene ring-modified g-C3N4 under visible-light irradiation photocatalytic HER rates [265].Copyright 2023, Royal Society of Chemistry
Other aromatic rings, taking triazole groups for instance, have also been investigated according to Wang and coworkers’ work [264].Annealing the freeze-drying mixture of urea and 3-amino-1,2,4-triazole (3-AT), authors successfully obtained the porous cyanamide-triazole-heptazine polymer (CTHPx, x is the mass ratio of urea to 3-AT) with both triazole groups and -C≡N groups.Controlled samples were pristine g-C3N4(CN), triazole groups-modified CN (THP), and cyanamide groups-modified CN (CHP).The corresponding triazole groups and -C≡N groups of CTHP30were identified by the FT-IR spectrums with the typical peaks at around 3400, 2175, and 739 cm-1assigning to –NH2, -C≡N, and N–N, respectively (Fig.25a).More detailed peak affiliation was analyzed from13C cross-polarization magic angle spinning (CPMAS) solid-state NMR spectra (Fig.25b).According to the UV–vis DRS, we can observe the enhanced solar absorption of these triazole groups- or/and -C≡N-modified g-C3N4, of which THP without cyano groups displayed the best visible-light absorption, followed by CTHP30(Fig.25c).However, the CB position of THP was only slightly above the theoretical H2evolution potential, which suggested an inferior driving force that was not beneficial for HER (Fig.25d).In contrast, the CTHP30exhibited a reasonably narrowed bandgap of 2.65 eV with sufficient driving force for HER reaction, indicating the necessity of multi-functional group modification on g-C3N4.Therefore, it showed the highest photocatalytic HER rate of 12,723 mmol h-1g-1, which was 7.34-fold higher than CN.Similar phenomena were also observed on the quinoline ring and naphthalene ring-modified g-C3N4with a five-fold higher HER rate than unmodified g-C3N4(Fig.25e) [265].
Very recently, it is worth mentioning that, similar to g-C3N4, the covalent organic frameworks (COFs) consisting of organic elements (such as C, N, O, H) via covalentπbonding to form a conjunction structure have boosted extensive research interest in the field of photocatalysis [266].Aminebased COFs, as one of the most important COFs, have suffered from less chemical stability in harsh conditions and insufficientπconjugation system with inherent polarization [267].Fortunately, similar to the boost photocatalytic activity induced by grafted function groups in g-C3N4matrix, the poor photocatalytic performance can also be well improved by the substitution of linkages by introducing the azole linkages [268].Furthermore, the grafted β-ketoenamine to imine moieties in the linkages was also efficient in generating a non-quenched excited state and a more favorable HOMO level, thus leading to an enhanced photocatalytic HER rate [269].It is the most exciting study that Wang’s group has demonstrated that the triazine-containing COFs framework could significantly boost the H2O2photosynthesis rate up to 2111 μM h-1due to the high-speed photocarrier transfer pathway of dual donor–acceptor structure [270], which provided us a new thinking of the combination of g-C3N4with functional COFs linkages groups.
6 Crystallinity with Extended Conjugation System

Fig.26 Defect control of crystallinity improvement (PHI type).Ideal crystalline g-C3N4 in a PHI-type and b PTI-type; DOSs of c PHI-CN and d PTI-CN; e XRD patterns and f photocatalytic HER performance of various g-C3N4 samples with different crystallinity [273].Copyright 2016, American Chemical Society.g HRTEM images of PHI-CN nanosheet and h its corresponding FFT patterns; i crystalline structure; j thickness [83].Copyright 2017, Wiley–VCH
As the concrete manifestation of broader and weakened (002) and (001) planes, the crystallinity of most defectengineered g-C3N4would reduce due to the disrupted periodicity induced by the internal vacancies or the external impurities during the intensive thermal annealing/etching process [104, 117, 271].Accompanying with enhanced surface area, unique nanostructure, and defect formation, their overall solar activity is generally enhanced [171].Being on the opposite side of defect engineering toward g-C3N4, a higher crystallinity normally indicates a more regular atomic arrangement with extended and fully condensed conjugation structure, which stabilizes theπ-electron system for fast charge mobility and improves the solar utilization due to the reduced bandgap and less photocarrier traps induced by defects.Therefore, it would be fancy if paying great attention to see if there is a balance between crystallinity and defect.To this end, this section will begin with the ideal crystalline types of g-C3N4including the poly-heptazine imides (PHI) type and poly-triazine imides (PTI) type, followed by giving typical samples of the combination of defect creation in high crystalline g-C3N4toward enhanced photocatalytic activity [83, 88, 106, 272, 273].The details were extended as follows:
6.1 Poly-heptazine Imides (PHI) with Weakened Interlayered van der Waals Interaction
The PHI-typed g-C3N4(PHI-CN) is the most widely investigated model for the current research study, which is an infinite repeat of the tri-s-triazine unit (Fig.26a).While PTItyped g-C3N4(PTI-CN) is composed of triazine unit connected with N atoms in the bridging site (Fig.26b).Generally speaking, PHI-CN is normally synthesized via a simple ion-thermal strategy using bulk g-C3N4and MCl (M = Li, Na, K) as precursors [83].Compared to PTI-typed g-C3N4(PTI-CN), most PHI-typed g-C3N4(PHI-CN) materials have demonstrated a significant improvement in solar activity.In a pioneered work, Lin and co-workers found that the PHICN had a smaller calculated bandgap than PTI-CN (1.17 vs.3.23 eV), indicating its superior visible-light response ability due to the extended conjugatedπsystem (Fig.26c–d) [273].Additionally, the intercalated Li+and Cl-in PTI-CN have little influence on narrowing the band compositions.Regarding XRD patterns, the PHI-CN assigned to g-CN-1 exhibited much higher crystallinity with sharper (002) and (001) peaks moving in the opposite direction when compared to its bulk counterparts (bulk g-CN, mpg-CN, and g-CN-2, Fig.26e).This was supposed to be the enhanced polymerization degree with fewer hydrogen bonds and strong chemical interaction between interlayers.While the PTI-CN assigned to PTI/Li+Cl-showed a distinct XRD pattern, which was also consistent with the previous report [81, 88, 274–276].Due to the structure and crystallinity difference, g-CN-1 displayed the highest photocatalytic HER rate of 770 μmol h-1, far more exceeding PTI/Li+Cl-, which indicated the advantage of PHI units over PTI motifs (Fig.26f).A subsequent study was performed to unravel the relationship between HER performance and PHI nanostructure, which employed the ultra-thin PHI-CN nanosheets as the target material [83].Its few-layered merit was reflected by the obvious (002) lattice fringes according to the HRTEM image and a layer thickness at around 0.98 nm (Fig.26g–j).Compared to the bulk PHI-CN, PHI-CN nanosheets exhibited a 2.5-time higher HER rate under visible-light irradiation, further confirming that nanostructure engineering is also important for solar applications.
Similar research has also been carried out by employing the ion-thermal strategy to prepare the high crystalline g-C3N4[277].As shown in Fig.27a, various precursors including cyanamide, dicyandiamide, melamine, and thiourea were annealed in the presence of eutectic NaCl/KCl salts, and the corresponding g-C3N4samples were donated as CND, CNC, CNM, and CNT, respectively.According to the HRTEM, one can see the obvious lattice stripes assigned to the (202) crystal plane of CNT, indicating its good crystallinity (Fig.27b).Besides, compared to the bulk g-C3N4, their XRD patterns revealed a new peak at around 8.0°, ascribing to a large interplanar packing distance of 1.104 nm due to the molten salt condition during the pyrolysis [278].Interestingly, the (002) peaks were witnessed with a positive shift, which evidenced the reduced interlayered distance.This was due to the induced high crystallinity that substantially suppressed the edge amino groups, weakening the interlayered van der Waals force and lowering the defect density, and thus the photocarrier separation efficiency would be boosted.This was in good accordance with the reduced EPR signal intensity of CND, CNC, CNM, and CNT atg= 0.002, attributing to dangling bonds or nitrogen defects (Fig.27c).After the analysis of Mott–Schottky plots and DRS results, the authors gave their detailed electronic band structures as displayed in Fig.27d.In contrast to the low crystalline bulk g-C3N4, all these crystalline g-C3N4exhibited a narrowed bandgap by 0.22–0.51 eV, indicating an enhanced visible-light responsive ability.Accordingly, CNT had the most negative CB value of - 1.43 V, which was a sign for the best candidate for the generation of ·O2-radical.However, its driving force for H2O2generation was the lowest as its most lifted VBM at around 0.82 V.Fortunately, CNT displayed the maximum H2O2production of 2.48 mmol g-1h-1with an apparent quantum efficiency of 22% (λ= 400 nm) among all samples, further suggesting the superior role of high crystallinity over the corresponding oxidization ability.
Different from the ion-thermal-assisted polymerization of PHT-CN, Chubenko et al.adopted a chemical vapor deposition (CVD) method to prepare the crystallized g-C3N4thin film up to 1.2 μm on the glass and silicon substrate [279].As shown in Fig.27e, the CVD furnace was divided into the former lower-temperature zone below 350 °C and later high-temperature zone in the range of 500–650 °C.The later zone was heated to the target temperature with the substrates inside followed by the continued heating of a lowtemperature zone containing melamine powder to provide the precursor atmosphere at 350 °C in dry Ar gas.Moreover, the crystallinity of g-C3N4reached the highest level when the target heating temperature was 600 °C even using the tiny amount sample on the glass substrate as reflected by the strong (002) diffraction peak and obvious (004) peak signal as shown in Fig.27f.Exhibiting the best crystallinity, g-C3N4synthesized under 600 °C also owns a moderate bandgap of 2.87 eV, which was 0.16 eV smaller than that obtained under 550 °C, still showing potential for future photocatalytic activity.
It is worth mentioning that the crystalline g-C3N4obtained via the ion thermal reaction using the metal salts as the solvent cannot avoid all surface defects, particularly the insertion of K atoms accompanying edge cyano groups.Although some research works aimed to achieve the antidefect engineering goal, this is almost not possible to realize this.In most cases, they were enhanced crystalline g-C3N4with tiny defects.Therefore, it provided us with an opportunity to optimize the photocatalytic activity of g-C3N4by balancing the crystallinity and defect types.The following section focuses on the combination of crystallinity and defects toward enhanced photocatalytic activity.
In continuing work, a strategy combining high crystallinity and N defects was proposed to boost the photocatalytic HER of PHI-CN [106].Herein, the resulting defective sample (D-CCN) with cyano group and the unpolymerized amino group was obtained after annealing the mixture of high crystalline g-C3N4(CCN) and NaBH4in N2(Fig.28a).Although the impurities have been imported into the D-CCN, they still preserved high crystallinity as evidenced by the clear lattice fringes of 1.09 nm assigned to thedspacing of in-plane (001) layers (Fig.28b).Intriguingly, the high crystallinity of D-CCN could reduce the unblocked channels across the 2D conjugatedπin-planes, which enabled smooth in-plane charge transport and easier excitation dissociation.While the decreased (002) spacing further shortened the lateral distance, it enhanced the charge transfer along the vertical direction.Additionally, the defects induced by the mid gaps could extend the visible-light absorption to 610 nm and promote the charge separation efficiency (Fig.28c).The authors claimed that the defect-modified D-CCN might be composed of two parts: (1) main framework with PHI-CN exhibiting an intrinsic bandgap of 2.63 eV and (2) partial matrix of functional groups grafted defective g-C3N4with a midgap of 1.87 eV.Due to the energy difference between these gaps, the electron flow could be expected as shown in Fig.28d, giving rise to the significantly promoted photocarrier separation efficiency.Therefore, we observed a substantial improvement of photocatalytic HER performance for D-CCN to 64 μmol h-1, which was 8 and 40 times higher than those of CCN and bulk g-C3N4with low crystallinity, respectively.

Fig.28 Defect control of crystallinity improvement (PHI type) with functional groups.a Illustration of fabrication strategy combining crystallinity and defect control; b HRTEM image of D-CCN; c DRS spectra of CCN and D-CCN, bandgap of D-CCN determined by Tauc plots (inset); d schematic illustration of the speculative band structure in D-CCN [106].Copyright 2019, Wiley–VCH
Liang et al.contributed outstanding research on combining the crystalline g-C3N4with W-doping in the cell void sites of PHI units via a solvothermal reaction employing the crystalline g-C3N4(CCN) and W(CO)6as starting materials [280].As we know, bulk g-C3N4(BCN) obtained from the traditional pyrolysis method suffered from an overloading of edge amino groups, which dramatically destroyed its crystallinity, worsening the photocarrier transfer pathway along the in-plane direction.In contrast, CCN was observed with fewer surface defects such as K atoms in the void and few cyano groups on the edge, which was demonstrated to narrow its bandgap and boost the photocarrier transfer process.According to the SEM and HRTEM mapping result, the W elements were distributed evenly without obvious nanoclusters aggregations.Along with the large W atomic radius, the W-doping position should be similar sites with that of K atoms in the void position.It was clear that the crystallinity of W-doped crystalline g-C3N4(CCN-W) did not change with a d-spacing of 0.98 nm assigned to the interlayered (100) planes, which was similar to that of CCN.Interestingly, an obvious XRD diffraction peak at around 8° was witnessed for both CCN and W-CCN, which were supposed to be the (100) planes due to the K-doping (Fig.29a).Also, there was a slight (002) peak shifting from 28.2° to 27.9° of CCN over CCN-W, suggesting a larger interlayered distance of CCN-W owing to the bigger W atomic radius.Remarkably, the EPR signal of CCN-W was weaker and stronger than that of BCN and CCN, respectively.This indicated that W6+-doping was beneficial to balance the N vacancy concentration and crystallinity.When it comes to the photocatalytic CRR activity, the dominant species were CO2-, HCO3-, and m-CO32-before irradiation for CCN-W as reflected by the in situ DRIFTS spectra (Fig.29b).The in situ FT-IR spectroscopy measurement recorded within 2 h of photocatalytic CRR activity further revealed the formation of bicarbonate b-HCO32-, HCOO-and –OCH3groups with their peaks located at 1100/1200/1420, 1370/1514/1578, and 1450 cm-1, respectively.As a result, CCN-W delivered the highest CO, CH4, and C2H4yields of 5.75, 4.45, and 1.17 μmol g-1h-1, respectively (Fig.29c).Notably, the introduction of active W-N6sites into CCN-W not only enhanced and activated the adsorption capacity for CO2and CO with a moderate affinity ability but also enriched photoelectrons, which was critically beneficial for the high collision possibility and low CRR barrier, thus leading to the main production of hydrocarbons with a high selectivity of 83%.In contrast, CCN displayed a lower CRR yield in comparison with the W-doped sample with a high selectivity of CO, further demonstrating the superior role of W-N6in boosting the photocatalytic activity of crystalline g-C3N4(Fig.29d).Thus, these pieces of work provide us with a new pathway balancing both crystallinity and defect creation, which is of vital importance for the future design of g-C3N4-based photocatalysts for solar applications.
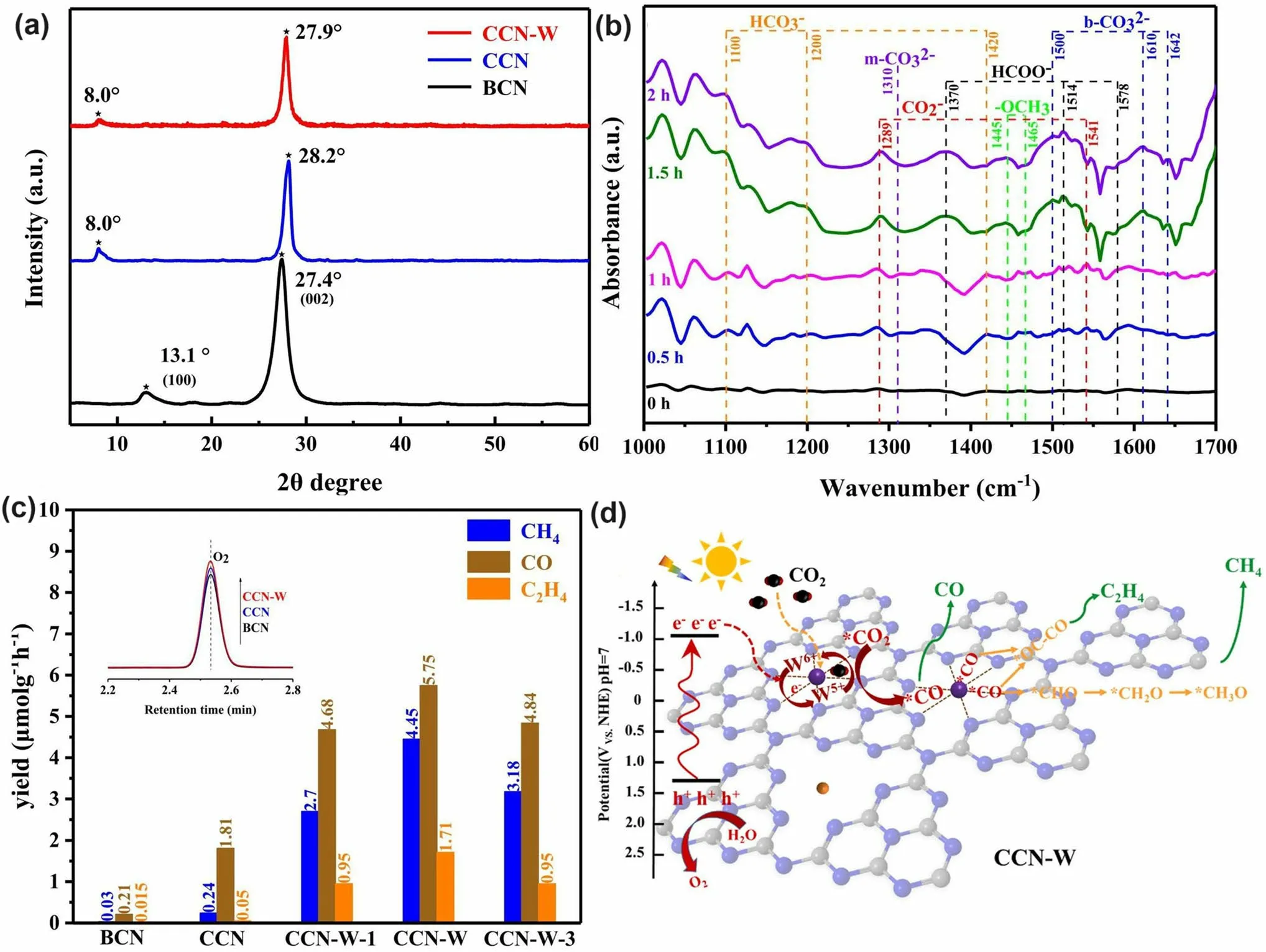
Fig.29 Defect control of crystallinity improvement (PHI type) with metallic doping.a XRD patterns of BCN, CCN, and CCN-W; b In situ DRIFTS of photocatalytic CRR intermediates in the absence (0–1 h) and presence (1–2 h) of LED illumination for CCN-W; c photocatalytic CRR products of various g-C3N4 samples under full spectral irradiation; d diagrammatic of multifunctional role of W-N6 active centers for photocatalytic CRR over CCN-W [280].Copyright 2022, Elsevier
6.2 Poly-triazine Imides (PTI) with Active {1010} Facets
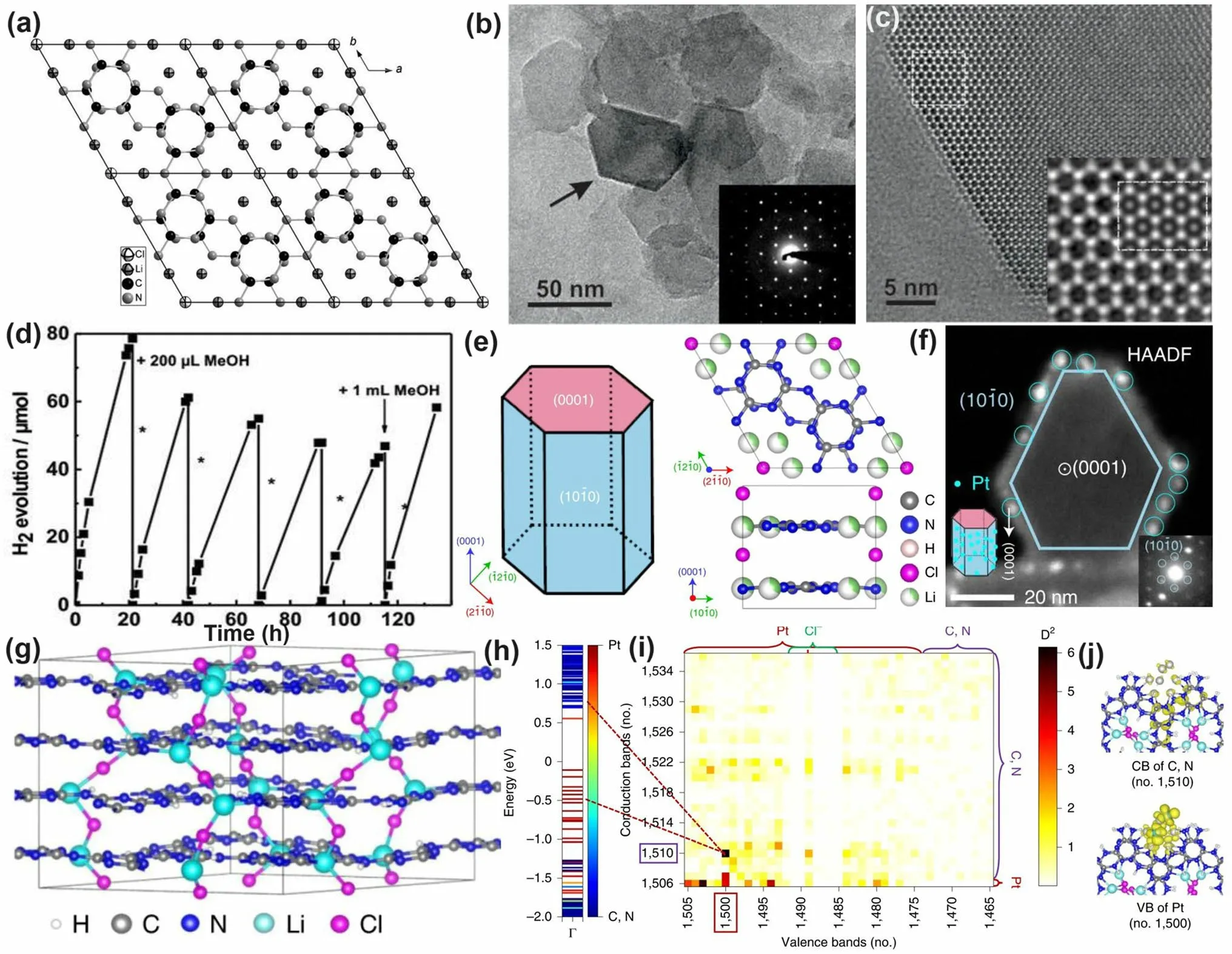
Fig.30 Defect control of crystallinity improvement (PTI type).a Parallel projection of PTI/Li+Cl- structure [272]; Copyright 2011, Wiley–VCH.b TEM image of exfoliated ultrathin PTI/Li+Cl- nanosheets; c HRTEM image of PTI/Li+Cl- nanosheets viewed along (001) direction; d photocatalytic HER activity of PTI/Li+Cl- [81]; Copyright 2014, American Chemical Society.e Left, schematic illustration of the PTI/Li+Cl- single crystal with the basal plane terminated by two {0001} planes and the side faces terminated by six equivalent {1010} facets.Right, the crystal structure of PTI/Li+Cl-; f HAADF-STEM image of a PTI crystal aligned close to the {0001} direction with Pt nanoparticles on prismatic {1010} facets; g computational PTI/Li+Cl- model; h projected band structure (Г-point, spin up) of Pt8@PTI/Li+Cl-; i transition dipole moments between VBs and CBs and j charge density of Pt8@PTI/Li+Cl- with band numbers of 1,510 (CB of C, N) and 1,500 (VB of Pt) [276].Copyright 2020, Springer Nature
Lotsch’s group first proposed the ion thermal method to obtain PTI-based carbon nitrides, which employed the eutectic mixture of LiCl and KCl as solvent [272].The synthetic route concluded two steps: (1) Pre-heating of dicyandiamide and molten salt under an inert Ar atmosphere at 400–500 °C; (2) Annealing above mixture under vacuum for a long time up to 48 h, the brownish PTI/Li+Cl-was obtained.Based on the XRD, TEM, and solid-state NMR spectroscopy analysis, PTI/Li+Cl-was found to exhibit a high crystallinity with ABA stacking and separated by the weak van der Waals forces.Additionally, the Li+and Cl-were located in the channels along theZ-axis direction (Fig.30a).Further investigation on the exfoliated ultra-thin PTI nanosheets showed that the hexagonal shape and triazine unit were kept as reflected by the HRTEM images in Fig.30b–c [81].Interestingly, the resultant PTI nanosheets enabled the H2evolution under solar irradiation (Fig.30d).However, its cycling HER activity in the TEOA solution suffered from severe performance decay, indicating instability in the basic environment.While changing to a methanol additive, the HER performance showed reasonable stability within 130 h, suggesting the promising potential in photocatalysis.
Further identification of the reactive planes of PTI/Li+Cl-during the photocatalytic HER has been performed by fabricating PTI/Li+Cl-single crystals via a modified above-mentioned synthetic method with a higher rating rate of 6 °C min-1and a shorter post-annealing time of 12 h [276].The corresponding PTI/Li+Cl-sample showed a regular hexagonal structure with prismatic {1010}planes and basal {0001} planes as shown in Fig.30e.According to previous reports, the poor solar water splitting performance of PTI-based g-C3N4can be enhanced by the photo-deposition of suitable co-catalysts such as Pt and Co clusters.The HAADF-STEM image of PTI/Li+Cl-displayed that the Pt clusters were deposited on the prismatic {1010} facets with negligible distribution on {0001} planes (Fig.30f).In addition, contrast samples PTI-xwith different S{1010}/S{0001}ratios were also obtained, where x is the annealing temperature.The higher S{1010}/S{0001}, the better overall water splitting performance is.Correspondingly, PTI-550, with the highest ratio, exhibited the best HER and OER rates of 189 and 91 μmol h-1, respectively.These results demonstrated the prismatic {1010} planes were the active facets, which was also confirmed by the transition dipole moments between the CB and VB using the Pt8cluster absorbed on the {1010} planes of PTI/Li+Cl-as a calculated model (Fig.30g–j).The photogenerated electrons were demonstrated to migrate from the Pt8energy levels (band no.1475–1505) to CB of g-C3N4(above band no.1510) on the active {1010} facets.Besides, recent advancements of crystalline g-C3N4in both PHI and PTI types are summarized in Table 4.

Table 4 Recent advancement of defective g-C3N4 with functional groups and highly crystalline g-C3N4 for different solar applications
6.3 Recent Discussion on Defect Traps with fs-TAS
Based on the above discussion, defect engineering has demonstrated an indispensable contribution to improving the solar utilization of g-C3N4toward various solar applications.With discreet regulations, mono/multiple types of defects (vacancies, dopants, and functional groups) can induce additional impurity energy levels, such as midgap states and subgap states in g-C3N4[102, 110, 171].Most cases emphasize the critical role of these defect states: (1) lowering the photocarrier excitation energy with extended visible-light absorption; (2) acting the temporary electron reservoir to accept the migrated electrons from CB, further inhibiting the photocarrier recombination rate.However, the side effects of these defect states in g-C3N4have drawn less research attention compared to those in other semiconductor-based systems, which mainly include: (1) deeper defect energy levels with insufficient redox driving force to restrain the formation of desired products; (2) trapped energy levels or detrimental surface states acting as photocarrier recombination centers that reduce the amount of thermodynamically satisfied electrons.
Despite few studies on g-C3N4deep into this concern, there are tremendous research experiences in other photocatalyst systems to be referred to [296–307].For example, the dangling bonds aroused during the defect manipulation might cause deteriorated surface states which would further lower the solar activity.The surface states have also been well studied in the application of photoelectrochemical water splitting.For instance, Benjamin and co-workers proposed an electrochemical EIS method to interpret the effect of surface states in the charge transfer process [305].These surface states were optimized with the accumulation of holes at the α-Fe2O3/electrolyte interface, which is beneficial for water oxidation.Furthermore, theoretical calculations verified the N-doping level is vital for the position of defect states in La2Ti2O7[304].Specifically, one N atom replacing the O atoms would lead to deep localized states, which was not good for photocatalytic activity.Two N atoms and one O vacancy induced a continuum energy band just below the CB of La2Ti2O7, which enabled a fast charge transfer rate and enhanced solar utilization.This finding allows us to develop advanced defect control with optimized defect states.Recently, several studies on identify the shallow defect states to explore the deep photocarrier transfer kinetics have emerged using the femtosecond transient absorption spectrometer (fs-TASM) [112, 144, 308]Typically, the time and space-resolved fs-TASM is composed of a femtosecond Ti/Sapphire regenerative amplifier laser system to generate a pulse of tens femtosecond and a data acquisition transient absorption spectrometer.After the amplifier and BBO crystals, the laser with a certain wavelength can be obtained as the pump, and the probe pulse can also generate a whitelight continuum spectrum.The pump and probe beams are focused onto the sample to get the temporal and spatial overlap (Fig.31a) [309].Very recently, Gao et al.fabricated the B and P co-doped g-C3N4(BPCN) and found the electrons can be transferred along the pathway of P → N → C → B according to the DFT calculations (Fig.31b) [112].Moreover, the smallest ΔGchange of 0.16 eV further indicated BPCN was more favorable in the absorption and desorption processes of active H*, and thus HER performance (Fig.31c).The femtosecond transient absorption spectra (fs-TAS) were employed to reveal the deep charge transfer dynamics.Specifically, for pristine BCN, only a negative signal was observed in the range of 420–800 nm, mainly due to the stimulated emission (SE, Fig.31d–e).However, for BPCN, both negative signal and positive signals were seen in the range of 420–640 and 640–800 nm, respectively (Fig.31g–h).The strongest positive absorption band of BPCN was ascribed to the excited states absorption (ESA) induced by the photogenerated electrons, indicating the fast charge excitation and separation processes.The authors continue to perform the kinetic decay curves to identify the lifetime species after excitation.According to the decay curves at 540 nm in Fig.31f, the corresponding shortest fast-trapping componentτ1of 2.16 ps for BPCN indicated the shallowest defect states compared to those of CN, B-doped g-C3N4(BCN) and P-doped g-C3N4(PCN).As for the 750 nm decay curves, CN didn’t exist due to its ignorable positive signal.Interestingly, the authors observed a shortestτ1of 0.3 ps and longestτ2of 31 ps for BPCN (Fig.31i), indicating its shallowest defect traps and longest charge separation lifetime due to the synergistic effect of the electron-rich P and electron-deficient B.Along with the optimized electronic band structure with smallest bandgap of 2.46 eV and electron transfer pathway, BPCN exhibited a superior photocatalytic HER rate of 4579 μmol h-1g-1.

Fig.31 Defect control of defect states.a Schematic representation of a fs-TASM setup [309].Copyright 2012, Elsevier.b Charge density difference maps of the as-obtained sample (yellow and cyan regions represented the electron accumulation and depletion; values at the atoms were the Bader charge); c free energy profiles of hydrogen absorption over the CN, PCN, BCN and BPCN for HER; time-dependent contour plots of fs-TAS (pump laser: 400 nm): d CN, g BPCN; TAS of e CN, h BPCN; Kinetics decay profiles of f CN and BPCN at 540 nm and i BCN and BPCN at 750 nm [112].Copyright 2024, Elsevier
Zhu’s group proposed more direct evidence probing the charge dynamics in the trap states for N vacant g-C3N4via the midinfrared femtosecond transient absorption spectroscopy (MIR fs-TAS) [144].Two control samples, bulk CN-550 obtained at 550 °C with deep trap states and mesoporous N vacant g-C3N4obtained at 630 °C with shallow trap states, were employed in this case (Fig.32a).According to MIR fs-TAS, both samples showed the presence of trapped electrons as their relatively strong absorption bands from 4500 to 5100 nm (Fig.32b-c).Their MIR decay kinetics curves reflected that CN-M-630 had much shorter lifetimes ofτ1andτ2than CN-550, suggesting the relaxation of electrons from CB to more shallow trap states (Fig.32d).A similar phenomenon was observed under visible light fs TA decay curves.In addition, the CN-M-630 displayed a prolonged longer lifetimeτ3, indicating a slow decay of the recombination process in the presence of hole-trapping solvent (methanol, Me-OH).To further reveal the charge transfer dynamics, the time-resolved PL spectrum was performed, whereτ1(short lifetime) andτ2(long lifetime) are assigned to the radiative and non-radiative decay of photocarriers from CB/defect states to VB, respectively.The CN-M-630 showed both shorterτ1(1.33 vs.2.59 ns) andτ2(8.70 vs.14.17 ns) with a decreased contribution ofτ1(51.0–49.4%), which demonstrated its lower quantity of quick recombined photocarrier and enhanced charge separation and transfer.Based on the above fs-TAS analysis, one can infer the shallow trap states in CN-M-630 enabled not only a fast charge separation and transfer process but also a suppressed photocarrier recombination, which is good for photocatalytic activity.While the deep trap states in CN-550 revealed a sluggish photocarrier transport and severe charge recombination process (Fig.32e).Inspired by this work, our group realized a precise defect control on g-C3N4with shallow defect states toward enhanced HER performance [91].Specifically, the S dopants and N vacancies were simultaneously introduced into hollow g-C3N4prisms via a dualsolvent-assisted synthetic strategy.By adding the ethylene glycol solvent into precursor formation and molten sulfur solvent into the pyrolysis process, the defective g-C3N4exhibited a moderate concentration of N vacancy and a high S-doping level.This has been demonstrated to be effective to induce shallow defect states, which enabled both a promoted solar harvesting ability and a moderate electron-trapping ability to avoid photocarrier recombination (Fig.32f).As a result, the resultant defective g-C3N4displayed a superior HER rate of 4219.9 μmol g-1h-1, which was 29.1-fold higher than unmodified g-C3N4.

Fig.32 Defect control of defect states.a Schematic illustration for the fabrication of CN-550 and CN-M-630; MIR fs-TAS for b CN-550 and c CN-M-630; d MIR fs-TA decay kinetics; e scheme of the photocatalytic mechanism of CN-M-630 (STS: shallow trap states; DTS: deep trap states) [144].Copyright 2022, Elsevier.f Electron-trapping ability of defect states with different positions [91].Copyright 2023, Wiley–VCH

Fig.33 Defect control of defect states.a, b Pseudocolor fs-TAS of 2 wt% Pt-deposited CN and CNKLi in 10 vol % TEOA aqueous solution under the pump excitation of 350 nm; c, d fs-TAS at different delay times of 2 wt% Pt-deposited CN and CNKLi in 10 vol% TEOA aqueous solution; e, f Corresponding kinetic decay and fitting curves at 640 nm of 2 wt% Pt-deposited CN and CNKLi in 10 vol% TEOA aqueous solution; g Schematic illustration of the proposed charge carrier dynamics of CN and CNKLi (Ex stands for excitation; Em stands for emission; DT stands for deep-trapped states, whereas ST is representative of shallowly trapped states); h schematic illustration of ultra-fast charge transfer in crystalline CNKLi than pristine one [308].Copyright 2022, American Chemical Society
It is very exciting to learn that the fs-TAS is a powerful tool to reveal the photocarrier transfer kinetics so that we can glimpse an insight into the real defect behavior.It has been discussed above that the crystalline PHI-CN is a good photocatalyst HER catalyst owing to its weaker interlayered distance and ordered atomic arrangement [106].Recently, excellent work on detecting the electron’s lifetime in defect states has been contributed by Ye et al.by analyzing the fs-TAS spectrums of crystalline g-C3N4obtained in the presence of KCl/LiCl mixture (CNKLi) and pristine g-C3N4(CN) [308].To simulate the real photocatalytic environment, the photocatalyst was deposited with 2 wt% Pt in 10% TEOA solution.As shown in Fig.33a–d, CNKLi exhibited a negligible simulated emission, indicating the fast charge carrier separation process.In addition, the signal at around 640 nm of CNKLi (Fig.33d) was significantly stronger than CN (Fig.33c), further suggesting the efficient absorption of photogenerated charge carriers.The authors continue to perform the kinetic decay and fitting curves at 640 nm to identify the lifetime species after excitation.According to the decay curves in Fig.33e–f, the corresponding short-lived and long-lived spectral component (τ1–τ4) can be obtained (Fig.33g).The sluggish photocarrier transfer and severe recombination progress of CN was evidenced by the high 21.7% ratio of short-livedτ1of 11.8 ps, whereas for CNKLi theτ1value dramatically reduced to 1.6 ps of 59.5%, indicating an ultra-fast charge generation and transfer process due to the high PHI crystallinity.Different from the long-livedτ2of 15.5 ns indicating severe photocarrier recombination of CN due to the deep-localized states, CNKLi exhibited shortτ2andτ3in the ps scale, suggesting the formation of shallow defect states.In this case, the crystalline g-C3N4with shallow defect states delivered the accelerated charge transfer kinetics via the advanced experimental fs-TA characterization, of which the photocatalytic activity would be boosted (Fig.33h).The above works provide us with new insight into the charge transfer dynamics in defective trap states by more convincing data rather than the qualitative results.
7 Conclusions and Outlook
Over the past 10 years, enormous attribution has been devoted to the defect-engineered g-C3N4to boost its solar utilization on light harvesting and charge transfer kinetics by optimizing the electronic structure, electronic conductivity, and electronic polarization.We highlight the regulation strategies of vacancy creation, impurities doping by hetero-atoms and metallic atoms, defect modification of grafted functional groups, and crystallinity control.Despite great advancements being made, there is still space for future breakthroughs in the research direction of defectengineered g-C3N4in the following aspects (Fig.34):
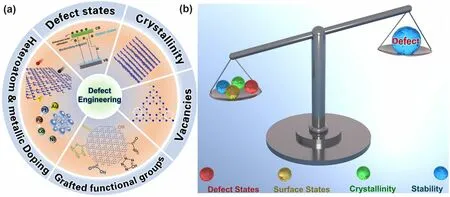
Fig.34 a Review summary; b defect design for future g-C3N4-based photocatalysis
(1) Further regulation on defect-associated energy levels to maximize the solar utilization, referring to shallow defect states and non-deterioration on surface states, preventing the traps from being the recombination centers.More importantly, the photocarrier transfer dynamics in defect states are meaningful to the deep understanding of defect creation, which needed to be characterized by more advanced optical techniques (fs-femtosecond transient absorption spectroscopy) or electrochemical method (EIS analysis).
(2) Discreet manipulation of defect concentration to maintain the crystallinity of g-C3N4at a reasonable level.As is known, there is a trade-off between defect engineering and crystallinity.The high crystallinity of PHI-based g-C3N4enables a better in-plane and interlayer charge transfer due to the reduced hanging bonds induced by the defects.Thus, the balance between defects and crystallinity of g-C3N4should be paid more attention.
(3) Defect stability should be given more emphasis in future g-C3N4-based studies.Due to the long-term solar irradiation, the more inert bulk g-C3N4itself would inevitably suffer from photocatalytic activity degradation.The vacancies, dopants, functional groups, and single/dual atoms might go through a more complex structure change, which needs to be detected via more advanced real-time technologies.
(4) A precise understanding of each defect type in multiple-defect-modified g-C3N4needs to be specified.The coupling of different defect types in g-C3N4is common and the benefits for performance improvement are obvious.However, it is difficult to use the control variables strategy to fabricate the target defective g-C3N4with a single defect or random mixing of defect types, making it hard to figure out which type/types are the most important.Building a precise calculated model as close to its experiment result to simulate the reaction process and catch a glimpse of real defect mechanism is a fantastic but challenging work, which deserves more research attention.
To this end, for a better future defect “customization” on g-C3N4, researchers need to fabricate the target g-C3N4via a precise control on both defect type and concentration in the experiment along with the guidance of theoretical calculations.More importantly, the desired “customization” goal must obey basic principles: (i) tunable electronic band structure with designed CBM and VBM for a required photocatalytic activity, giving sufficient redox driving force; (ii) shallow defect states and optimized surface states to avoid the severe photocarrier recombination in both bulk phase and surface, maximizing the benefits of defects; (iii) optimized crystallinity with appropriate interlayered interaction and good balance with defects to guarantee the fast photocarrier transfer pathway, accelerating the redox kinetics; (iv) robust stability to maintain a high activity producing fuels, removing pollutants, and showing great potential for large-scale use.
In summary, we review the background and research history with significant progress, challenges, and corresponding solutions of defect-engineered g-C3N4toward enhanced solar utilization on various applications.In addition, recent inspiring work on tracing the charge dynamics of trapping states is also emphasized.Furthermore, future design strategies for more effective defective g-C3N4have also been proposed.We believe with synergetic efforts on defect controls and advanced characterization techniques, more breakthroughs in highly efficient g-C3N4-based photocatalysts in various solar applications can be achieved.
AcknowledgementsD.W.Su would like to acknowledge the support of the Australia Research Council (ARC) through the Discovery Project (DP230101040).X.C.Gao would like to thank the Natural Science Foundation of Shandong Province (ZR2022QB139, No.ZR2020KF025) and the Starting Research Fund (Grant No.20210122) from the Ludong University.X.G.Ma would like to thank the Natural Science Foundation of China (12274190) from the Ludong University.We also thank the support of the Shandong Youth Innovation Team Introduction and Education Program and the Special Fund for Taishan Scholars Project (No.tsqn202211186) in Shandong Province.
Declarations
Conflict of interestThe authors declare no interest conflict.They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http:// creat iveco mmons.org/ licen ses/ by/4.0/.
- Nano-Micro Letters的其它文章
- A Review on Engineering Design for Enhancing Interfacial Contact in Solid-State Lithium–Sulfur Batteries
- Hierarchically Structured Nb2O5 Microflowers with Enhanced Capacity and Fast-Charging Capability for Flexible Planar Sodium Ion Micro-Supercapacitors
- Superhydrophobic Surface-Assisted Preparation of Microspheres and Supraparticles and Their Applications
- Proof of Aerobically Autoxidized Self-Charge Concept Based on Single Catechol-Enriched Carbon Cathode Material
- Bioinspired Multifunctional Self-Sensing Actuated Gradient Hydrogel for Soft-Hard Robot Remote Interaction
- Solvation Engineering via Fluorosurfactant Additive Toward Boosted Lithium-Ion Thermoelectrochemical Cells

