Blockade of Rho-associated kinase prevents inhibition of axon regeneration of peripheral nerves induced by anti-ganglioside antibodies
Andrés Berardo ,Cristian R.Bacaglio ,Bárbara B.Báez Rubén Sambuelli,Kazim A.Sheikh,Pablo H.H.Lopez
Abstract Anti-ganglioside antibodies are associated with delayed/poor clinical recovery in Guillain-Barrè syndrome,mostly related to halted axon regeneration.Crosslinking of cell surface gangliosides by anti-ganglioside antibodies triggers inhibition of nerve repair in in vitro and in vivo paradigms of axon regeneration.These effects involve the activation of the small GTPase RhoA/ROCK signaling pathways,which negatively modulate growth cone cytoskeleton,similarly to well stablished inhibitors of axon regeneration described so far.The aim of this work was to perform a proof of concept study to demonstrate the effectiveness of Y-27632,a selective pharmacological inhibitor of ROCK,in a mouse model of axon regeneration of peripheral nerves,where the passive immunization with a monoclonal antibody targeting gangliosides GD1a and GT1b was previously reported to exert a potent inhibitory effect on regeneration of both myelinated and unmyelinated fibers.Our results demonstrate a differential sensitivity of myelinated and unmyelinated axons to the pro-regenerative effect of Y-27632.Treatment with a total dosage of 9 mg/kg of Y-27632 resulted in a complete prevention of anti-GD1a/GT1b monoclonal antibody-mediated inhibition of axon regeneration of unmyelinated fibers to skin and the functional recovery of mechanical cutaneous sensitivity.In contrast,the same dose showed toxic effects on the regeneration of myelinated fibers.Interestingly,scale down of the dosage of Y-27632 to 5 mg/kg resulted in a significant although not complete recovery of regenerated myelinated axons exposed to anti-GD1a/GT1b monoclonal antibody in the absence of toxicity in animals exposed to only Y-27632.Overall,these findings confirm the in vivo participation of RhoA/ROCK signaling pathways in the molecular mechanisms associated with the inhibition of axon regeneration induced by anti-GD1a/GT1b monoclonal antibody.Our findings open the possibility of therapeutic pharmacological intervention targeting RhoA/Rock pathway in immune neuropathies associated with the presence of anti-ganglioside antibodies and delayed or incomplete clinical recovery after injury in the peripheral nervous system.
Key Words: anti-ganglioside antibodies;anti-glycan antibodies;axon regeneration;ganglioside;Guillain-Barré syndrome;nerve repair;ROCK;Y-27632
Introduction
After an axonal lesion of peripheral nerve,successful reconnection with endtarget organs such as the muscle and skin involves the concerted action of different cellular processes,including the appropriate axon regrowth,and the pathfinding that guarantees an accurate and timely efficient reinnervation (Akram et al.,2022).Axon regeneration and target reinnervation are guided by growth cones (GCs),specialized structures generated at the tips of regenerating axons that promote their extension by providing actin-guided extension of microtubules (Klimovich et al.,2021;Leite et al.,2021;Poitras and Zochodne,2022).Delayed or deficient target reinnervation usually results from multiple factors including the action of axon regeneration inhibitors,a diverse family of molecules with the ability to negatively modulate the GC cytoskeleton,ultimately leading to halted axon regeneration (Akram et al.,2022).Several studies have established the relationship between the presence of high titers of anti-Gg Abs and poor outcome/incomplete recovery in patients with GBS (Shastri et al.,2023;Thomma et al.,2023;Zhu et al.,2023).Passive immunization studies using monoclonal antibodies (mAbs) or patient-derived anti-ganglioside antibodies in an animal model of axon regeneration as well as exposure to regenerating dorsal root ganglion explants later confirmed that these Abs can act as inhibitory cues to halt axon regeneration by generating dystrophic GCs at the tip of regenerating axons (Kamakura et al.,2005;Lehmann et al.,2007;Lopez et al.,2010).The molecular mechanisms triggering the generation of dystrophic GCsin vivoby anti-Gg Abs leading to inhibition of axon regeneration have not yet been fully resolved,butin vitrostudies using primary dorsal root ganglion neuron (DRGn) cultures confirmed a critical role for the small GTPase RhoA and its associated kinase (ROCK) in a neurite outgrowth paradigm (Zhang et al.,2011).In addition,treatment of DRGn with a mAb targeting gangliosides GD1a and GT1b (anti-GD1a/GT1b mAb) induced GC collapse by targeting its cytoskeleton via RhoA-dependent and independent pathways in a time-dependent manner.Thus,exposure of DRGn to anti-GD1a/GT1b mAb triggers the early collapse of actin lamellipodia in a RhoA-independent manner followed by retraction of actin filopodia and microtubule disassembly via RhoA-dependent mechanisms (Rozes Salvador et al.,2016).Also,treatment of DRGn cultures with mAb1B7 induces inhibition in a neurite outgrowth paradigm via phosphorylation/inactivation of CRMP-2 at serine 555 downstream of RhoA/ROCK,while pharmacological inhibition of RhoA or ROCK prevents this effect.Interestingly,in vivoelectroporation of DRGn with an expression vector carrying a mutant form of CRMP-2 at residue serine 555 confirmed the role of RhoA/ROCK/CRMP-2 pathwayin vivo,opening the possibility for a therapeutic pharmacological intervention (Rozes Salvador et al.,2016).
Like anti-Gg Abs,the vast majority of inhibitors of axon regeneration described so far halt neurite outgrowth via activation of the small GTPase RhoA/ROCK signaling pathways,therefore it is not surprising that its pharmacological modulation has consolidated as a potential therapeutic target over the past years.Several studies have shown that selective blockade of this pathway has positive effects on axon regeneration (Jia et al.,2016;Kalpachidou et al.,2019;Xiang et al.,2021).In the same line,microinjection of a constitutively active form of RhoA,as well as the application of lysophosphatidic acid,which activates intracellular Rho,induces collapse of the GC and neurite outgrowth in PC12 and N1E-115 cells (Jalink et al.,1994).In contrast,the administration of clostridium botulinum C3 transferase,a specific RhoA inhibitor,which stimulates the proliferation of neurites from DRGn cultures,has shown encouraging results in preclinical animal studies and human clinical trials for the treatment of acute spinal cord injuries (Kalpachidou et al.,2019).In vitrostudies have shown that treatment with Y-27632,a specific pharmacological inhibitor of ROCK,prevents the effect of axon regeneration inhibitors on central and peripheral neurons (Joshi et al.,2019;Mertsch et al.,2021).Recently,specific inhibitors of ROCK such as fasudil,Y-27632 or FSD-C10 have been proposed as a possible therapeutic strategy to promote axonal growth in lesions of the nervous system (James et al.,2010;Xin,2015;Devaux et al.,2017).
The aim of this work was to perform a proof of concept study to evaluate the effects of the pharmacological blockade of the RhoA-ROCK pathway by administration of Y-27632 on the inhibitory effect triggered by passive immunization with anti-GD1a/GT1b mAb in an animal model of axon regeneration of peripheral nerves.
Methods
Anti-ganglioside mAb
The generation,purification,and specificity of the mAb anti-GD1a/GT1b IgG2b (anti-GD1a/GT1b mAb) was reported in previous studies (Lunn et al.,2000;Gong et al.,2002),including its ability to halt axon regeneration of myelinated and unmyelinated axons in an animal model (Lehmann et al.,2007).Large scale production using a hollow fiber bioreactor was performed at the Johns Hopkins Cell Culture Facility (Baltimore,MD,USA).An irrelevant mouse IgG (cat # 107-14,Southern Biotech,Birmigham,AL,USA) was used as negative control Ab.Saline phosphate buffer (PBS) was used as diluent.
Sciatic nerve-crush model
A standardized mouse sciatic nerve-crush model was used (Lopez et al.,2010).Male 10-12 week old C57BL/6J mice (in house breeding,up to 4 mice per cage) were maintained on a 12-hour light/dark cycle (lights on at 7 a.m.) with food and waterad libitumunder specific pathogen free (SPF) conditions (originally purchased from Charles River,Wilmington,MA,USA;strain code: 632) weighting 24 g were anesthetized by intraperitoneal administration of a cocktail of 100 mg/kg ketamine+10 mg/kg xylazine (Richmond VetPharmaTM,Buenos Aires,Argentina).Male mice were used due to differences in gene expression in dorsal root ganglion neurons in response to nerve injury (Jang et al.,2021).Once anesthesia and deep analgesia were induced,an incision in the skin was made in the gluteal region to separate the muscular layers.After the sciatic nerve was approached,the right sciatic nerve was crushed 35 mm rostral to the middle toe for 30 seconds using fine forceps on day 0.Separation of proximal and distal endoneurial contents confirmed complete crush.After the nerve crush,the wound was closed,allowing the animals to recover.Mice received intraperitoneal analgesia (acetaminophen at 100 mg/kg,Roemmers,Buenos Aires,Argentina) for 3 days.On day 21,mice were perfused intracardiacally with PBS followed by 4% paraformaldehyde.Tibial nerve segments were harvested at 20 mm distal to the crush site,postfixed with a mixture of 3% glutaraldehyde and 4% paraformaldehyde at 4°C and processed for epon embedding.Skin reinnervation was examined in foot pads of hind paws post-fixed with 4% paraformaldehyde at 4°C using immunohistochemistry as described below.Animal procedures were fully reviewed and approved by the Institutional Animal Committee (IACUC),School of Chemistry,National University of Córdoba on July 13,2021 (Protocol 2021-1070) and conduced in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8thed.,National Research Council,2011).All experiments were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines (Percie du Sert et al.,2020).
Treatments with anti-GD1a/GT1b mAb and Y-27632
Each experiment comprised of four groups of animals,i.e.,control IgG,anti-GD1a/GT1b mAb,anti-GD1a/GT1b mAb+Y-27632,and Y-27632 (each experiment was run in duplicate,n=3/4 mice per group).Twenty-four hours after nerve crush,mice received a single intraperitoneal (IP) injection of 1.5 mg of anti-GD1a/GT1b mAb targeting an epitope shared by gangliosides GD1a and GT1b (anti-GD1a/GT1b mAb and anti-GD1a/GT1b mAb+Y-27632 groups) or 1.5 mg of an unrelated mouse IgG (Cat# 107-14,Southern Biotech,Birmingham,AL,USA) (control IgG group) in 1 mL of PBS.A drug control group named Y-27632 was treated with Y-27632 2HCl,a specific pharmacological inhibitor of ROCK (named Y-27632) (Cat# S1049 purchased from SelleckchemTM,Houston,TX,USA),and a group combining treatments with anti-GD1a/GT1b mAb and Y-27632 (named 1B7+Y-27632),with the exception of the higher dose,received 10 single 1 mL doses consisting of either 1.5,0.9,or 0.5 mg/kg of Y-27632 2HCl in PBS every other day (starting on day 1) which accounts for a total dose of 15,9,and 5 mg/kg,respectively.On day 21,mice were perfused intracardiacally with PBS and 4% paraformaldehyde,then tibial nerves were harvested at 20 mm distal to the crush,post-fixed and processed for morphometric analysis.
Morphometric analysis of regenerating myelinated fibers in tibial nerves
Post-fixed tibial nerves (see description above) were embedded in epon overnight,and 1 µm cross-sections obtained using a ultramicrotome were stained with toluidine blue (Sigma,St.Louis,MO,USA) as described (Joshi et al.,2015).Nerve sections were imaged at 40× magnification using a bright field microscope (Zeiss,Oberkochen,Germany).Nerve reconstruction was achieved by image stitching using the open-source software package Fiji version 1.53t (https://imagej.nih.gov/ij/download/src/;Schindelin et al.,2012).For morphometric analysis,we manually counted all myelinated regenerating sprouts in a single whole cross-section image of the nerve composed of two merged individual images.
Morphometric analysis of skin reinnervation
The post-fixed distal hindlimbs of animals were extracted and decalcified using sodium citrate solution+formic acid (Sigma) for 72 hours.The hind paws were then isolated from the hindlimbs,cryoprotected with 30% sucrose and cross-sectioned at 25 µm thickness using a cryostat (Leica,Deer Park,IL,USA).We quantified the intraepidermal density of regenerated fibers (suraldependent innervation area of foot pad,sciatic nerve branch) identified by immunohistochemistry with primary rabbit polyclonal antibodies against the marker PGP 9.5,which detects unmyelinated fiber in the epidermis (Cell Marque,Rocklin,CA,USA,Cat# 318A,RRID: AB_1160836),used at 1:250 dilution and incubated over night as described (Collongues et al.,2018).Anti-PGP 9.5 antibody binding was detected and developed with HiDef DetectionTMHRP Polymer System from Cell Marque (Sigma) according to the manufacturer’s instructions.Chromogenic reaction was visualized using DAB Substrate Kit Cell Marque™ chromogen,Cat# 957D-3 (Sigma).These sections were lightly counterstained with hematoxylin and eosin (Sigma).We manually counted the number of regenerating epidermal fibers crossing a 60 µm length linear section at the dermal-epidermal junction on images taken at 63× magnification using a brighfield microscope (Zeiss).
Analysis of functional skin reinnervation
The mechanical sensitivity of the reinnervated skin was tested at 21 days post-crush by gently applying a fine blunt pin to the plantar surface of the paw without moving the paw (Pinprick test).The most lateral part of the plantar surface of the hind paw,corresponding to the sensory field innervated by the sural nerve,was divided into five areas indicative of short (plantar) and long (toe) functional reinnervation distance,respectively.The pinprick stimuli were applied from the most lateral toe to the heel of the paw.A response was considered positive when the animal briskly removed its paw,and the response was graded 1 for this area,and then tested for the next one.A final score was achieved with the summation of grades obtained from the responsive areas (maximum score of 5).If none of the stimuli elicited a positive response,the overall grade was 0 (Jha et al.,2021).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 7.0 for Windows (GraphPad Software,San Diego,CA,USA,www.graphpad.com).All values are represented as mean ± standard error of the mean (SEM).For comparison between groups,two-way analysis of variance (ANOVA) followed by Tukey’spost hoctest was used.Pvalues ≤ 0.05 were taken as significant.
Results
Y-27632 treatment and regeneration of myelinated nerve fibers
We first analyzed the pro-regenerative effect of the intraperitoneal administration of Y-27632,a specific pharmacological inhibitor of ROCK,in an established animal model of axon regeneration where the potent inhibitory effect of anti-GD1a/GT1b mAb was previously documented (Lehmann et al.,2007).Based on the work published by James et al.(2010),we performed the first experiment with the administration of a total dose of 15 mg of Y-27632 per animal over a period of 21 days.We observed a significant reduction (~60%) in the number of regenerated fibers in the tibial nerves from mice treated with Y-27632 when compared to control vehicle-treated nerves,indicative of a severe toxic effect of this dosage (data not shown).Therefore,we decided to scale down the total dose of Y-27632 to 9 mg.We still observed a significant although diminished reduction in the toxic effect of the treatment on regeneration of myelinated fibers when compared to control nerves receiving only vehicle (Figure 1A).As expected,the administration of a single dose of 1.5 mg of anti-GD1a/GT1b mAb induced a robust inhibition of axon regeneration of myelinated fibers in tibial nerves.However,the combined treatment of Y-27632 (total dose of 9 mg) and anti-GD1a/GT1b mAb did not result in a significant improvement of axon regeneration (Figure 1AandB).In conclusion,the total dosage of 9 mg of Y-27632 exerts a mild toxic effect on axon regeneration of myelinated fibers with no improvement in the regeneration of mice exposed to anti-GD1a/GT1b mAb.Given the toxicity of high dose Y-27632 (9-15 mg) on myelinated nerve fiber regeneration,we decided to decrease the total dosage to 5 mg to test its efficacy to promote axon regeneration of myelinated nerve fibers.In contrast to the toxic effects of high dose Y-27632,treatment with a dose of 5 mg of Y-27632 did not display toxicity on regenerating myelinated fibers compared to vehicle-treated control group (Figure 2B).Figure 2Adepicts representative 1 µm-thick plastic-embedded sections from tibial nerves stained with toluidine blue.The morphometric analysis indicates a pronounced inhibitory effect of anti-GD1a/GT1b mAb on the regeneration of myelinated fibers when compared to control mice receiving irrelevant IgG.Notably,the immunization of anti-GD1a/GT1b mAb in combination with Y-27632 treatment (5 mg total dosage) resulted in significant improvement of axon regeneration compared to anti-GD1a/GT1b mAb treatment alone.However,this anti-GD1a/GT1b mAb-mediated effect was not completely reversed with 5 mg dosage of Y-27632.Altogether,treatment with Y-27632 shows a dose-dependent toxicity on regeneration of myelinated fibers in peripheral nerves,however,we identified 5 mg as a suitable total dosage to partially overcome anti-GD1a/GT1b mAbdependent inhibition of axon regeneration.

Figure 1|Administration of a total dose of 9 mg of Y-27632 does not prevent inhibition of axon regeneration induced by anti-GD1a/GT1b mAb.
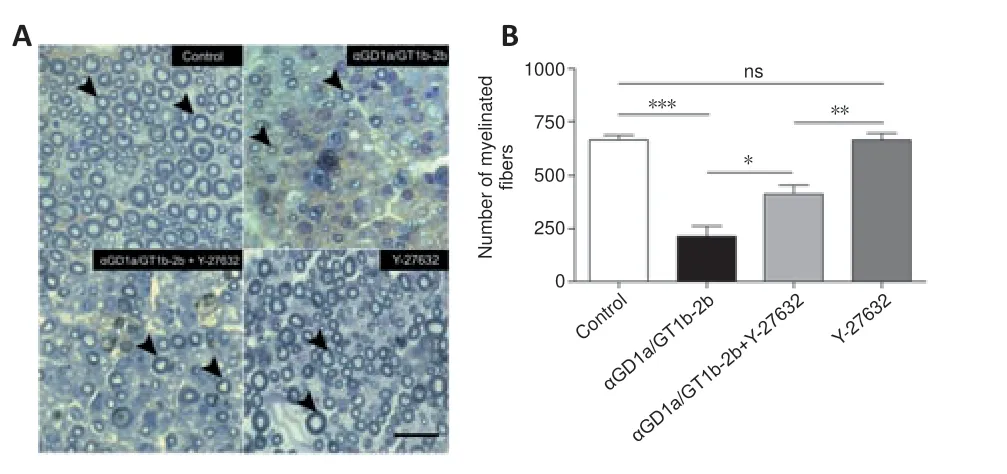
Figure 2|Administration of a total dose of 5 mg of Y-27632 partially prevents inhibition of axon regeneration induced by anti-GD1a/GT1b mAb.
Y-27632 treatment and regeneration of unmyelinated nerve fibers
It was previously documented that administration of anti-GD1a/GT1b mAb in an animal model of axon regeneration inhibited regeneration not only of myelinated axons but also unmyelinated fibers (Lehmann et al.,2007).Thus,we decided to study the intraepidermal density of regenerated unmyelinated fibers in areas of hind paw innervated by the sural branch of the sciatic nerve.Figure 3Adepicts representative photomicrographs of unmyelinated fibers,at the dermal/epidermal junction,identified by the use of antibodies against the marker PGP 9.5.Morphometric studies indicated absence of toxic effects due to the administration of a total dose of 9 mg/kg of Y-27632 when comparing the number of regenerated fibers to control group receiving vehicle (Figure 3B).Interestingly,while a pronounced inhibitory effect on skin reinnervation was observed in the epidermis from mice exposed to anti-GD1a/GT1b mAb compared to control IgG-treated group,the combinatory treatment of anti-GD1a/GT1b mAb and 9 mg Y-27632 resulted in a complete reversal of the inhibitory effect of anti-GD1a/GT1b mAb.In sum,Y-27632 has no toxic effect on regeneration of unmyelinated sensory fibers and it completely reverses the anti-GD1a/GT1b mAb-mediated inhibition of axon regeneration of unmyelinated fibers.

Figure 3|Administration of a total dose of 9 mg of Y-27632 completely reverses the inhibitory effect of anti-GD1a/GT1b mAb on the regeneration of sensory unmyelinated fibers.
Y-27632 treatment and functional sensory reinnervation of the skin
Additionally,we analyzed the functional sensory reinnervation of skin in these groups of animals by studying the mechanical sensitivity in the skin.Paw withdrawal (scaled 1 to 5) was categorized by studying the paw retraction in response to pinprick in five different areas indicative of short (plantar) and long (toe) functional reinnervation distance,respectively.The findings indicate a significantly reduced sensitivity in the paw from animals receiving anti-GD1a/GT1b mAb compared to the control group (P<0.05;Figure 4).In addition,while the Y-27632 group (dose of 9 mg/kg) did not differ from the control vehicle-treated group,the combinatory treatment of anti-GD1a/GT1b mAb and Y-27632 improved the functional reinnervation in the skin to levels similar to the IgG-treated control group.In short,a total dose of 9 mg/kg of Y-27632 has no toxicity for functional sensory reinnervation of the skin and it reverses anti-GD1a/GT1b mAb-mediated inhibition of unmyelinated sensory axons.

Figure 4|Administration of a total dose of 9 mg of Y-27632 completely prevents the inhibitory effect of anti-GD1a/GT1b mAb on the functional sensory reinnervation of the skin.
Discussion
Taking into account a vast evidence for the use of pharmacological inhibitors of RhoA/ROCK signaling pathways as potential targets for improving axon regeneration in the CNS,the objective of this study was to perform a proof of concept study to demonstrate the effectiveness of Y-27632,a selective pharmacological inhibitor of ROCK,in an animal model of axon regeneration of peripheral nerves,where the passive immunization with a mAb targeting gangliosides GD1a and GT1b was previously reported to exert a potent inhibitory effect on regeneration of both myelinated and unmyelinated fibers (Lehmann et al.,2007).Our results demonstrate a differential susceptibility of myelinated and unmyelinated axons to the administration of Y-27632.Treatment with a total dosage of 9 mg/kg of Y-27632 resulted in a complete prevention of anti-GD1a/GT1b mAb-mediated inhibition of axon reinnervation of unmyelinated fibers to skin.Moreover,functional studies confirmed the recovery of cutaneous mechanical sensitivity induced by Y-27632,which mostly involves unmyelinated fibers,demonstrating an efficient target reinnervation by c-fibers from dorsal root ganglion.In contrast,treatment with the same dosage of Y-27632 appears mildly toxic for the regeneration of myelinated fibers,without further improvement of axon regeneration on nerves exposed to anti-GD1a/GT1b mAb compared to control nerves.Based on these observations,we scaled down the dosage of Y-27632 to 5 mg/kg.Interestingly,we found a significant although not complete recovery of regenerated myelinated axons exposed to anti-GD1a/GT1b mAb in distal segments of the crushed sciatic nerves.Additionally,we did not find evidence of toxicity with this dosage,i.e.reduction in the number of myelinated fibers in control groups exposed to Y-27632 alone.Overall,these findings supportin vivoinvolvement of RhoA/ROCK signaling pathways in the molecular mechanisms associated with the anti-GD1a/GT1b mAb-mediated inhibition of axon regeneration in this paradigm.
Activation of RhoA/ROCK is a common downstream signaling pathway in which multiple inhibitors of axon regeneration from CNS converge.A previous study has identified that mAbs and patient-derived polyclonal anti-Gg antibodies inhibit neurite outgrowth of DRGnin vitroin a RhoA/ROCK-dependent manner (Zhang et al.,2011).Further studies focusing on the GC morphology from DRGn identified that anti-GD1a/GT1b mAb negatively modulates their cytoskeleton through the RhoA-dependent and independent pathways (Rozes Salvador et al.,2016).Thus,while retraction of actin filopodia and microtubule depolymerization leading to GC collapse rely on the activation of RhoA/ROCK pathways,the later depends on the phosphorylation/inactivation of the downstream effector CRMP-2;retraction of actin lamellipodia was not susceptible to pharmacological inhibition of RhoA or ROCK,despite both treatments completely prevented inhibition of neurite outgrowth by anti-GD1a/GT1b mAb.Additionally,the role of phosphorylation/inactivation of CRMP-2 at serine 555 signaling pathway downstream of ROCK activation have been demonstratedin vivoin regenerating axons secondary to anti-GD1a/GT1b mAb treatment (Rozes Salvador et al.,2016),where the generation of dystrophic GC was previously documented (Lehmann et al.,2007).Altogether,these findings could explain,in part,why treatment with a total dosage of 5 mg/kg of Y-27632 produced just a partial (but not complete) improvement in the regeneration of myelinated fibers.Actin dynamics at the GCin vivo,in particular the actin lamellipodia network,plays a pivotal role in GC motility and extension towards the target organ;but its collapse secondary to anti-GD1a/GT1b mAb exposure was not prevented by Y-27632.Therefore,the molecular mechanisms leading to inhibition of axon regeneration by anti-Gg Abs seems to differ significantly from other inhibitors of axon regeneration,that commonly exert a retraction of actin lamellipodia at GCin vivoandin vitro,restricting microtubule protrusion and limiting axon extension by a RhoA/myosin II-dependent mechanisms (Nichol et al.,2016;Stern et al.,2021).Thus,despite RhoA activation is a key mediator to halt axon regeneration by anti-Gg Abs,additional intracellular mediators of outgrowth inhibition could be involved.For instance,inhibition of axonal growth can occur through a second parallel pathway,where GSK-3b is activated through the tumor suppressor phosphatase and the tensin homologue or R-Ras-GAP in neurons from peripheral nervous system (PNS;Gobrecht et al.,2014).Therefore,the regenerative response may require the inactivation not only of ROCK but also of GSK-3b at the level of PNS and central nervous system (CNS;Leibinger et al.,2017;Nieuwenhuis and Eva,2022).Conversely,RhoA inactivation seems sufficient to promote axon regeneration of unmyelinated fibers and their efficient skin reinnervation.Altogether,these findings highlight the need for a detailed understanding of the cell biology of regenerating PNS neurons prior to analyzing the therapeutic potential of Y-27632 to promote nerve repair.In this sense,future studies should also define whether anti-Gg Abs targeting individual gangliosides such as GM1,GD1a or GT1b share similar molecular mechanisms to limit axon regeneration and whether this is a feature common to all autoimmune neuropathy-associated anti-Gg Abs.
Another important aspect of our study relates to the dose-dependent toxic effect of Y-27632 selectively observed on myelinated fibers.We initiated our studies with a dosage of 15 mg/kg Y-27632 based on the work by James et al.(2010),who found a beneficial effect on axon regeneration of unmyelinated fibers by Y-27632 on cisplatin-induced peripheral neuropathy.At this dosage,Y-27632 displayed a high toxic effect on myelinated fibers in our model,so this dosage was discontinued.Subsequent studies using treatments of Y-27632 at 9 and 5 mg/kg over a 21 days lapse confirmed a dose-dependent toxicity.Interestingly,the toxic effect is dependent on the cell phenotype,since a differential response to Y-27632 was observed between unmyelinated and myelinated axons.Few studies have reported that inhibition of ROCK by Y-27632 displayed negative effects on axon regeneration in the CNS (Narumiya and Thumkeo,2018).However,the effects of modulating RhoA/ROCK signaling pathways (including administration of Y-27632) on non-neuronal cells show opposite results.Thus,activation of RhoA/ROCK in monocytes is sufficient to improve adhesion and transendothelial migration,while inhibition by Y-27632 can reduce the monocytic recruitment in the inflamed tissue (Honing et al.,2004).In the same line,macrophage-specific RhoA knockout negatively modulates peripheral nerve repair by delaying wallerian degeneration and nerve regeneration in mice (Xu et al.,2021).Also,RhoA activation in Schwann cells reduces its migration and promotes normal myelination mediated by neurotrophins acting through the low affinity neurotrophin p75NTR while in CNS stop astrogliosis (Yamauchi et al.,2004;Stern et al.,2021).On the other hand,Melendez-Vasquez et al.(2004) reported that Y-27632-mediated ROCK inhibition regulates Schwann cell myelination and formation of associated axonal domains,which could also explain the differential response of myelinated and unmyelinated fibers to Y-27632 treatment.Taken together,these results suggest that the RhoA/ROCK signaling pathways are actively involved in the process of nerve repair in neuronal as well as non-neuronal cells from the nervous system,raising a note of caution about the possible deleterious effects of pan-inhibition of RhoA to promote axon regeneration;and at the same time,highlight the need for understanding the complete spatiotemporal dynamics of RhoA signaling in generating a microenvironment that minimizes side effects.On the other hand,the lack of complete recovery of myelinated axon counts in nerves exposed to anti-GD1a/GT1b mAb and lower doses of Y-27632 (5 mg/kg) could reflect a differential susceptibility of motor (which accounts for 30 % of total myelinated fibers) versus sensory fibers to the Y-27632 treatment as previously reported by Joshi et al.(2015),a hypothesis that needs to be further confirmed in future studies.
A final note of caution for the interpretation of our study relates to sex differences as the base for opposite response of human neuthropils to chemorepulsion,since only males were used in our study (Consalvo et al.,2022).Ruts et al.(2012) demonstrated that myelinated and unmyelinated fibers from the skin are affected diffusely in GBS and its variants,including the pure motor form,correlating their density with the severity of the pain in the acute phase and the long-term disability.Selective damage of small unmyelinated fibers was also confirmed by Yuki et al.(2018).While the role of anti-Gg Abs in unmyelinated fiber injury in patients with GBS is not clear,based on our findings a potential therapeutic benefit by treatment with low dosage of Y-27632 in GBS patients with predominant affection of small fibers can be speculated.
This study has some limitations that should be noted.The time-lapse to study axon regeneration was limited to 21 days,however,shorter periods (i.e.14 days,average time to reinnervate muscle by myelinated fibers in this model) could provide further evidence about the efficiency of the pharmacological inhibition of ROCK.Additionally,in light of the moderate toxicity observed on axon regeneration of myelinated fibers in mice administered with higher doses of Y-27632,shorter administration protocols could provide evidence about a possible time-dependent toxic effect by this inhibitor.Another limitation is the lack of studies demonstrating muscle reinnervation by myelinated fibers,a process required for functional recovery,which could be potentially affected by Y-27632 administration.Future inclusion of these functional studies could provide a more comprehensive analysis of the potential of Y-27632 as a therapeutic strategy.Finally,in relation to the partial recovery of axon regeneration of myelinated fibers exposed to anti-GD1a/GT1b mAb in mice administered with Y-27632,it will be interesting to explorein vivothe contribution of RhoA/ROCK-independent molecular mechanisms of inhibition of axon regeneration by anti-ganglioside antibodies.
In conclusion,our work provides additional and novel information on the modulatory role exerted by Y-27632 on the RhoA-ROCK signaling pathway during the process of nerve repair in an experimental model of axon regeneration in PNS associated with the presence of high titer of circulating anti-Gg Abs.Additional studies are necessary to further understand the mechanism(s) of action(s),given the multiple cellular effects related to this pathway and the differential susceptibility of different cells in order to consider Y-27632 as a potential pharmacological intervention in anti-Ggrelated autoimmune neuropathies.
Author contributions:AB,CRB,and PHHL designed the experiments.AB,CRB,BBB,RS,and PHHL performed the experiments.AB,CRB,BBB,and PHHL analyzed the bulk of the experiments.KAS was responsible for mAb production.PHHL guided the study.AB,KAS,and PHHL wrote the manuscript.All authors read and approved the final manuscript.
Conflicts of interest:The authors declare no conflict of interest.
Data availability statement:No additional data are available.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Could mammalian inorganic polyphosphate be a crucial signaling molecule in neurological disorders?
- Use of an immunocapture device to detect cytokine release in discrete brain regions
- New immune regulators of sciatic nerve regeneration? Lessons from the neighborhood
- Multifunctional glycolipids as multi-targeting therapeutics for neural regeneration
- Astrocytes dynamically regulate the blood-brain barrier in the healthy brain
- Epigenetic memory of drug exposure history controls neural stem cell quiescence in the adult brain

