The relationship between DNA fragmentation and the intensity of morphologically abnormal human spermatozoa
Mercedes González-Martínez ,Pascual Sánchez-Martín ,Carmen López-Fernández ,Stephen D.Johnston ,Jaime Gosálvez
1Clínica Ginemed,Unidad de Reproducción,Sevilla,Spain
2Department of Biology,Universidad Autónoma de Madrid,Cantoblanco,Madrid,Spain
3School of Environment,The University of Queensland,Gatton,Australia
4School of Veterinary Science,The University of Queensland,Gatton,Australia
ABSTRACT Objective: To determine the relationship between teratozoospermia and sperm DNA fragmentation (SDF) in the human ejaculate.Methods: This retrospective study included 100 normozoospermic men as a control cohort (abnormal forms >14%),210 patients with a high level of abnormal forms (≤4%) and 65 patients presenting with a moderate level of abnormal forms (>4% to ≤14%) based on the World Health Organization definitions.Sperm morphology was assessed using bright field microscopy.Sperm DNA fragmentation was assessed using the sperm chromatin dispersion assay.Non-parametric analyses were conducted to examine the relationship between abnormal sperm morphology and sperm DNA fragmentation;receiver operating characteristic (ROC)analyses were conducted to assess sensitivity and specificity of this relationship.Results: A correlation analysis revealed that the higher the proportion of abnormal spermatozoa in the ejaculate,the higher the level of SDF (Spearman's Rho=-0.230;P<0.001).Significant differences in the proportion of SDF were found when all cohorts were compared (P<0.001);these significant differences were also retained when the different cohorts were compared pairwise.ROC analysis showed a moderate but significant predictive value for SDF to differentiate patients with different levels of teratozoospemia.Conclusions: Although analysis of a more continuous range of values for teratozoospermia would help further clarify any causal relationship with SDF,there is clearly a synergistic or coincident affiliation between these variables that needs to be acknowledged by the clinician when interpreting the spermiogram.
KEYWORDS: Teratozoospermia;Sperm morphology;Sperm DNA fragmentation;Male factor;Human reproduction;Human fertility
1.Introduction
Teratozoospermia has been defined as the presence of morphologically abnormal spermatozoa in the ejaculate,most likely resulting from defective cellular differentiation during spermiogenesis.The specific origins of such sperm cell abnormalities are typically obscure but have been linked to variables such as environmental contaminants and stressors,patient age and/or genetic driven factors[1-4].A lack of proper sperm chromatin maturation during spermiogenesis,may also have direct consequences on chromatin stability and DNA damage[5].Consequently,as the majority of abnormal sperm morphologies have been associated with spermiogenic dysfunction,it is not surprising that both phenomena can coexist when measured in the ejaculate[6-9].Although the precise relationship (causal or otherwise)between the incidence of sperm DNA fragmentation (SDF) and sperm morphology remains to be elucidated,from a clinical perspective,the presence of either high levels of abnormal sperm morphology and/or high levels of SDF is clearly indicative of male infertility[10,11].
Significance
The direct relationship between teratozoospermia and sperm DNA fragmentation in the human ejaculate is unclear.The results of this study have demonstrated that men with high levels of abnormal sperm morphology in their ejaculate,also appear to have a higher risk of sperm DNA damage.A synergistic affiliation exists between the intensity of teratozoospermia and sperm DNA fragmentation in the human ejaculate.This finding has clinical consequences for the interpretation of the spermiogram,the underlying aetiology of the pathology and diagnosis of patient fertility.
The aim of the present investigation was to investigate whether there was a direct correlation between an increase in the incidence of an abnormal sperm morphology (teratozoospermia) in the ejaculate and SDF.We have assumed,as the most parsimonious hypothesis,that SDF will increase,as the level of abnormal spermatozoa increases in the ejaculate.
2.Subjects and methods
2.1.Study design,patient cohorts and inclusion criteria
This retrospective study was conducted from 2019 to 2022 and consisted of a normozoospermic control cohort and two experimental cohorts,samples of which were obtained from a Spanish Fertility Clinic located in Seville,Spain and analysed at the Department of Biology,Autonomous University of Madrid,Spain.For the purposes of the present study and in accordance with the standards of the World Health Organization (WHO)[12],the experimental control cohort consisted of 100 normozoospermic men with >14% normal morphological sperm in their ejaculate;the individual level of abnormal forms was not scored,but only whether it was >14%.The experimental cohorts consisted of men presenting with either a high level of abnormal sperm morphology (HLab;n=218) characterised by ≤4% of normal spermatozoa in their ejaculate or men presenting with a moderate level of abnormal sperm morphology (MLab;n=65),characterised by a range of normal spermatozoa from >4%to ≤14%.These cohort thresholds were also chosen based on the WHO recommendations,which set a lower threshold level of normal sperm in 1999 [12] at 15%,then subsequently reduced this value to 4% in 2021[13].In both HLab and MLab cohorts,men presenting with levels of motility higher than 42% and defined as the sum of progressive and non-progressive motility,were included in the study,whereas men with severe asthenozoospermia,oligozoospermia and oligo-astheno-teratozoospermia also defined by the WHO criteria were excluded.
2.2.Semen analysis
Semen samples were collected at the clinicviamasturbation into sperm safe plastic containers that were kept at room temperature for 30 min to a maximum of 1 h prior to liquefaction.Ejaculates showing abnormal sperm morphology were assessed under bright field microscopy using a Nikon Eclipse microscope equipped with a high-resolution Nikon 12bits CCD (Nikon DS-Q) and using 40×fluorite objective.After semen smears were aired dried,they were fixed in methanol and stained using Diff-Quik (Medion Grifols Diagnostics,Düdingen,Switzerland).All samples were analysed using 100× immersion oil objectives and 200 spermatozoa were scored.SDF was assessed using the Sperm Chromatin Dispersion test (Halosperm;Halotech DNA,Madrid,Spain) as per the manufacturer’s instruction.Samples were assessed for morphology and SDF by the same embryologist (MG-M).SDF was expressed as percentage of sperm showing a fragmented DNA molecule after the observation of 300 spermatozoa.
2.3.Statistical analysis
Statistical analysis was conducted using SPSS (IBM SPSS Statistics Package v26,NY,USA).Data were tested for normal distribution.To compare the values of SDF,all three cohorts were considered as independent variables,and the Kruskal-Wallis H test was used.To compare the values of SDF associated with two independent cohorts,the W Wilcoxon test was employed.Correlation analysis was performed using Spearman test.The predictive values of SDF to differentiate HLab,MLab and control cohorts were calculated using a receiver operating characteristic (ROC) curve analysis based on information provided by the area under the curve (AUC).Standard error and 95% confidence interval (CI) was used in this study.
2.4.Ethics statement
All data included in this study had patient consent and ethical authorisation of both the clinic from which the samples were collected (Clínica Ginemed,Unidad de Reproducción,Sevilla) and the University of Seville (Permit number-3375125c8b5f1d04c951 1825aef98d309135328c) human ethics committees.All procedures followed were in accordance with the Helsinki Declaration of 1975,as revised in 2000.
3.Results
3.1.The correlation between teratozoospermia and SDF
To establish an overall general perspective with respect to how the presence of abnormal sperm morphology interacted with SDF,a correlation analysis was first performed using data from individuals of all three cohorts;this analysis revealed a significant negative correlation (Spearman's Rho=-0.363;P<0.001).Given the percentage of normal spermatozoa of the control cohort were only scored by the clinic as having levels of normal sperm >14%,rather than their specific individual level,this represented a limitation on our correlation analysis and is therefore likely to be understating the strength of any correlation.Consequently,we performed a second correlation analysis which only included the HLab and MLab cohorts which resulted in a weaker but significant negative correlation (Spearman's Rho=-0.230;P<0.001) (Figure 1).Together these analyses suggest that when abnormal sperm morphology is considered as a continuous series,the lower the value of normal spermatozoa in the ejaculate,the higher the level of SDF.Figure 2 shows the distribution of the SDF values within each cohort (HLab,MLab and control).Significant differences in the percentage of SDF were found when all cohorts were compared;these differences were also maintained when the different cohorts were compared pairwise.

Figure 1.The distribution of sperm DNA fragmentation(SDF) and percentage of normal spermatozoa data observed in individuals with high (HLab: ≤4% normal -black circles)and moderate (MLab: >4% to <14% -red circles) numbers of abnormal spermatozoa.Blue dotted line -Spearman's Rho=-0.230;P<0.001.
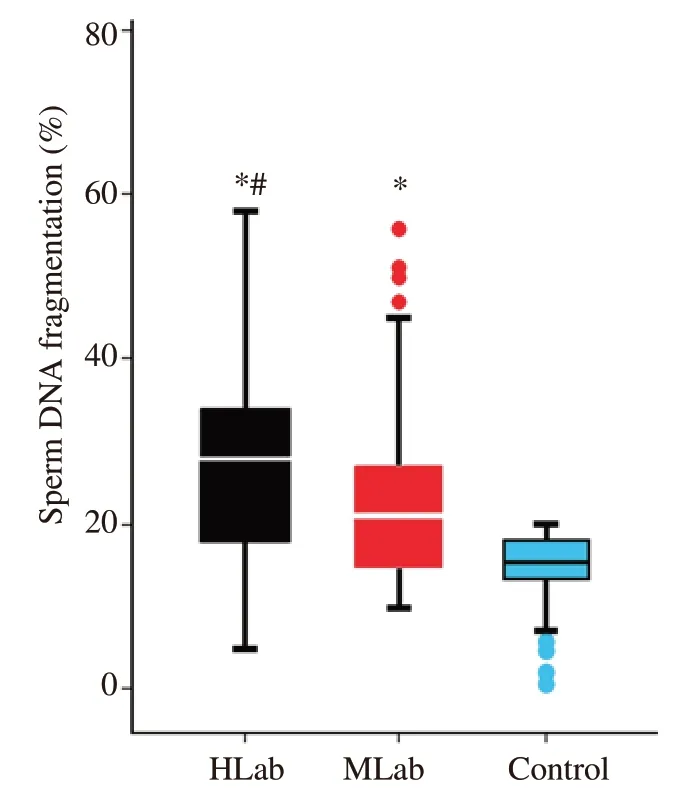
Figure 2.Box and whisker diagram representing the distribution of sperm DNA fragmentation (SDF) observed in males with high (HLab -black) and moderate (MLab -red) levels of abnormal forms and fertile sperm donors(Control -blue).*P<0.05: compared to the control;#P<0.05: compared to MLab.
3.2.Risk prediction with ROC curves
ROC curve analyses were conducted to assess the cut-off values to determine the level of SDF that might be used to differentiate the different levels of teratospermia cohorts (HLab,MLab and control)(Figure 3).Results of the different comparisons are shown in Table 1.Pvalues revealed significant differences between all cohorts except when MLab are compared with the control.The AUC values ranged from 0.58 to 0.70 and were only moderately predictive.In fact,the cut-off values for SDF derived from to each comparison were quite similar,independent of the cohort analysed.Cut-off values and the levels of sensitivity and specificity are showed in Table 1.
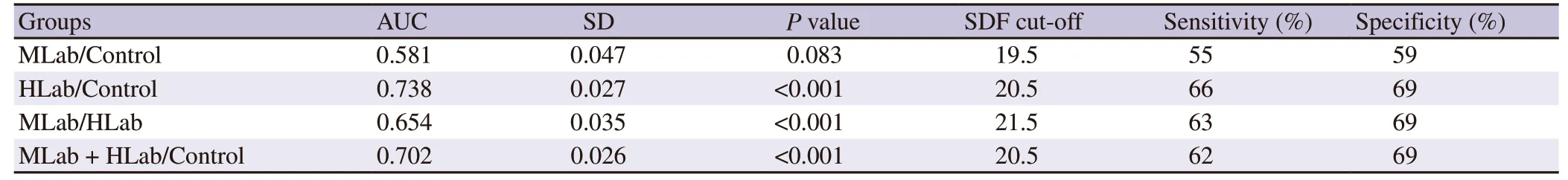
Table 1.Comparative receiving operator characteristics obtained values associated to area under the curve.
4.Discussion
The study has shown that after comparing men with high and moderate levels of morphologically abnormal spermatozoa in their ejaculates,an increasing level of sperm morphological abnormalities was correlated with a corresponding increase in the level of sperm DNA fragmentation,i.e.,men with high levels of abnormal sperm morphology in their ejaculates appear to have a higher risk of sperm DNA damage,andvice-versa.Statistically significant differences were also found when SDF values observed in men categorised as having moderate (MLab) and high (HLab) levels of morphological alterations were compared with the control sample.While these findings are consistent with results reported elsewhere that demonstrate a strong correlation between the presence of abnormal sperm morphology and sperm DNA quality[14-16],our study has further revealed that the level of abnormal spermatozoa and SDF show a significant continuous increase with increasing levels of abnormal sperm forms.Consequently,poor reproductive outcomes in patients with teratozoospemia are not only a consequence of elevated numbers of abnormal spermatozoa but may also be co-dependent on the level of SDF.This finding has clinical consequences for the interpretation of the spermiogram,the underlying aetiology and the subsequent diagnosis of the patient’s fertility.
The cut-off level of SDF,as revealed by the ROC curve analysis to discriminate individuals with a high level of abnormal forms (HLab)compared to that of the fertile men (control),resulted in an AUC of 0.738 with an SDF discrimination value for SDF between both cohorts of 20.5%;these values are very similar to those previously reported[17] (AUC=0.746 and SDF discriminatory value for SDF=18%).While the AUC in both studies might be regarded as moderate,an identical tendency nevertheless exists for an increased level of abnormal forms when individuals are classified as presenting with an altered level of SDF.
While the severity of sperm abnormalities and their relationship with SDF have not been fully investigated in humans,there are some examples in animal models[18].In the case of Holstein bulls,two different forms of sperm abnormalities have been defined and classified as being either major or minor sperm abnormalities[19].Descriptions of the different morphological variations have been summarised by Encisoet al[20] who showed that the major sperm abnormalities were associated with a mean SDF value of(85.0±5.0)%,whereas the minor sperm abnormalities showed a significantly lower mean value of (17.8±5.5)%.These observations suggest the same tendency as described in the current study,where not only the severity of the abnormality,but also the type of morphological variation may influence the SDF.In addition,in the case of Holstein bulls,Saileret al[21] showed that sperm head morphology was not only related to the severity of SDF but also to fertility.Further studies that utilise data that more precisely defines the type of sperm morphology in humans are needed to better evaluate their impact on reproductive outcomes and their relationship with SDF.This will require the andrologist to conduct a more detailed analysis of sperm morphology in each patient.
The close relationship between sperm morphology and chromatin integrity suggests a strong,yet undefined biological relationship between these two parameters.Teratozoospermia is related to the level of aneuploidy and aneuploidy in SDF,which tends to be higher in aneuploid spermatozoa[22].Therefore,it is possible that some of the detected fragmented DNA may be related to the presence of variable levels of sperm aneuploidy in the ejaculate[23-25].Murielet al[22] used three-color fluorescence in situ hybridization experiments that targeted chromosome X,Y and 18 and was performed on slides that were pre-processed using the sperm chromatin dispersion assay to identify spermatozoa with and without fragmented DNA.These authors found that spermatozoa with fragmented DNA present with a 4.4× fold increase in the rate of diploidy and a 5× fold increase in disomy with respect to sperm free of SDF.The overall aneuploidy rate was 4.6× fold higher in spermatozoa with fragmented DNA.Additionally,a higher level of sperm DNA damage was associated with sperm presenting aneuploid sex chromosomes produced during first and second meiotic divisions.In comparison to the findings of Murielet al[22],the observed increase of SDF in morphologically abnormal spermatozoa observed in the current study,may indicate,as Muriel and co-workers state,“that the occurrence of these morpho-anomalies during sperm maturation may lead to sperm DNA fragmentation as part of a genomic screening mechanism developed to genetically inactivate sperm with a defective genomic makeup.”
Interestingly,the susceptibility of morphologically abnormal sperm to DNA damage when teratozoospermic patients are cryopreserved and thawed was found to be significantly higher than that observed in ejaculates that contained morphologically normal spermatozoa[26].This finding suggests that defective protamination and chromatin compaction may produce a low level of DNA protection during physiochemical trauma associated with sperm cell cryopreservation,a phenomenon that is essentially congruent with compromised sperm maturation.Additionally,it is feasible that sperm DNA with deficient protamination is more susceptible to reactive oxygen species damage.In fact,it has been shown that poor quality spermatozoa containing partially decondensed chromatin are more susceptible to DNA damage[27].
Finally,it should be noted that teratozoospemia is frequently present in combination with other sperm alterations,such as those with asthenozoospermia,and oligozoospermia (oligo-asthenoteratozoospermic men),so that full elimination of these factors during sperm evaluation is difficult to control,especially if they are present at sub-detectable levels.While this possible interactive synergy of differing pathologies needs to be acknowledged as a limitation in the interpretation of our findings,results presented in the current study reinforce the idea that complex and possible interactive or synergistic scenarios,in this case teratozoospermia and SDF,can affect male fertility,such that the ultimate influence of a single abnormality on reproductive outcome is difficult to ascertain.
Conflict of interest statement
The authors declare no conflicts of interest.
Funding
This study received no extramural funding.
Authors’ contributions
Mercedes González-Martínez performed sperm DNA fragmentation analysis and data searching.Pascual Sánchez-Martín carried out patient recruitment and study design.Carmen López-Fernández performed sperm DNA fragmentation analysis.Stephen D.Johnston was responsible for data interpretation,draft and final manuscript writing.Jaime Gosálvez performed study design,data analysis and first draft production.
 Asian Pacific Journal of Reproduction2024年1期
Asian Pacific Journal of Reproduction2024年1期
- Asian Pacific Journal of Reproduction的其它文章
- Promotion of sexual and reproductive health in Pakistan-The role of technology and online awareness
- Adding chitosan nanoparticles of green tea extract in diluent and thawing temperatures ameliorate the post-thawed quality of Boer buck semen
- Pro-fertility effect of Ficus carica fruit extract in streptozotocin-induced male rats
- Subsequent pregnancy outcomes and fertility rates in the case series that underwent bilateral hypogastric artery ligation (BHGAL) due to severe postpartum hemorrhage
- Exploring the relationship between ambient sulfur dioxide and semen quality parameters: A systematic review and meta-analysis
- Engineering of ovarian tissue for ovarian dysfunctions: A review
