The evolution of cancer genomic medicine in Japan and the role of the National Cancer Center Japan
Teruhiko Yoshida, Yasushi Yatabe, Ken Kato, Genichiro Ishii, Akinobu Hamada, Hiroyuki Mano,Kuniko Sunami, Noboru Yamamoto, Takashi Kohno
1Department of Genetic Medicine and Services, National Cancer Center Hospital, Tokyo 104-0045, Japan; 2Department of Diagnostic Pathology, National Cancer Center Hospital, Tokyo 104-0045, Japan; 3Clinical Research Support Office, Clinical Research Coordinating Section, Biobank Translational Research Support Section, National Cancer Center Hospital, Tokyo 104-0045, Japan; 4Department of Pathology and Clinical Laboratories, National Cancer Center Hospital East, Chiba 277-8577, Japan;5Division of Molecular Pharmacology, National Cancer Center Research Institute, Tokyo 104-0045, Japan; 6National Cancer Center Research Institute, Tokyo 104-0045, Japan; 7Department of Laboratory Medicine, National Cancer Center Hospital, Tokyo 104-0045, Japan; 8Department of Experimental Therapeutics, National Cancer Center Hospital, Tokyo 104-0045, Japan; 9Division of Genome Biology, National Cancer Center Research Institute, Tokyo 104-0045, Japan
ABSTRACT The journey to implement cancer genomic medicine (CGM) in oncology practice began in the 1980s, which is considered the dawn of genetic and genomic cancer research.At the time, a variety of activating oncogenic alterations and their functional significance were unveiled in cancer cells, which led to the development of molecular targeted therapies in the 2000s and beyond.Although CGM is still a relatively new discipline and it is difficult to predict to what extent CGM will benefit the diverse pool of cancer patients, the National Cancer Center (NCC) of Japan has already contributed considerably to CGM advancement for the conquest of cancer.Looking back at these past achievements of the NCC, we predict that the future of CGM will involve the following:1) A biobank of paired cancerous and non-cancerous tissues and cells from various cancer types and stages will be developed.The quantity and quality of these samples will be compatible with omics analyses.All biobank samples will be linked to longitudinal clinical information.2) New technologies, such as whole-genome sequencing and artificial intelligence, will be introduced and new bioresources for functional and pharmacologic analyses (e.g., a patient-derived xenograft library) will be systematically deployed.3) Fast and bidirectional translational research (bench-to-bedside and bedside-to-bench) performed by basic researchers and clinical investigators, preferably working alongside each other at the same institution, will be implemented; 4) Close collaborations between academia, industry, regulatory bodies, and funding agencies will be established.5) There will be an investment in the other branch of CGM, personalized preventive medicine, based on the individual’s genetic predisposition to cancer.
KEYWORDS Cancer genomic medicine; biobank; patient-derived xenograft; multi-gene panel test; whole genome sequencing
A brief overview of the history of cancer genomic medicine
Several different terms have been used to describe cancer genomic medicine (CGM), including precision, tailored,personalized, and stratified medicine.Recently, “precision medicine” has become the favored term, likely because it was used by U.S.President Obama in the 2015 State of the Union Address1.Because medicine has strived to be individualized and precise since the age of Hippocrates, a more appropriate term would be modern or next-generation personalized medicine, with a special emphasis on the active use of“omics” approaches.In Japan CGM is often incorporated into the names of government committees or public documents(e.g., Genome Medicine Council or Action Plan for Whole Genome Analysis 2022)2; however, it is also recognized that other omics-derived information should be an integral part of CGM3.In this review, we have selected CGM to highlight genomics as the current focus of cancer precision medicine.
CGM was neither “built in a day” nor merely imported to Japan from abroad.Instead, CGM is the product of ~ 40 years of long but exciting international collaboration and competition in cancer research, aiming to achieve “bench-to-bedside” translation of cancer therapies.In the age of data-driven science,CGM has also been fueled by an increased understanding of unmet clinical needs, ideas, and clinicopathologic information.Moreover, the path to CGM has been cleared by the multiple technological and informational developments in genomic analysis.Although CGM is an exciting newcomer to clinical oncology, CGM is neither a perfect nor a final solution in the fight against cancer.Thus, in this review we begin by summarizing the history of CGM, including the preclinical stages,before discussing the next potential breakthroughs needed to address the current problems affecting cancer medicine.
Figure 1 (left) provides a brief and partial history of CGM.In our view the origin of CGM dates back to approximately 1900, when the laws of Mendelian inheritance were independently rediscovered by three scholars.Then, Watson and Crick unveiled the DNA double helix in 1953.Knudson4proposed the two-hit theory in 1971, which served as the first monumental milestone in CGM.The two-hit theory was developed during the detailed clinical observations of patients with hereditary retinoblastoma and proven 15 years later in 1986 when Dryja cloned the first tumor suppressor gene(RB1)5.
In contrast, CGM targeting of somatic (i.e., non-hereditary)genetic changes began with the translation of new knowledge in molecular virology into human molecular oncology.Indeed, successive discoveries in the somatic and functional activation of oncogenes in cancer cells formed the basis of molecular target therapy in the 2000s.The landmark discovery involved imatinib, which was approved by the U.S.Food &Drug Administration (U.S.FDA) in 2001 for the treatment of chronic myeloid leukemia (CML).The availability of imatinib completely transformed the standard of care for CML, which at that time was only curable with bone marrow transplantation6.This discovery arose from a combination of the genomic analyses performed in the 1960s, karyotyping, and the identification of thec-Ablproto-oncogene from the retroviral counterpart in the 1980s.

Figure 1 Brief global chronology of CGM (left) and at the NCC in Japan (right, blue).TCGA, The Cancer Genome Atlas; NCCHE, NCC Hospital East; C-CAT, Center for Cancer Genomics and Advanced Therapeutics; ICGC ARGO, International Cancer Genome Consortium Accelerating Research in Genomic Oncology.
Moreover, it soon became clear that the activation of oncogenes and inactivation of anti-oncogenes or tumor suppressor genes were only one part of the entire landscape of cancer development and progression, and the “Hallmarks of Cancer”have increased from 6 in 20007to 8 in 20118and then 14 in 20229.
The Human Genome Project was launched in 1990 with a view of offering a comprehensive and systematic understanding of human genes.The Human Genome Project generated a plethora of human reference sequences10and ushered in the age of genomic biology and medicine.Following the introduction of the first commercial microarray technology in 199411,deployment of next-generation sequencing (NGS) in 200512,13transformed research and clinical care in oncology by enabling the personalized sequencing of genomes, which is the crux of CGM.
The unmet clinical needs in Japan since the 1980s
An enormous number of researchers in academia and industry,physicians, patients, and their families worldwide have contributed to the history of molecular oncology (summarized in Figure 1).The National Cancer Center (NCC) of Japan has been at the forefront of this international effort since the 1980s, which has been supported by the First Comprehensive 10-year Strategy for Cancer Control (1984–1993) in Japan(Figure 1, right), and superseded by 3 additional 10-year strategies, with the expected completion of the latest strategy in March 202414-16.
One of the major reasons for the continual investment in CGM by the Japanese government is the rapid increase in cancer-related deaths, although the age-adjusted mortality has actually declined, reflecting the rapid aging of the Japanese population.Cancer remains the number one killer in Japan,not only among the elderly, but also among children and adults 40–70 years of age (the group that maintains Japan’s infrastructure).In 2021 cancer was estimated to be responsible for approximately 378,600 deaths, which represents 26.5% of deaths occurring within the total Japanese population of 125.7 million people.The estimated number of cancer cases was approximately 1,009,800, which is equivalent to an incidence of 1 in 2 Japanese individuals developing cancer at some point during their lifetime17.In fiscal year 2019, the medical cost of cancer was 36.5 billion USD, equivalent to ~ 15% of the total national medical cost of 246 billion USD18.
Although the 5-year relative survival rate for all cancers has increased steadily and reached 64.1% for patients diagnosed in Japan from 2009–2011, survival rates and quality-of-life vary widely depending on the organ, type, and stage of cancer17.In the 1980s the Japanese government had the foresight to understand that conducting basic research to elucidate the molecular mechanisms underlying cancer development and progression is essential to truly revolutionizing patient outcomes.As a result, the number of publications reporting important discoveries in cancer research authored by Japanese investigators increased considerably.This in turn contributed to the establishment of the concept of multi-step carcinogenesis19, which laid the ground for CGM developments in the 2010s.
Evolution of the NCC biobank
Creation of the NCC biobank and implementation of broad/biobank consent
One of the successful aspects of the NCC infrastructure that fueled advances in CGM since the early stages was the development of a biobank.To date, the NCC biobank is one of the best in Japan, both in terms of the number and the quality of samples collected from cancer patients.Although the bioresource collection initially relied on individual study protocols,the bioresource collection gradually spread throughout the NCC Hospital, and in 1994 became routine practice in the Pathology Division because pathologists had become aware of the emerging potential of information gathered using genomics and other “omics” approaches in clinical research and pathologic diagnosis.Most cancer tissue samples for pathologic diagnosis are collected and stored as conventional formalin-fixed paraffin-embedded (FFPE) specimens in every pathology laboratory as a part of the medical record.The pathologists of the NCC Hospital, however, made additional efforts to improve the quality of antigen preservation and the extraction of DNA from paraffin-embedded samples20.The pathologists also began storing snap-frozen tissue samples in liquid nitrogen.
A key driver of the NCC biobank evolution was the introduction and revision of ethical research guidelines at the direction of the Japanese government.Early guidelines stated that germline research should be regulated more strictly by germline-specific ethical guidelines (first introduced in 2001)21,22because of the potential impact of heritable information on other family members, the risk of social discrimination, and other consequences of information misuse.Thus,in the early days, standard protocols requested informed consent for a specific research purpose.Moreover, many research ethics committees even requested a defined list of the genes to be analyzed in each protocol.At the time there were several arguments against broad consent; however, versatility is crucial if a biobank is to be useful in unforeseen, future research applications.After a period of negotiation, broad consent was authorized for the NCC biobank in 2002.This form of consent permitted the somatic analysis of left-over clinical specimens(mostly cancer tissues, plasma, and serum samples) via an optout process for each specific research protocol, including those planned and initiated after sample donation.
In 2011, a major revision to broad consent (now termed“biobank consent” in the NCC) was implemented in the NCC biobank.This revision meant that sample donors now needed to provide opt-in informed consent for research involving germline analyses and one-time peripheral blood drawing for purely research purposes in addition to the use of left-over samples23.A new staffing role (Research Concierge)was created to inform patients of the terms of NCC biobank informed consent during the initial visit to the NCC Hospital;however, individuals who had difficulty communicating in Japanese, individuals who were donors for hematopoietic cell transplantation, or individuals who attended the outpatient clinic for hereditary cancer syndromes were exempt from this procedure.
Status of the NCC biobank today
When we decided to switch from opt-out to opt-in informed consent, we did not know how many patients would agree to participate in the biobank project; however, we were astonished that approximately 90% of the patients kindly gave their consent and continue to give their consent to this day.The NCC is affiliated with two hospitals (the NCC Hospital at the Tsukiji campus in Tokyo and the NCC Hospital East at the Kashiwa campus, Chiba prefecture).Since its creation, the NCC biobank has operated as a single integrated project using the same protocol.The total number of patients who have donated their blood and/or tissue samples to the NCC biobank(based on broad or biobank consent) since 2002 is approximately 119,500 at the time to writing; approximately 72,500 patients were collected at the Tsukiji Hospital and approximately 47,000 patients were collected at the Kashiwa Hospital.Most samples were stored as FFPE tissue specimen blocks(4.27 million samples) or left-over plasma or serum samples(620,000 samples).Figure 2 summarizes the current stocks of the other two major sample types stored in the NCC biobank:(1) peripheral blood samples donated for research purposes;and (2) frozen tissue samples stored in liquid nitrogen.
Because the one-time peripheral blood samples are being used for germline analyses, all information relating to these samples is pseudonymized in compliance with the Ethical Guidelines for Human Genome/Gene Analysis Research21,22,which were already in place when the current NCC biobank protocol (based on opt-in biobank consent) was approved in December 2010.The blood samples are processed by the biobank laboratory staff soon after blood was drawn to obtain plasma, DNA, and RNA lysate samples.
The snap-frozen cancer tissue samples, paired with neighboring non-cancerous tissue samples obtained from the same organ, are an invaluable resource for cancer research.The pathologists at the NCC hospitals have made considerable efforts to preserve the quality of these samples.Invaluable information gathered over years of working at the NCC Biobank has been published as The Guidelines on the Handling of Pathological Tissue Samples for Genomic Research by the Japanese Society of Pathology24.An important factor in ensuring high sample quality is the macroscopic decision at the time of sampling by specialized pathologists,who consider two often competing requirements: (1) cancerous, non-cancerous, necrotic, highly degenerated, hemorrhagic tissues should be distinguished accurately to ensure that the samples have high scientific value and adequately address multiple research questions; and (2) biobank sampling should not perturb the pathologic diagnosis by avoiding lesions that are too small or too close to the surgical margins(i.e., in accordance with the principle of non-maleficence in research ethics).As a consequence, 40%–50% of all surgical cases are deemed bio-bankable, which is especially true for patients who receive an early diagnosis or are treated with neoadjuvant chemoradiotherapy.

Figure 2 Current status of the NCC biobank.The inventories of the two major samples collected in the biobank are shown as of 31 October 2022.In addition to the 1) peripheral blood samples drawn for research purposes and 2) frozen cancer tissue samples, other left-over samples,such as FFPE blocks and plasma, are also used based on the biobank consent.
Clinical information
Establishing a link between clinical samples and health information (medical data linkage) is a critical and integral aspect of how biobanks contribute to medical research.The NCC biobank has installed two major paths for accessing high quality clinical and pathologic information by taking advantage of a single institutional biorepository of a leading comprehensive cancer hospital.This biorepository displays the hospital-based cancer registry data in a standard format that can be easily accessed using the biobank catalogue, thus facilitating research planning and design, and provides access to medical records on the basis of biobank consent, which allows use of the pseudonymized samples (i.e., the samples are coded and linkable to the medical records, which include follow-up information obtained after sample collection).
In accordance with the Cancer Control Act of 200725, the Japanese government designated 408 hospitals as hubs (designated cancer care hospitals) for promoting high-quality cancer care in each area of the country.These hospitals provide highly specialized patient care while fulfilling patient informational needs and training health professionals.Maintaining the hospital-based cancer registry, which is described in the Cancer Registration Promotion Act, is one of the requirements for designated cancer care hospitals.The hospital-based cancer registry was the first nationwide uniform registry system to be implemented in Japan for all types of cancer26,27.The items collected have been standardized and comprise 99 items,as follows: (i) patient identification and demographic information (e.g., name, date of birth, and name of the treating hospital); (ii) tumor information, such as the date of diagnosis, primary tumor site, International Classification of Diseases for Oncology (ICD-O-3) morphology/histology codes, UICC clinical and pathologic TNM staging (cTNM, pTNM), extent of disease (clinical/pathologic); (iii) information relating to initial treatment, such as date and modality of treatment, with or without palliative care; (iv) survival information, such as date of the last follow-up, date of death; and (v) administrative information, such as the names of the registrars, attending physicians, as well as referring and treating hospitals.Importantly,the data in the hospital-based cancer registry are curated from the medical records by professional registrars, who have completed the basic training course offered by the NCC.In addition to the rigorous training of tumor registrars, the quality of data within the registry is also ensured by consistency- checking standard software and the extensive support provided by the NCC staff26.Through a collaboration with the NCC section in charge of the cancer registries, the NCC biobank has linked the database catalog with information contained within the hospital-based cancer registry.Moreover, biobank users who need additional clinicopathologic information can collaborate with the NCC physicians to search and analyze medical records according to the approved research protocols.
The National Center Biobank Network (NCBN)
There are six national centers (NCs) in Japan that conduct specialized basic and clinical research on diseases that have a significant health impact on the country.The NCC was established in 1962 as the first of the NCs.The Japanese government subsequently established the National Cerebral and Cardiovascular Center in 1977, the National Center for Neurology and Psychiatry in 1986, the National Center for Global Health and Medicine in 1993, the National Center for Child Health and Development in 2002, and the National Center for Geriatrics and Gerontology in 2004.Because the NCs had each developed their own biobanks, the NCBN was launched in 2011 “to establish a shared biobank and develop a structure to facilitate industry-academia-government cooperation regarding bioresources through broad joint research”28.Thus, the NCBN forms part of a joint initiative to facilitate the one-stop access to the NC bioresources.The NCBN offers an open-access catalogue database, which links the biobanks of the six NCs, including the NCC biobank29.Typically, the NCBN database catalogue includes the following: (1) basic patient information, such as age and gender; (2) medical questionnaire information, such as medical and family histories,a history of allergies, and a history of alcohol consumption and/or cigarette smoking; (3) disease-relevant information for primary diseases and co-morbidities, such as the ICD10 code or the corresponding Medical Information System (MEDIS)number; and (4) biological sample information, such as type of specimen (e.g., plasma/serum, spinal fluid, DNA, or tissue),date of collection, and method of storage.NCBN is also in the process of creating a system that can provide both bioresources and genomic data to its users30.
Use of the NCC biobank
As previously mentioned, the vast collection of bioresources provided by the NCC biobank has enabled basic researchers to elucidate molecular mechanisms and identify therapeutic and diagnostic targets; however, the biobank samples are also being used by hospital staff to answer a variety of clinical questions.Specifically, approximately 30% of the blood samples collected from 107,637 patients since October 2022 have been used in various studies, including studies conducted by physicians(Figure 2).Moreover, both the frozen cancer tissues and the blood samples designated for germline analysis are a critical resource for the next stage of CGM, whole genome sequencing(WGS), as discussed below.
Between April 2010 and October 2022, research using biobank samples has led to the publication of approximately 1020 papers in international journals31.This number corresponds to a cumulative impact factor of 8936.117 and a total of 42,064 citations.Of the papers published, 60% arose as a result of collaborative research with other institutions,16% of which were from the private sector.The NCC biobank has the largest collection of blood and the frozen tissue samples derived from cancer patients in Japan.The above statistics imply that the NCC biobank is routinely accessed by researchers, resulting in a large number of publications,including researchers in collaboration from academia and industry outside the NCC.
Of note, the NCC ensures that all the individual research protocols that were approved by the Research Ethics Committee for the use of the NCC biobank samples and data are listed on the NCC homepage with lay summaries32.As a result, patients can opt-out (withdraw) their previous biobank consent at any point.Therefore, the informed consent process in the NCC biobank is two-tiered: (1) an initial opt-in for current and future research projects; (2) followed by an opportunity to opt-out at any time after reviewing a specific research protocol on the NCC website.
Japan patient-derived xenografts (J-PDX)
The J-PDX library deserves special attention as one of the most recently developed bioresources to be provided by the NCC.PDX has been increasingly considered as a powerful new bioresource for increasing the success rate of cancer drug research and development by offering a more clinically relevant alternative to conventional cancer cell line-based research models; however, a large, pan-cancer PDX repository, including PDX models for rare cancer types, specific to the needs of the Japanese population was not available.
Information regarding the possible use of cancer tissue samples to establish the PDX resource has been added to the latest NCC biobank consent form.The largest PDX library that has been established, which is based in part on the biobank consent, is the J-PDX library.The J-PDX library is Good Laboratory Practice (GLP)-compliant and formed in collaboration with a clinical laboratory company33.As of December 28, 2022, a total of 557 PDXs have been successfully established from tumor tissues donated by 1,791 patients with various cancer types, including rare and pediatric cancers.According to the Patient-derived Cancer Model (PDCM) Finder, 4932 xenograft models have been established to date (data release 3.1| 2022-12-06)34.The size and quality of the J-PDX models are comparable to those of the top PDX libraries in the world, such as the NCI Patient-derived Models Repository(PDMR)35and the Mouse Models of Human Cancer database(MMHCdb) hosted by The Jackson Laboratory (USA)36.
The J-PDX library has a number of valuable applications,such as enabling a pre-clinical study to select target cancer types for a clinical trial (“PDX basket trial;” Figure 3A).In addition, pre- and post-treatment PDX models of a patient registered in a clinical trial can serve as “an avatar in a co-clinical study” to search for predictive biomarkers (Figure 3B)37.Unlike the established clonal cancer cell lines, the preserved heterogeneity of the PDX models may reveal important information, such as determining the mechanisms for overcoming drug resistance38.
Discovery and translation of the RET fusion in lung adenocarcinoma(LADC)
Over the past two decades, the NCC biobank has facilitated the discovery of many driver gene mutations and their functions.Among these, the identification of theRETgene fusion in LADC may be one the most illustrative examples of how the NCC has advanced CGM.Lung cancer is the leading cause of cancer deaths in Japan.LADC is the most common histologic type of lung cancer, affecting approximately 40% of lung cancer patients.A number of studies have analyzed somatic mutations in LADC, and showed that these mutations assume a typical “long tail distribution” (ranging from few common to many infrequent oncogenic drivers), as seen in most, if not all,other cancer types39.EGFR[31% according to the Catalogue Of Somatic Mutations In Cancer (COSMIC) as of January 2023],KRAS(17%), andALKfusion (5%) mutations in patients with LADC have been observed in a mutually- exclusive manner,suggesting the mutations to be critical driver mutations.In fact, tyrosine kinase inhibitors targeting EGFR or ALK proteins have shown to yield highly-effective treatment responses in the percentage of the patients with the EGFR for ALK fusion mutations, respectively40.
To search for new driver mutations, including fusions, RNA was extracted from 319 frozen LADC tissue samples stored in the NCC biobank41.The RNA sequencing of 30 samples revealed a novel chimeric fusion betweenKIF5BandRETin 1 patient.The follow-up RT-PCR analyses identified the same fusion transcript in 5 other patients [6/319 (1.9%)].The fusion was the result of an inversion between the long and short arms of chromosome 10.This finding was validated by fluorescencein situhybridization (FISH) as a split in the signals associated with the probes flanking the translocation sites.Combined with the results of two other studies published back-to-back in the same journal42,43, the prevalence ofRETfusion in LADC,including theKIF5B-RETandCCDC6-RETfusions, was estimated to be 1%–2% in Asian and non-Asian populations.Importantly,RETfusions were found in LADC tissues lackingEGFR,KRAS,HER2, orALKmutations or fusions, suggesting thatRETfusions are a novel driver of LADC.
Mechanistically, the coiled-coil domains of the partner protein, KIF5B, induces dimerization of RET proteins, resulting in constitutive activation of RET kinase.The same mechanism has been observed inALKfusions.The oncogenic activity of theRETfusion and inhibition by the RET kinase inhibitor, vandetanib, have been validated in a number of studies,including those studies involving NIH3T3 cell transformation,IL3-independent growth of Ba/F3 cells, and transgenic mouse models41-44.

Figure 3 (A) PDX-based preclinical drug testing and screening.The figure shows an example of a “PDX basket trial” to prioritize cancer types in the planning of clinical trials.A “PDX umbrella trial” is also possible to screen various drugs for the same type of cancer but with different genomic alterations or biomarkers.IND, investigational new drug.(B) Co-clinical PDX study.Pre-treatment PDX, and paired post-treatment PDX in the case of treatment failure, will serve as versatile “avatars in co-clinical studies” to elucidate mechanisms of drug resistance and development of predictive biomarkers.
Following the February 2012 publication of theKIF5BRETfusion discovery, a landmark nationwide clinical study,LC-SCRUM-Japan [a part of SCRUM-Japan45and expanded internationally as LC-SCRUM-Asia (UMIN000036871)], was launched (in February 2013)46to screen driver mutations,includingRETandROS1fusions andBRAFmutations in lung cancer.Because vandetanib was approved by the U.S.Food and Drug Administration (FDA) for the treatment of thyroid cancer, an investigator-initiated clinical trial, the LURET study, was launched in parallel with LC-SCRUM-Japan.Screening of 1,536 patients identified 19RETfusion cases.For the 17 of 19 eligible patients, the response rate of vandetanib was 53%, with a progression-free survival period of 4–7 months47.One patient with aCCDC6-RETfusion showed a strong initial response to vandetanib but developed resistance at 38 weeks of treatment.Targeted deep sequencing of a biopsy specimen obtained from the vandetanib-resistant tumor revealed the first example of a secondaryRETmutation (S904F), which affected the activation loop of the RET kinase48.Another secondary acquiredRETmutation (V804L), was identified in a non-small cell lung cancer patient with aKIF5B-RETfusion who progressed after exhibiting an initial partial response to vandetanib for 13 months49.The patient then participated in phase I of a phase I/II clinical trial (LIBRETTO) for a RET-specific kinase inhibitor, LOXO-292, which was designed to overcome drug resistance caused by mutation of the RET V804 gatekeeper residue.The patient experienced a rapid clinical and biochemical response, which was indicative of a partial response.Because of the high therapeutic efficacy in the LIBRETTO trial (i.e., an 85% objective response for treatment-naïve cases)50, LOXO-292, also known as selpercatinib, was approved by the FDA in 2020, and subsequently by the Pharmaceuticals and Medical Devices Agency(PMDA) in 2021, which led to selpercatinib reimbursement by the National Health Insurance System (NHIS) in Japan.
Figure 4 provides an overview ofRETfusion research in lung cancer.This example elegantly illustrates the value of NCC biobank samples.Research that started with the genomic/transcriptomic analysis of patient samples stored in the NCC biobank was rapidly and successfully translated into standard patient treatment.Such a success story was only made possible by the close collaboration between the NCC Research Institute, the NCC Hospital East, the NCC Hospital,and the pharmaceutical industry, each of which was independently strong and instrumental in this collaborative process.Moreover, identification of resistance mechanisms and the development of methods to overcome resistance highlights how the iteration of bidirectional translational research(i.e., bench-to-bedside and bedside-to-bench) drives the evolution of CGM for patient benefit.
It was the little robber-maiden, who had got tired of staying at home; she was going first to the north, and if that did not suit her, she meant to try some other part of the world
TheRETfusion has been observed not only in lung cancer,but also in a small subset of other common cancers, such as colorectal, breast, and pancreatic cancers; importantly, these cancers also respond to treatment with RET kinase inhibitors50.Consequently, the FDA approved the use of selpercatinib as a tumor-agnostic treatment forRETfusion-positive solid tumors in 202251.
Development and translation of the NCC Oncopanel
As demonstrated by the success of the LC-SCRUM-JapanRETfusion detection program, screening for oncogenic alterations is a key aspect of CGM.Therefore, the NCC launched a new project to implement an NGS-based multi-gene panel in the Oncology Clinic52, as shown in Figure 5.Part of this project involved the development of the NCC Oncopanel System to examine both the somatic and germline alterations of 114 (now 124) genes.The feasibility and utility of the NCC Oncopanel System were examined in patients with advanced solid tumors undergoing treatment at the NCC Hospital.The Trial of OncoPanel for Gene-profiling to Estimate both Adverse events and Response (TOP-GEAR) during cancer treatment (UMIN000011141) study was initiated in July 2013 and analyzed 131 cases in the first phase, which ended in October 2014.Actionable mutations were detected in 45%of the patients, 11 (8%) of whom were enrolled in phase I clinical trials of drugs targeting specific driver mutations(i.e., matched therapy).The median progression-free survival time was 5.5 months for patients receiving matched therapy and 1.9 months for patients undergoing nonmatched treatment53.

Figure 4 Brief history and key publications of RET fusion from NCC.Discovery and its translation to clinical trials resulting in the standard of care are shown.PMDA, Pharmaceuticals and Medical Devices Agency, Japan.TKIs, Tyrosine Kinase Inhibitors.
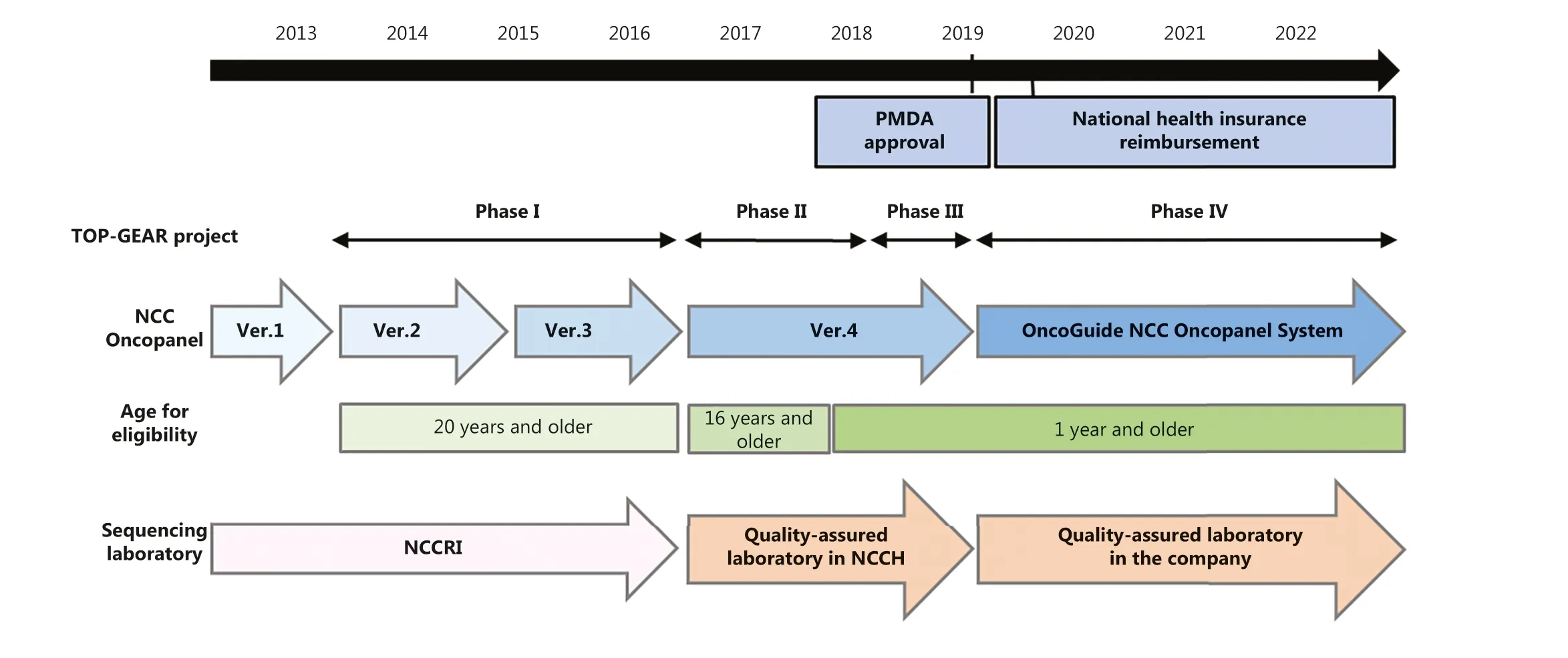
Figure 5 Development, approval, and health insurance coverage of the NCC Oncopanel system.The initial clinical study of the multi-gene panel test was performed for patients with ≥ 20 years of age, but the multi-gene panel test was also approved for pediatric solid tumors.The commercial name of the NCC Oncopanel System is “OncoGuide NCC Oncopanel System”.PMDA, Pharmaceuticals and Medical Devices Agency, Japan; NCCRI, NCC Research Institute; NCCH, NCC Hospital.
In the second phase of TOP-GEAR (initiated in May 2016), the NCC Oncopanel System was used in a qualityassured clinical laboratory in compliance with international quality standards.The laboratory was established and operated in the NCC Hospital in collaboration with a Japanese diagnostics company.By May 2017, > 200 patients had participated.Of the 230 cases, for which FFPE tumor tissues with a tumor cell content ≥ 10% were available,gene profiling data were obtained successfully for 187(81.3%) patients.Actionable mutations were identified in 111 (59.4%) patients, and 25 (13.3%) of the 187 patients received matched therapy54.Based on its success, the NCC Oncopanel System was then incorporated into a new regulatory pathway (SAKIGAKE), which was developed by the Ministry of Health, Labor, and Welfare (MHLW) in Japan to accelerate clinical development.
The third phase of TOP-GEAR involved a multi- institutional study (initiated in April 2018), which aimed to validate the clinical utility of the NCC Oncopanel System within the framework of the Advanced Medical Care B system in Japan.To this end, approximately 350 patients > 16 years of age were enrolled at 50 hospitals across Japan.
The NCC Oncopanel System was approved by the PMDA on December 25 2018, followed by approval of the FoundationOne CDx of Foundation Medicine (USA) the next day.On June 1 2019, reimbursement by the NHIS was implemented for both multi-gene panel tests as Comprehensive Genomic Profiling(CGP).This event marked the point when CGM first became publicly accessible in Japan.
Incidentally, during the feasibility testing of the NCC Oncopanel System in the diagnosis of pediatric cancer, the TOP-GEAR study identified two cases with pediatric lung cancer, which were postulated to have occurred through vaginal transmission of cancer cells from mothers with cervical cancer to their infants55.At this point, it became clear that CGP contributed to CGM not only in terms of improving the diagnosis and treatment of patients, but also in terms of expanding our understanding of cancer etiology in a real-world setting.
National data sharing scheme of CGM in Japan
Several reports from Japan and other countries have suggested that only 10%–20% of patients would be able to access matched therapy (including a clinical trial setting) following CGP54,56,57.This current low level of access to matched therapy has generated considerable debate in the regulatory approval and institution of the coverage provided by NHIS.Reimbursement has been justified by the need to accumulate, share, and repurpose genomic and clinical information for the future innovation of cancer medicine in both the academic and industrial sectors.The MHLW of Japan established the Center for Cancer Genomics and Advanced Therapeutics(C-CAT), as a national datacenter for CGM, in June 2018.Clinical information and genomic data gathered from CGP tests are securely transferred to the C-CAT.These data can now be openly shared with academic institutions and industry for the research and development of drugs and medical devices.Moreover, the real-world data accumulated over the years will be a critical resource to evaluate the contribution of genomic medicine to the patient benefits by various measures.The Japanese CGM scheme, with C-CAT at its core, has been reviewed in this issue and elsewhere3,58,59.
Prospect of WGS in CGM
Opportunities and expectations of WGS
It is now possible to identify cancer-causing mutations in approximately 40%–50% of cancer patients54,56,57.Thus, the major barrier to accessing appropriate treatment following multi-gene testing is not the lack of targets but the lack of drugs for those patients.For the remaining 50%–60% of patients,for whom multi-gene testing reveals no useful information,a leap of innovation may be required to discover alternative effective therapeutic targets.Based on the growing expectation for data-driven science approach these days, upgrading existing molecular analysis techniques to a more comprehensive approach may be required to benefit the majority of cancer patients, although it may not solve all the unanswered questions for the conquest of cancer.Thus, WGS should be used to bring the genomic DNA sequencing data to the theoretical completion for each patient.Such a strategy should also strive towards bringing together data from multi-omics analyses(e.g., RNA sequencing and epigenomics), to enhance the value of WGS.
Figure 6 summarizes the advantages and weakness of target-based sequencing and WGS60-63.One possible scenario in the near future may be the concurrent use of both modalities, probably as a hybrid between multi-gene panel testing and WGS of selected cases, that is, until a technological breakthrough improves the clinical utility of WGS, especially in relation to read depth and length, and the ease of WGS data handling.
Action Plan by the Japanese government
In September 30 2022 the MHLW announced the “Action Plan for Whole Genome Analysis 2022”2, which is an updated version of the plan publicized in December 201964.The target diseases of the Action Plan are cancer and intractable diseases;the latter is defined as “rare diseases in which the pathogenic mechanism is not clear and the treatment methods are not established and which require long-term medical treatment having contracted the disease”65.The project, in which NCC researchers have been playing an important role, started in 2020 with the retrospective analysis of samples from 550 cases with advanced cancer and 3,247 cases with hereditary cancer syndromes (the cancer part of the project).Similarly,the non-cancer part of the project involved the sequencing of samples from approximately 2,500 cases, including monogenic, multi-factorial, or difficult-to-diagnose cases2.
In 2021 the Action Plan was updated to include prospective cases, with the aim of returning the research results to the patient for a potential clinical benefit.Again, the NCC Hospital researchers led some of the key subprojects because the NCC Hospital is a Designated Core Hospital for CGM and receives a large volume of cancer patients.The NCC Hospital Expert Panel examines approximately 30 cases each week and recommends treatment options based on the CGP results.The NCC Hospital has a well-developed and active biobank of cancer tissues (see the NCC biobank sections, above).and has a long history of participating in cancer genome analyses and collaborating with the NCC Research Institute and others, as well as excellent human resources.
The basic scheme of the national WGS project within the 5-year Action Plan is shown in Figure 7.The current version of the Plan introduced the concept of Industry and Academia Forums to emphasize the importance of data sharing between these entities, with the aim of advancing the treatment of cancer and intractable diseases.
Importantly, the Action Plan has proposed that Divisions specialized for Ethical, Legal, and Social Issues (ELSI) and for Patient and Public Involvement (PPI) should be set up within the Project Implementation Organization for WGS.Their activities will be essential to promote the WGS project and ensure that it is fully understood and trusted by society66.Discussion points will include standardization of informed consent forms, access to genetic counseling,information security, privacy protection, no discrimination based on genomic information, education, and how to provide support and information about WGS to the general public.

Figure 6 General characteristics and approximate comparisons of target (multi-gene panel), whole exome, and whole genome sequencing.CoDx, companion diagnostics; R&D, research and development.

Figure 7 Scheme of the Whole Genome Analysis Project in Japan (Plan).The organizational outline is shown in Action Plan for Whole Genome Analysis 20222.R&D, research and development.
Germline predisposition and prevention; the other branch of CGM
The current primary aim of CGM is to optimize and develop treatments based on somatic (cancer-cell-derived) genomic information.In the “Action Plan for Whole Genome Analysis 2022”2, CGM is defined as “the medicine to select the treatment by knowing the characteristics of the cancer of each patient based on the genetic changes through genomic analyses of the cancer (patient)”.The reference to “cancer (patient)”may indicate that CGM should implicate both somatic and germline analyses (e.g., pharmacogenetics and indication for PARP inhibitors).
Another powerful application of CGM is in early diagnosis and prevention based on the stratification of genetic risk of cancer development.At present, a major focus of medical genetics is the relatively rare (considered to affect < 5% of all cancer patients) hereditary cancer syndromes (HCS).The American Society of Clinical Oncology released a statement on the genetic testing for cancer susceptibility in as early as 1996 (Figure 1)67,68.In Japan, however, the true potential of“the other branch of genomic medicine” has been somewhat overlooked, especially in the oncology field.This is exemplified by the fact that the first reimbursements offered by the NHIS were for single gene germline tests for retinoblastoma and medullary thyroid cancer in 2016, followed by multiple endocrine neoplasia type 1 and BRCA1/2 in 2020.The NHIS has also reimbursed patients with positive BRCA1/2 genetic test results for risk-reducing surgery and magnetic resonance imaging (MRI) for the early diagnosis of breast cancer; however, relatives with pathogenic variants but without evidence of cancer are currently not reimbursed for these genetic tests or other HCS preventive measures.In a sense, this situation may appear understandable, considering the limited resources to sustain the umbrella-type health insurance system.It will be increasingly important to assess the long-term impact of optimal investments in preventive medicine on the NHIS and the economy overall69.
Nevertheless, the multi-gene testing performed to screen somatic driver mutations for treatment selection has already identified pathogenic germline variants [or presumed germline pathogenic variants (PGPVs) in the case of tumor-only tests]in many patients who would have never been suspected of HCS based on traditional diagnostic or testing criteria70,71.WGS may further accelerate and expand the “genome-first”trend because paired somatic and germline sequencing is the norm for CGM by WGS, which may unveil a multitude of genetic diseases (not only HCS)72,73.
Moreover, it is likely that rare HCS and “common cancer”are not dichotomous.Instead, they probably appear at either end of a continuous risk spectrum74,75.Along this spectrum,there must exist a “medium-high risk group” for which preventive intervention (ranging from focused surveillance to risk-reducing surgery or chemo/immuno-preventative therapy) may be clinically indicated (Figure 8).
WGS may therefore herald a new era in the other branch of CGM, too, in the realm of preventive medicine.The germline CGM will benefit individuals and families with a clinically significant cancer predisposition by supplying a wealth of information on germline variants (e.g., structural, splicing,and other gene regulatory variants) and polygenic risk data along the continuous spectrum of genotype-phenotype relationships.

Figure 8 Genetic architecture of cancer predisposition: a spectrum rather than a dichotomy.In addition to the classic genetic high-risk groups, moderate-risk groups may benefit from genome-based “precision preventive medicine.” GWAS, genome-wide association studies;WGS, whole genome sequencing.
Conflict of interest statement
AH: received research grants from AstraZeneca, Boehringer Ingelheim, Chugai Pharma, Daiichi-Sankyo, Eisai, Lilly, Konica Minolta, Tosho, Healios, and Chordia Therapeutics.
KS: received honoraria from Sysmex, Chugai Pharma, Astra-Zeneca, Novartis, Eisai, Riken Genesis, Eli Lilly, Pfizer, illumine and a research grant from Sysmex.
TK: received advisory board compensation from Lilly Japan and research grants from Sysmex and Chugai Pharma.
All other authors declare no conflicts of interest.
Author contributions
Conceived and designed the review: Teruhiko Yoshida, Noboru Yamamoto and Takashi Kohno.Wrote the paper, searched the literature and made the illustrations in each part and edited the whole manuscript: Yasushi Yatabe, Ken Kato, Genichiro Ishii, Akinobu Hamada, Hiroyuki Mano and Kuniko Sunami.
 Cancer Biology & Medicine2024年1期
Cancer Biology & Medicine2024年1期
- Cancer Biology & Medicine的其它文章
- Mission of the National Cancer Center Hospital in Japan to promote clinical trials for precision medicine
- Large-scale loss-of-function perturbations reveal a comprehensive epigenetic regulatory network in breast cancer
- Genomic medicine and cancer clinical trial in Thailand
- The role of intestinal flora on tumorigenesis, progression,and the efficacy of PD-1/PD-L1 antibodies in colorectal cancer
- Emerging mechanisms and implications of cGAS-STING signaling in cancer immunotherapy strategies
- Improving the value of molecular testing: current status and opportunities in colorectal cancer precision medicine
