RNA sequencing of exosomes secreted by fibroblast and Schwann cells elucidates mechanisms underlying peripheral nerve regeneration
Xinyang Zhou, Yehua Lv, Huimin Xie, Yan Li, Chang Liu, Mengru Zheng, Ronghua Wu, Songlin Zhou, Xiaosong Gu1,,Jingjing Li, Daguo Mi
Abstract Exosomes exhibit complex biological functions and mediate a variety of biological processes, such as promoting axonal regeneration and functional recovery after injury.Long non-coding RNAs (lncRNAs) have been reported to play a crucial role in axonal regeneration.However, the role of the lncRNA-microRNAmessenger RNA (mRNA)-competitive endogenous RNA (ceRNA) network in exosome-mediated axonal regeneration remains unclear.In this study, we performed RNA transcriptome sequencing analysis to assess mRNA expression patterns in exosomes produced by cultured fibroblasts (FC-EXOs) and Schwann cells (SCEXOs).Differential gene expression analysis, Gene Ontology analysis, Kyoto Encyclopedia of Genes and Genomes analysis, and protein-protein interaction network analysis were used to explore the functions and related pathways of RNAs isolated from FC-EXOs and SC-EXOs.We found that the ribosome-related central gene Rps5 was enriched in FC-EXOs and SC-EXOs, which suggests that it may promote axonal regeneration.In addition, using the miRWalk and Starbase prediction databases, we constructed a regulatory network of ceRNAs targeting Rps5, including 27 microRNAs and five lncRNAs.The ceRNA regulatory network,which included Ftx and Miat, revealed that exsosome-derived Rps5 inhibits scar formation and promotes axonal regeneration and functional recovery after nerve injury.Our findings suggest that exosomes derived from fibroblast and Schwann cells could be used to treat injuries of peripheral nervous system.
Key Words: ceRNA network; exosomes; fibroblast cells; Gene Ontology (GO); Kyoto Encyclopedia of Genes and Genomes (KEGG); protein-protein interaction (PPI)networks; RNA-seq; Schwann cells
Introduction
Traumatic peripheral nerve injury impairs sensory-motor function, resulting in a serious economic burden on society.Therefore, the potential mechanism of peripheral nervous system (PNS) regeneration and repair should be explored.The lack of peripheral nerve repair is mainly due to the fact that the regenerated axons need to traverse a relatively long distance to reinnervate the target tissue (Stratton et al., 2017).Studies have found that certain non-neuronal cells play a very important role in peripheral nerve axon regeneration (Zigmond and Echevarria, 2019; Feng et al., 2023; Sarhane et al., 2023).A number of studies have focused on a novel mechanism of intercellular communication within PNS that involves direct transfer of molecules from glial cells to axons via extracellular vesicles called exosomes(Lopez-Leal and Court, 2016; Pascual et al., 2020).Schwann cell exosomes(SC-EXOs) have been shown to accelerate the rate of regeneration in the PNS by regulating Rho activity (Contreras et al., 2022; Zhang et al., 2023).In addition, fibroblast exosomes (FC-EXOs) promote axonal regeneration in a neurologically inhibited environment and inhibit scar formation by regulating Wnt signaling (Tassew et al., 2017; Zhao et al., 2023).Thus, we hypothesized that a combination of exosomes produced by a variety of cells types might activate different neuronal signaling pathways and help repair and regenerate damaged neurons.This study focused on two major nonneuronal cell exosome populations that affect peripheral nerves: FC-EXOs and SC-EXOs.The aim of the study was to explore the interaction network and identify hub genes that regulate exosomes from different cellular sources during regeneration after nerve injury, to lay a theoretical foundation for RNA therapy based on exosomes derived from multiple cell types.
For severed axons to regenerate, they must undergo calcium signaling regulation, cytoskeletal reorganization, axonal mRNA transport, protein transport, and up-regulation of mRNA translation (Whitworth et al., 2017).The cellular stress caused by axon severance may limit synthesis of the new proteins required to maintain axon regeneration (Asghari Adib et al.,2018; Fricker et al., 2018).However, studies have found that nerves exhibit extensive axon germination at the site of injury, and non-neural cells near the regenerated buds seem to generate a substantial amount of rough endoplasmic reticulum and free ribosomes that are taken up by the injured axons (Müller et al., 2018; Di Paolo et al., 2021).For example, the soluble growth factor Neuregulin 1 (NRG1) is strongly up-regulated in and released by Schwann cells immediately after nerve injury (El Soury et al., 2018).Furthermore, NRG1 up-regulation regulates the expression of ribosomal RNA processing genes, suggesting that NRG1 promotes cell survival and stimulates protein expression.Notably, Schwann cells can deliver ribosomes to axons,thereby providing neurotrophic and physical support for axon regeneration(Sotelo et al., 2014).In addition, Schwann cells produce ribosome-containing exosomes that can penetrate the myelin sheath and enter axons, indicating that ribosomal proteins are important factors involved in axon regeneration (Di Paolo et al., 2021).However, exosome-mediated extracellular communication,ribosomal delivery, and glial cell-neuron regulation mechanisms in the PNS remain largely uncharacterized.
The ability of peripheral nerves to regenerate after injury also depends on the extent of injury and scarification (Ghosh et al., 2020).Tissue remodeling and scar formation are inherent parts of wound healing, and incorrect or incomplete repair may lead to the formation of intranerve scarring accompanied by fiber wrapping, further hindering the growth and regeneration of new axons and ultimately impairing normal function.Studies have shown that activin A, a key factor secreted by neurofibroblasts, can promote Schwann cell proliferation and migration through the activity of ALK4, ALK5, and ALK7 (Li et al., 2022).Schwann cells can also interact with fibroblasts directly or indirectly through the EphB receptor to induce nerve bridge formation and promote axon regeneration (Qu et al., 2021).A recent study showed that, when axons are damaged, FC-EXOs can activate a specific gene expression pattern or regulatory mechanism that inhibits scar growth,as well as interact with other non-neuronal exosomes, to regulate protein synthesis and promote axon regeneration in the PNS (D’Ambrosi and Apolloni,2020).However, the mechanism underlying this effect remains unclear.
Changes in the expression levels of various signaling factors, as well as molecular cascade reactions, are critical to the regeneration of damaged peripheral neurons.The poor regenerative ability of the adult mammalian nervous system after injury results from two characteristics of the mature nervous system: inhibition by the external environment and a reduction in the innate regenerative ability of mature neurons compared with newborn neurons (Park et al., 2008).Therefore, we hypothesized that exosomes play an important role in regulating axon regeneration.To determine the mechanism underlying this effect, we performed deep RNA sequencing analysis of SCEXOs and FC-EXOs in this study, complemented by a series of bioinformatics analyses, to explore the regulatory networks that integrate signals from exosomes derived from different cellular sources to promote regeneration of damaged nerve axons.
Recent evidence has emphasized the importance of competitive endogenous RNA (ceRNA) networks in biological processes.These networks link the functions of noncoding RNAs, such as microRNAs or long noncoding RNAs,to that of protein-coding mRNAs, enabling large-scale regulation of the transcriptome (Zhou et al., 2019).However, the regulatory mechanism of the lncRNA-miRNA-mRNA network during axonal regeneration after peripheral nerve injury is not well understood.In addition, previous studies have focused on the mechanism of individual components of the lncRNA-miRNA-mRNA axis(Wang et al., 2020; Yao et al., 2021).However, the role of the ceRNA network in regenerative repair of the nervous system remains unclear.Therefore, the purposes of this study were to determine whether FC-EXOs and SC-EXOs share a common ceRNA, and to explore whether therapy based on exosomes derived from multiple sources could promote axonal regeneration after peripheral nerve injury.
Methods
Ethics statement
All experimental animal protocols were approved by the Animal Ethics Committee of Nantong University (approval No.S20180102-152) on January 2, 2018 and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (8thed., National Research Council, 2011).This study is reported in accordance with the ARRIVE 2.0 guidelines (Animal Research:Reporting ofIn VivoExperiments; Percie du Sert et al., 2020).
Workflow
Figure 1 shows the data analyses that were performed as part of this study,including bioinformatics tools, predictive databases, and analyses.The genome sequencing results were processed and grouped.The degenes group included all genes that were differentially expressed in FC-EXOs compared with SC-EXOs.The fibroblast (C>20) (the average expression of genes included in the analysis was greater than 20 FPKM) group and Schwann cell (C>20)group included protein-coding genes whose expression levels were greater than 20 in FC-EXOs and SC-EXOs.The unique FC∩SC (the intersection of the Fibroblast group and the Schwann cell group) (C>20) group included proteincoding genes whose expression levels were greater than 20 in both FC-EXOs and SC-EXOs.The unique fibroblast (C>20) group included protein-coding genes whose expression level was greater than 20 in FC-EXOs and less than 20 in SC-EXOs, and the unique Schwann cell (C>20) group included genes that exhibited the opposite expression pattern.The (HUB50-Fibroblasts)∪(HUB50-Schwann cells) (C>20) group included all 50 hub genes from both the fibroblast (C>20) group and the Schwann cell (C>20) group.To ensure the reliability of the data, at least three biological replicates were performed for the RNA sequencing analysis.To eliminate errors and omissions in the analysis process, at least three bioinformatics analyses were performed on the RNA sequencing data from October 2021 to February 2022.
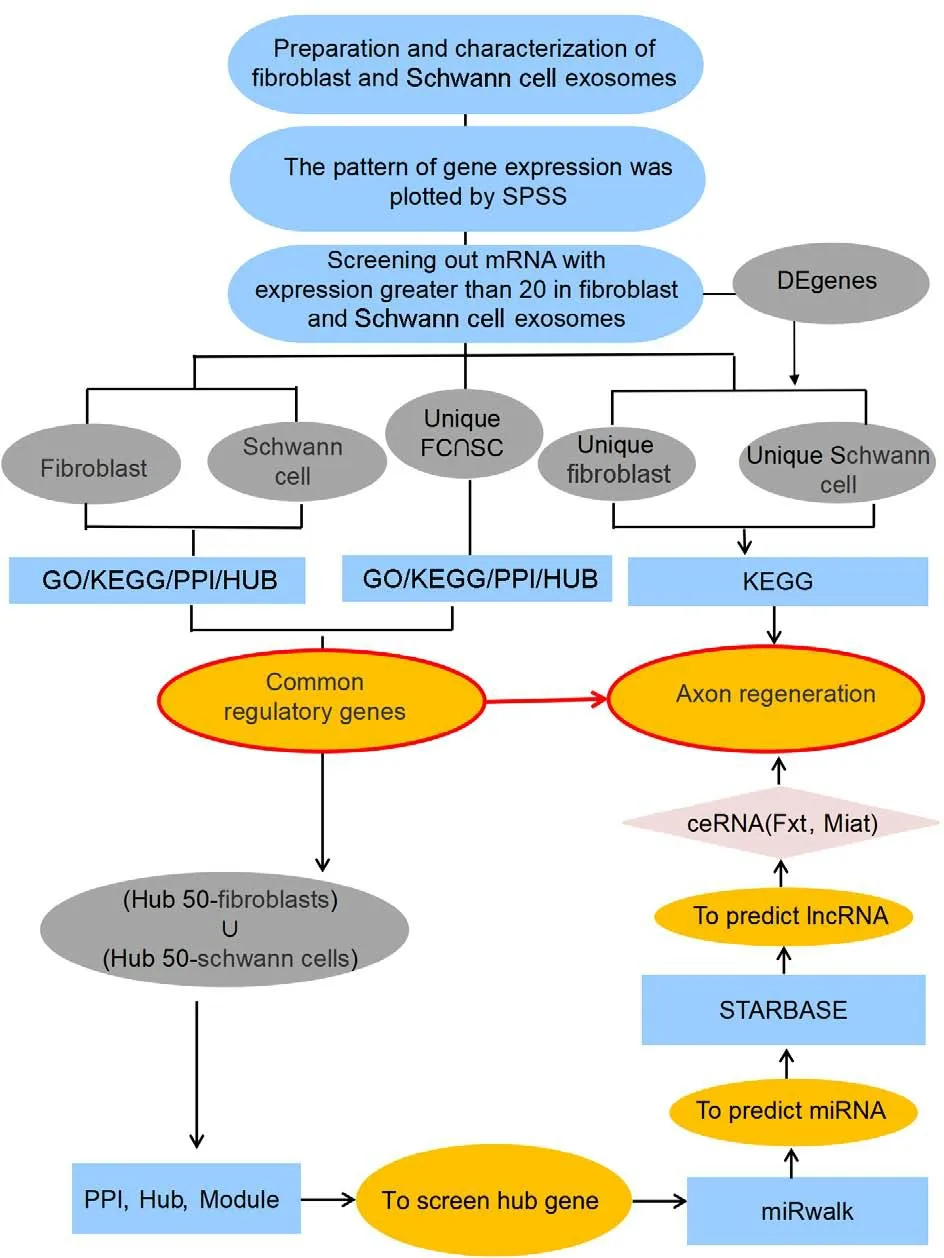
Figure 1 |Study flowchart.
Isolation and culture of dorsal root ganglion neurons, Schwann cells, and fibroblasts
Dorsal root ganglion (DRG) neurons, Schwann cells, and fibroblasts cells were obtained from 1-day-old male SD rats provided by Nantong University Laboratory Animal Center (license No.SCXK (Su) 2014-0001).The rats (weight 3–5 g) were housed at 24°C and 55 ± 5% humidity with a 12-hour diurnal cycle and normal ventilation.First, the rats were anesthetized by isoflurane(RWD, Shenzhen, Guangdong, China) inhalation at an induction dose of 4% and a maintenance dose of 2%.The entire sciatic nerve on both sides was harvested, cut into small pieces, and incubated in type IV collagenase(3 mg/mL, Worthington, Lakewood, NJ, USA) at 37°C for 1 hour with slight shaking every 20 minutes.After centrifugation at 1235 ×gfor 5 minutes,the precipitate was cultured in DMEM (Sigma, St.Louis, MO, USA, Cat#D5671) and F12 (Sigma; 3:1) with neuregulin (MedChem Express, Monmouth Junction, NJ, USA; 50 ng/mL), forskolin (MedChem; 5 µM), N2 supplement(Gibco, Grand Island, NY, USA; 1%), and penicillin/streptomycin (1%, Sigma-Aldrich).After 30–45 minutes of culture at 37°C in a 5% CO2incubator (Thermo Fisher Scientific, Waltham, MA, USA), fibroblasts and Schwann cells were isolated and purified based on differential digestion and differential adhesion.Most of the Schwann cells were in the supernatant and were collected for further culture, while most of the fibroblasts were attached to the bottom of the dish and were harvested using the same method described above.The medium used to culture the fibroblasts contained 10% FBS rather than neuregulin and forskolin, which allowed the fibroblasts in our mixed neural cultures to outperform all other cell types (Gnavi et al., 2015; He et al., 2020).To isolate DRG neurons from rats anesthetized with isoflurane, sterile scissors were used to cut open the spinal canal along the midline of the spinal cord,and sterile tweezers were used to remove the DRG).The DRG was then cut into small pieces, followed by incubation in collagenase (Worthington) at 37°C for 30 minutes, with slight shaking every 15 minutes.Then, 0.25% trypsin(Gibco) was added directly to the culture dish, and the cells were incubated at 37°C for 2 minutes to allow digestion to occur.Next, the solution was centrifuged at 1235 ×gfor 5 minutes, the supernatant was discarded, and the precipitate was resuspended in 5 mL of 15% BSA.After centrifugation at 1112 ×gfor 5 minutes, the precipitate was placed in Neurobasal medium(Invitrogen, Carlsbad, CA, USA) containing B27 (2%), GlutaMAXTMSupplement(1%), and penicillin (100 µg/mL)/streptomycin (1%) and cultured at 37°C in a 5% CO2incubator.Schwann cells and fibroblasts cells were visualized using a Zeiss-ax10 microscope (Carl Zeiss, Oberkochen, Germany).
Co-culturing DRG neurons with fibroblasts or Schwann cells
Fibroblasts and Schwann cells were resuspended in DMEM complete medium containing 2 mM L-glut, sodium pyruvate, and 10% FBS (Sigma).The genespecific siRNA and the control si-NC (Ribobio, Guangzhou, Guangdong, China)were suspended in LipofectamineTMRNAiMAX (Invitrogen) and Opti-MEM(Gibco) and incubated at room temperature for 15 minutes before being applied to the cells.The cells were seeded at a density of 6 × 104cells in the upper chamber of each Transwell chamber (FALCON, Corning, NY, USA, Cat#353095), with a chamber aperture of 0.4 µm and transfected with siRNA at a final concentration of 100 nM.After transfection for 12 hours, the DMEM complete medium was replaced, and the cells were reincubated.After 48 hours of transfection, DRG neurons were inoculated at a density of 0.5 ×104in the lower chamber of the Transwell chamber (FALCON) for co-culture experiments.After 24 hours of co-culture, DRG neurons were subjected to immunofluorescence staining.
Quantitative reverse transcription-polymerase chain reaction
A Trizol kit (Sigma, Cat# T9424) was used to extract total RNA from fibroblasts and Schwann cells.The purified total RNA was reverse transcribed using a HiScript III 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, Jiangsu, China).Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)was performed on a StepOneTMReal-Time PCR apparatus (Thermo Fisher Scientific) using a 2×AceQ qPCR SYBR Green Master Mix (high ROX premix)kit (Vazyme).The qRT-PCR conditions were as follows: 95°C for 2 minutes,followed by 40 cycles of 95°C for 30 seconds and 60°C for 30 seconds.Data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH)expression using the 2–∆∆CTmethod.The primers sequences were as follows:Rps5-Forward: 5′-CGG AAC ATC AAG ACC ATC GC-3′, Rps5-Reverse: 5′-AGG AGT TGG AGG AGC CCT TG-3’; GAPDH-Forward: 5′-TGG AGT CTA CTG GCG TCT T′, GAPDH-Reverse: 5′-TGT CAT ATT TCT CGT GGT TCA-3′.
Immunocytochemical staining
Cultured DRG neurons, Schwann cells, and fibroblasts were first fixed in 4% paraformaldehyde (Sigma-Aldrich) for 30 minutes.They were then incubated with bovine serum albumin (5%; from Invitrogen) for 1 hour at room temperature to block nonspecific binding.Then, the cells were incubated with antibodies to the fibroblast marker Thy-1 cell surface antigen(anti-CD90) (mouse, 1:1000, Abcam, Cambridge, MA, USA, Cat# ab225,RRID: AB_2203300), the Schwann cell marker S100 calcium-binding protein(Anti-S100) (rabbit, 1:1000, Sigma, Cat# SAB5500172) and the DRG neuron marker β3-tubulin (rabbit, 1:1000, Cell Signaling, Danvers, MA, USA, Cat#5568S) at 4°C for 12–16 hours.Then, the cells were incubated with Alexa Fluor 594 goat anti-mouse IgG (1:400, Abcam, Cat# ab150116, RRID: AB_2650601)and goat anti-rabbit IgG H&L Alexa Fluor 488 (1:500, Abcam, Cat# ab150077,RRID: AB_2630356) secondary antibodies for 2 hours at room temperature.Nuclei were labeled with Hoechst 33342 (1:1000, Abcam, Cat# ab145597).The morphology of Schwann cells and fibroblasts was observed under a TCS SP2 confocal microscope and a Zeiss-ax10 fluorescence microscope (Carl Zeiss).Cell type and axon length statistics were confirmed using ImageJ 1.8.0(National Institutes of Health, Bethesda, MD, USA; Schneider et al., 2012).
Exosome isolation and storage
Fibroblasts and Schwann cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S).When the cells reached 80% confluency, they were washed with PBS, and the medium was replaced with serum-free DMEM containing L-glutamine and 1 g/L BSA(FALCO).After 2 more days of culturing, the conditioned culture medium(CM) was collected and centrifuged at 300 ×gfor 10 minutes, 1000 ×gfor 10 minutes, and 10,000 ×gfor 30 minutes.After filtration through a 0.2-µm filter to remove cellular debris and impurities, the supernatant was centrifuged at 15,000 ×gfor 90 minutes.Finally, the exosome precipitate was resuspended in 200 µL sterile PBS and stored at –80°C.All centrifugation steps were carried out at 4°C (Zavattiet al., 2020).
Nanoparticle tracking analysis and particle size analysis
The exosomes collected from fibroblasts and Schwann cells were diluted to 1 × 109/mL in PBS and then injected into a NanoSight 500 (Version 2.2;NanoSight, Wiltshire, UK) using a 1-mL syringe to capture the Brownian motion of the exosomes.Subsequently, the nanoparticle diameters and nanoparticle concentrations were calculated using the Stoke-Einstein equation (Sancho-Albero et al., 2019).
Transmission electron microscopy
The exosomes collected from fibroblasts and Schwann cells were diluted in sterile PBS (Sigma-Aldrich), then dropped onto a Formvar®-coated copper grid (150 Mesh, Ted Pella Inc., Redding, CA, USA) and fixed with 4%paraformaldehyde (Invitrogen).After drying under an infrared lamp, the grids were stained with 2% uranyl acetate (Invitrogen) containing 0.7 M oxalates(Invitrogen) at pH 7.0.Then, the morphology and structure of the exosomes were observed using a Philips 201 transmission electron microscope (Philips/FEI Inc., BriarcliffManor, NY, USA; Aziz et al., 2018).
Western blotting
Proteins isolated from the exosomes collected from fibroblasts and Schwann cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred to a nitrocellulose membrane (Bio-Rad,Hercules, CA, USA).The membrane was blocked with 5% non-fat dry milk (BBI Life Sciences, Shanghai, China) at room temperature for 2 hours with slow shaking.Next, the membrane was washed with TBST to remove the residual non-fat dry milk and incubated overnight at 4°C with an anti-CD63 antibody(rabbit, 1:1000, Abcam, Cat# ab134045, RRID: AB_2800495) and an anti-CD9 antibody (rabbit, 1:1000, Abcam, Cat# ab236630, RRID: AB_2922400).Then,the membrane was incubated with an HRP-conjugated Affinipure Goat Anti-Rabbit IgG (H&L) (1:5000, Proteintech, Chicago, IL, USA, Cat# SA00001-2,RRID: AB_2722564) antibody at room temperature for 3 hours.The protein signals were visualized using an enhanced chemiluminescence detection system (Beyotime Biotechnology, Shanghai, China) and captured with a digital chemiluminescence scanner (C-Digit, LI-COR Biosciences, Lincoln, NE, USA).The relative intensities of the immunoblot bands were quantitated using Image Studio software (version 5.2, LI-COR Biosciences; Wang et al., 2019).
RNA-sequencing analysis
Several biological replicates of this study were performed, and the bestquality FC-EXOs and SC-EXOs were selected for RNA-sequencing analysis.The RNA integrity was determined using an Agilent 2200 Bioanalyzer (Ruibo,Guangzhou, China), and all samples had an RNA integrity index greater than 9.Next, the RNA-seq library was prepared according to the following steps:reverse transcriptional synthesis of double-stranded DNA, terminal repair,sequencing linker linking, PCR amplification, and library quality control.Subsequently, RNA-seq library sequencing was carried out by Guangzhou Ruibo Biotechnology Co., Ltd.using sequencing mode was PE150 (Ruibo).Ultimately, we identified 40,155 genes, including mRNAs, lncRNAs, circular RNAs, and miRNAs.
Data processing and differential expression analysis
First, we normalized the expression levels of the 26,504 FC-EXOs genes and 33,505 FC-EXOs genes using the limma package in the R software (https://www.r-project.org/; Ritchie et al., 2015).Then, the limma package in R software was used to identify differentially expressed genes in terms of foldchange (FC; Fibroblast relative to Schwann cell) and to draw the corresponding heatmaps and volcano maps.The thresholds used were as follows:| logFC |>2(Ambroise et al., 2011) andP< 0.05 (Perneger and Combescure, 2017).
Gene function and pathway enrichment analysis
mRNAs (C>20) from Schwann cells and fibroblasts were divided into five groups (Figure 1) by intersectional analysis (http://bioinformatics.psb.ugent.be/webtools/Venn/), including the Fibroblast group, the Schwann cell group,the Unique FC∩SC group, the Unique fibroblast group, and the Unique Schwann cell group.Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed for the five groups of mRNAs,as well as the predicted mRNAs by miRwalk predictive database, using the cluster profile R package and DAVID database (https://david.ncifcrf.gov/;Sherman et al., 2022).Gene function was assessed by GO terms, including the following three aspects: biological process (BP), molecular function (MF),and cellular composition (CC) The KEGG analysis was performed to identify possible pathways, and the corresponding bar and bubble plots were drawn in R software.In the diagrams, color is closely related to theP-value of each functional pathway, and the length of the line or the size of the dot represents the number of genes involved.The cut-offvalue was set atP< 0.05.As thePvalue decreased, the result became more significant; and as the count increased, the more genes corresponded with each GO or KEGG term (Jiang et al., 2020).
Protein-protein interaction
Genes from the Fibroblast group, the Schwann cell group, and the Unique FC∩SC group were uploaded to the online database STRING (https://stringdb.org/; Szklarczyk et al., 2021), the species was set asRattus norvegicus, and the interaction score was adjusted to 0.9 (Szklarczyk et al., 2019).Proteinprotein interaction (PPI) networks constructed using the STRING database were further processed using cytoHubba in Cytoscape software 3.9.0 (https://cytoscape.org/; Shannon et al., 2003).A module analysis was conducted using the MCODE plug-in in Cytoscape to screen for hub genes and function modules based on the interaction score.Among the 11 topological analysis methods included in cytoHubba, the Degree method (Chin et al., 2014) was selected to score and rank the interactions among genes.
miRNA naming and ceRNA network construction
To facilitate subsequent systematic analysis, miRNA names were assigned based on the NCBI (https://www.ncbi.nlm.nih.gov/gene/) and miRBase(https://www.mirbase.org/) databases (Kozomara et al., 2019).According to the ceRNA theory, lncRNAs may target miRNAs to induce the release of specific mRNAs, thereby leading to upregulation of the expression of that mRNA.To identify the target mRNA (Rps5), first, we used Cytoscape to select the top 50 most significantly differentially expressed mRNAs from the(Hub 50-fibroblast) ∪ (Hub 50-Schwann cells) group and screened for the hub gene based on the highest score in the Degree topology analysis.Next,40 miRNAs that could regulate rps5 were identified using miRwalk (http://mirwalk.umm.uni-heidelberg.de/; Sticht et al., 2018).Then, we used StarBase(http://starbase.sysu.edu.cn/) to predict the upstream lncRNAs of these 40 miRNAs and determine which of these lncRNAs appeared in the FC-EXO and SC-EXO sequencing results (Li et al., 2014).Finally, Cytoscape software (https://cytoscape.org/; Shannon et al., 2003) was used to visualize the ceRNA network.
Statistical analysis
FPKM was used to normalize gene length and library size using R language.Quantitative results were analyzed for significance by one-way analysis of variance and Dunnett’s multiple comparison test.Genes obtained from the sequencing data were screened and processed three times using SPSS 25.0(IBM, Armonk, NY, USA) to eliminate errors and omissions.For example, the fibroblast (C>20) group and Schwann cell (C>20) group included proteincoding genes whose expression levels were greater than 20 inFC-EXOs and SC-EXOs, respectively.Differences were considered significant atP< 0.05.
Results
Acquisition and identification of exosomes
To determine whether exosomes derived from fibroblast and Schwann cells can stimulate axonal regeneration, we purified exosomes from the conditioned medium of fibroblasts and Schwann cells, respectively.As shown in the flowchart in Figure 2A, primary cells were isolated from 1-week-old SD rats, and culturedin vitro.When the cell density reached 80%, the exofree serum medium was replaced with fresh medium and the cells were cultured for another 48 hours, after which the supernatant was collected to purify the exosomes.Immunofluorescence was used to assess the purity of the cells.The fibroblast cells showed strong positive expression of CD90, with a cell purity of 90% (Figure 2B).Similarly, the Schwann cells showed strong positive expression of S100 and 85% purity (Figure 2C).The CD90+fibroblasts were shaped like shuttles or irregular triangles, with an oval nucleus in the center and cytoplasmic protrusions that grew radially (Figure 2D).The nuclei of the S100+Schwann cells were oval, with the long axis parallel to the axon,and there was little cytoplasm around the nucleus (Figure 2E).Thus, we considered the status and density of these two cell types adequate for use in subsequent experiments.Under transmission electron microscopy, FCEXOs presented a typical round or oval cup-mouth structure (Figure 2F).As shown in Figure 2G, nanoparticle tracking analysis showed that the average size of the FC-EXOs was between 100 and 150 nm.In addition, western blot verified the expression of exosome markers and showed that CD9 and CD63 were significantly expressed in FC-EXOs (Figure 2H).The SC-EXO identification results were consistent with FC-EXO results, suggesting that both types of exosomes could be used for subsequent sequencing analyses.
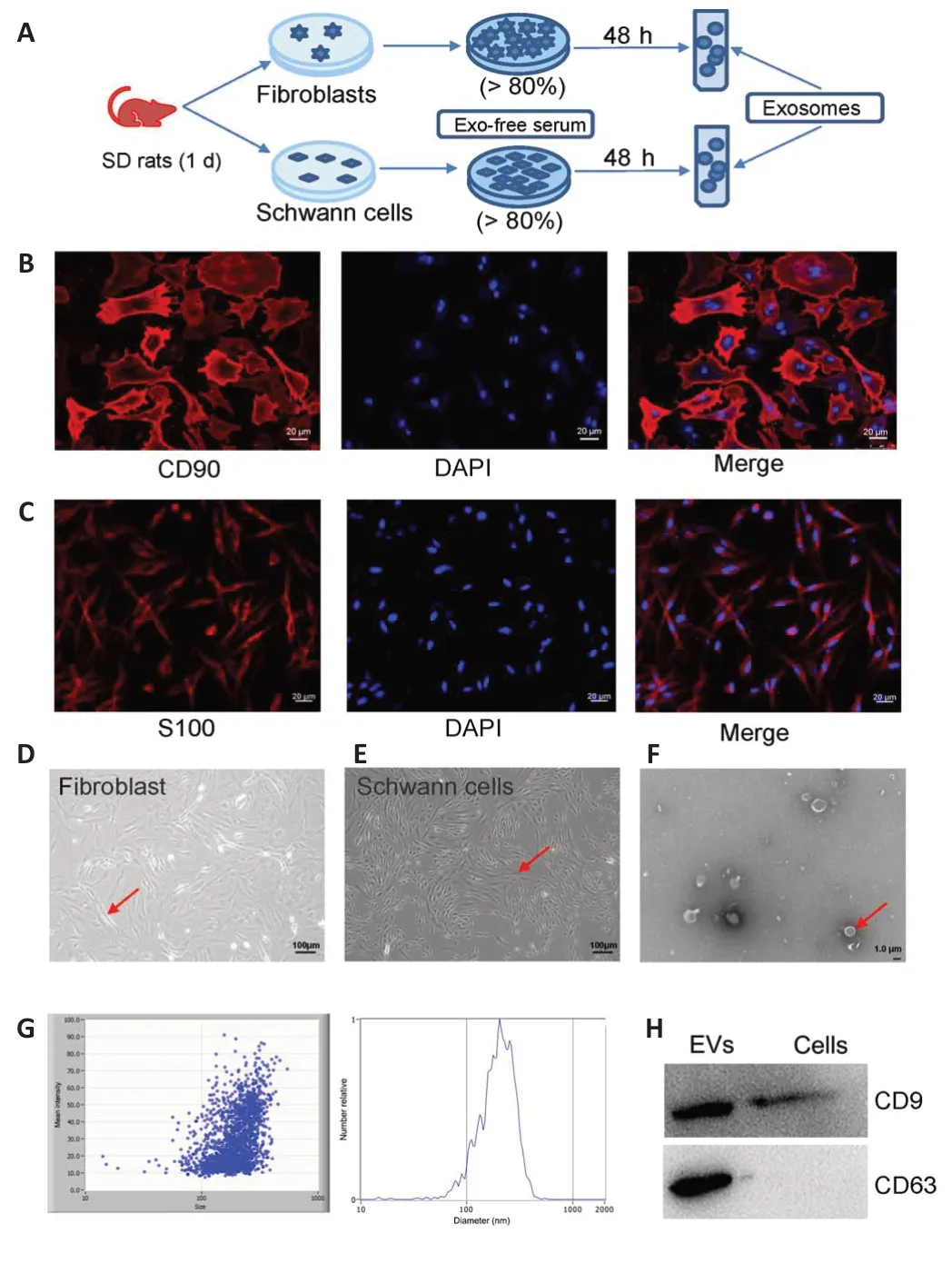
Figure 2 | Immunofluorescence detection of CD90+ fibroblast cells and S100+Schwann cells.
Screening and grouping of FC-EXO and SC-EXO mRNAs
To understand the functions and mutual regulatory mechanisms of FCEXOs and SC-EXOs in axon regeneration, RNA-seq was performed, yielding 26,504 genes in the FC-EXOs and 33,505 genes in the SC-EXOs, which were normalized before further analysis.On the basis of the proportions of different gene types, and considering that mRNAs accounted for half of the total number of genes identified from the RNA-seq analysis, we selected 17,907 fibroblast mRNAs and 20,021 Schwann cell mRNAs for further analysis.Next, we plotted the expression distribution of the mRNAs using SPSS (Figure 3A and B).Applying the cut-offcriterion of a gene expression level greater than 20 counts, yielded a list of 1663 FC-EXOs mRNAs (Figure 3C) and 1189 SC-EXOs mRNAs (Figure 3D).Finally, these mRNAs were divided into the Fibroblast (>20) group, Schwann cell (>20) group, Unique Schwann cell group (>20), Unique FC^SC (>20) group, and Unique Fibroblast (>20) group by intersection analysis (Figure 3E).The series of systematic bioinformatics analyses described below was conducted to identify the functions and regulatory mechanisms of exosomes derived from fibroblasts and Schwann cells.We believe that this will provide new insight into the potential therapeutic use of multi-source exosomes to promote axon regeneration.
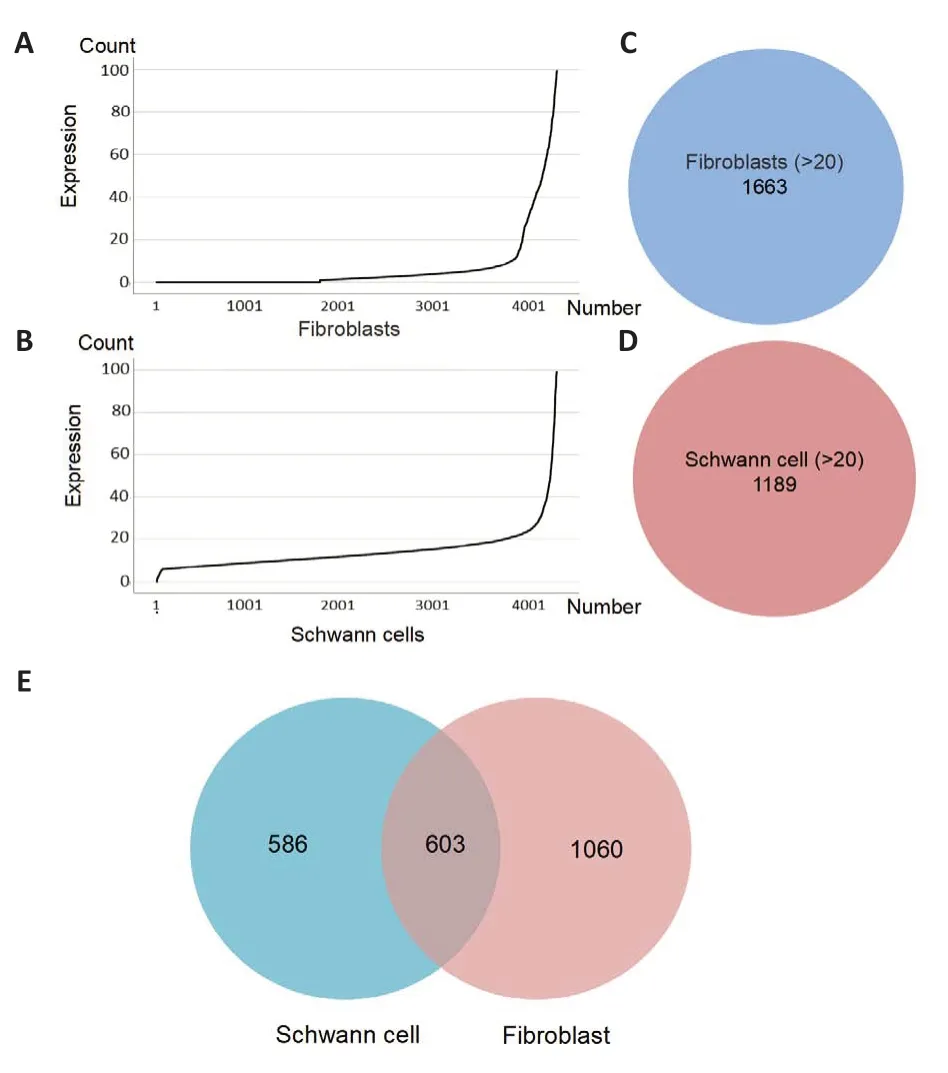
Figure 3 | Gene expression distribution, as well as preliminary screening andgrouping.
Identification of differentially expressed genes and functional analysis of FC-EXOs and SC-EXOs
To obtain a preliminarily understanding of the important functions carried out by FC-EXOs and SC-EXOs, we used Limma packets (|log2FC|> 2 andP-value <0.05) to analyze genes that were differentially expressed in FC-EXOs compared with SC-EXOs.Figure 4A and B shows the 152 up-regulated mRNAs and 349 down-regulated mRNAs in the form of a volcano map and heatmap, where the gray dots represent genes with no difference in expression, the red dots represent genes that were up-regulated in the Fibroblast group, and the blue dots represent genes that were down-regulated in the Fibroblast group.Next,to predict potential biological functions and identify axonal regenerationrelated molecular pathways in which these differentially expressed genes(DEGs) were involved, we performed KEGG functional enrichment analysis using the DAVID database; aP-value < 0.05 was considered statistically significant.The KEGG analysis results showed that the relatively significantly up-regulated DEGs were significantly enriched in Amyotrophic lateral sclerosis, Thyroid hormone synthesis, Cardiac muscle contraction, Apoptosis,and Adrenergic signaling in cardiomyocytes (Figure 4C).Furthermore, the significantly down- genes were enriched in ECM-receptor interaction, Inositol phosphate metabolism, Non-homologous end-joining, Neuroactive ligandreceptor interaction, Axon guidance, Calcium signaling pathway, Metabolic pathways, Other glycan degradation, Protein digestion and absorption, ABC transporters, and many more (Figure 4D).Next, KEGG enrichment analysis was carried out for the Unique fibroblast group (Figure 4E) and the Unique Schwann cell group (Figure 4F) to rule out differences between different algorithms.Comparison analysis showed that the results were consistent with the functional enrichment results for the differentially expressed genes.For example, the DEGs in the Unique fibroblast group were closely related to heterogeneous protein-related diseases such as neurodegenerative lesions,as well as proteolysis and apoptosis, and the DEGs in the unique Schwann cell group were associated with energy metabolism.Certain FC-EXOs can promote nerve and blood vessel regeneration and reduce scarring (Zhang et al., 2021).Schwann cells (SCs) transfer ribosomes to axons and enhance regeneration through extracellular vesicles called exosomes, which contain mRNA, miRNAs, and proteins.In addition, Schwann cells and fibroblasts have mutually regulatory effects (Zhao et al., 2022).Therefore, we hypothesized the existence of a regulatory network that can promote continuous axon regeneration and reduce scarring by regulating both FC-EXOs and SC-EXOs.
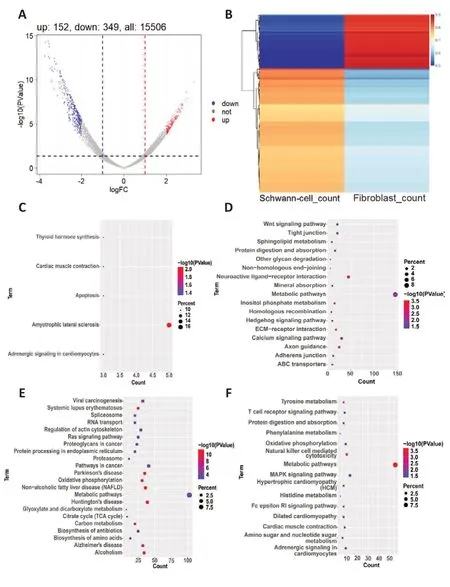
Figure 4 | Identification of differentially expressed genes (DEGs) and function analyses.
Functional enrichment analysis of the Fibroblast, Schwann cell, and unique FC∩SC groups
The first top terms for each biological function, including BP, CC, and MF,are displayed in Figure 5A–C.GO analysis of the genes from the Fibroblast group (Figure 5A) identified biological functions (BPs) related to translation,substantia nigra development, cell-cell adhesion, regulation of translational initiation, nucleosome assembly, aging, ATP synthesis-coupled proton transport, formation of translation preinitiation complex, hydrogen ion transmembrane transport, and liver regeneration.The molecular functions(MFs) of these genes included extracellular exosome, focal adhesion,cytoplasm, myelin sheath, nucleus, membrane, mitochondrion, mitochondrial inner membrane, extracellular matrix, and cytosol.Finally, the terms enriched in the MF category included poly(A) RNA binding, structural constituent of ribosome, protein binding, protein complex binding, NADH dehydrogenase(ubiquinone) activity, cadherin binding involved in cell-cell adhesion, mRNA binding, unfolded protein binding, actin filament binding, and translation initiation factor activity.Similarly, in the Schwan cells group (Figure 5B), the enriched GO functions were related to immune response, response to drugs,fatty acid transport, ventricular cardiac muscle tissue morphogenesis, negative regulation of protein ubiquitination, synaptic transmission, glutamatergic,skeletal muscle contraction, response to insecticide, and regulation of muscle contraction in the BP category; extracellular region, extracellular space, axon,cytoplasm, troponin complex, extracellular exosome, mitochondrion, keratin filament, collagen trimer, and endoplasmic reticulum in the CC category; and pyridoxal phosphate binding, G-protein-coupled receptor binding, copper ion binding, troponin T binding, structural molecule activity, enzyme binding,growth factor activity, hormone activity, peptidase inhibitor activity, and calcium-dependent protein binding in the MF category.To compare and explore the mechanisms in both FC-EXOs and SC-EXOs, we also performed GO functional enrichment analysis of the Unique FC∩SC group (Figure 5C) and found enrichment in translation, liver regeneration, ATP synthesis-coupled proton transport, cytoplasmic translation, ribosomal small subunit assembly,rRNA processing, response to ethanol, aging, hydrogen ion transmembrane transport, and ribosomal small subunit biogenesis in the BP category;extracellular exosome, focal adhesion, membrane, cytosolic large ribosomal subunit, myelin sheath, ribosome, cytoplasm, cytosolic small ribosomal subunit, mitochondrion, and nucleus in the CC category; and poly(A) RNA binding, structural constituent of ribosome, protein binding, mRNA binding,RNA binding, actin filament binding, large ribosomal subunit rRNA binding,large ribosomal subunit rRNA binding, proton-transporting ATP synthase activity, rotational mechanism, and translation initiation factor activity in the MF category.Subsequently, as can be seen in Figure 5D–F, the results from the KEGG enrichment analyses were visualized to identify molecular pathways related to axon regeneration.On the basis of KEGG analysis of the fibroblast(Figure 5D), Schwann cell (Figure 5E), and unique FC∩SC (Figure 5F) groups,the functions enriched in both FC-EXO and SC-EXO DEGs were closely related to ribosomes.This finding further confirms our hypothesis that SC-EXOs and FC-EXOs likely perform their functions or regulate each other through a shared set of ribosomal genes.
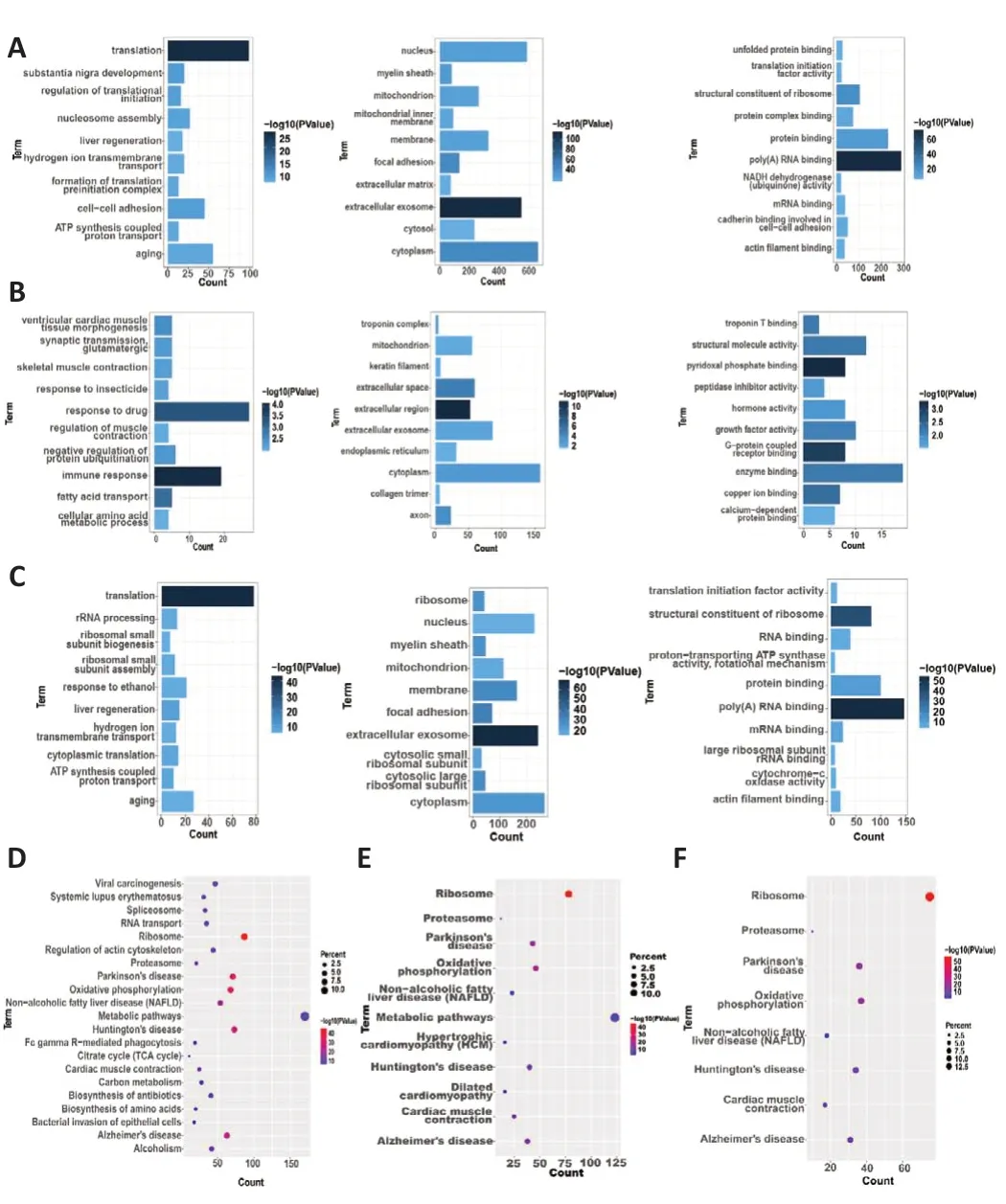
Figure 5| Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO)functional annotations indicating a common mechanism.
Establishment and optimization of PPI networks for candidate genes
On the basis of the results from the STRING database analyses and followed by optimization with the cytohubba plugin in Cytoscape, the PPI networks for the Fibroblast group, the Schwann cell group, and the Unique FC∩SC group, each of which contains the top 50 genes, were constructed, as shown in Figure 6A–C.For better visualization, Cytoscape software was used to reconstruct the concentric circles based on the interaction networks of the top 50 participants in each group (Figure 6D–F).According to the scores calculated using the Degree plugin, as the dot becomes redder and closer to the center, the score increases, and vice versa.In the end, 10 genes in the innermost circle of each concentric circle were selected as important candidate genes, including Uba52, Rps5, Rpl22, Rps8, Rpl18, Rpl11, Rps23,Rps3, Rpl4, and Rack1 in Fibroblast group (Table 1); Uba52, Rps5, Rpl22,Rpl18, Rpl11, Rps23, Rps8, Rpl4, Rps3, and Rpl5 in Schwann cell group (Table 2); and Uba52, Rps5, Rpl22, Rpl18, Rpl11, Rps8, Rps23, Rps3, Rpl4, Rpl5, and Rpl18a in the Unique FC∩SC group (Table 3).The important candidate genes in each group clearly belong to the ribosome family of genes.This provides preliminary supporting our hypothesis that FC-EXOs and SC-EXOs may promote axon protein synthesis and inhibit scar formation through certain ribosomal genes.However, the mechanism still remained unclear.
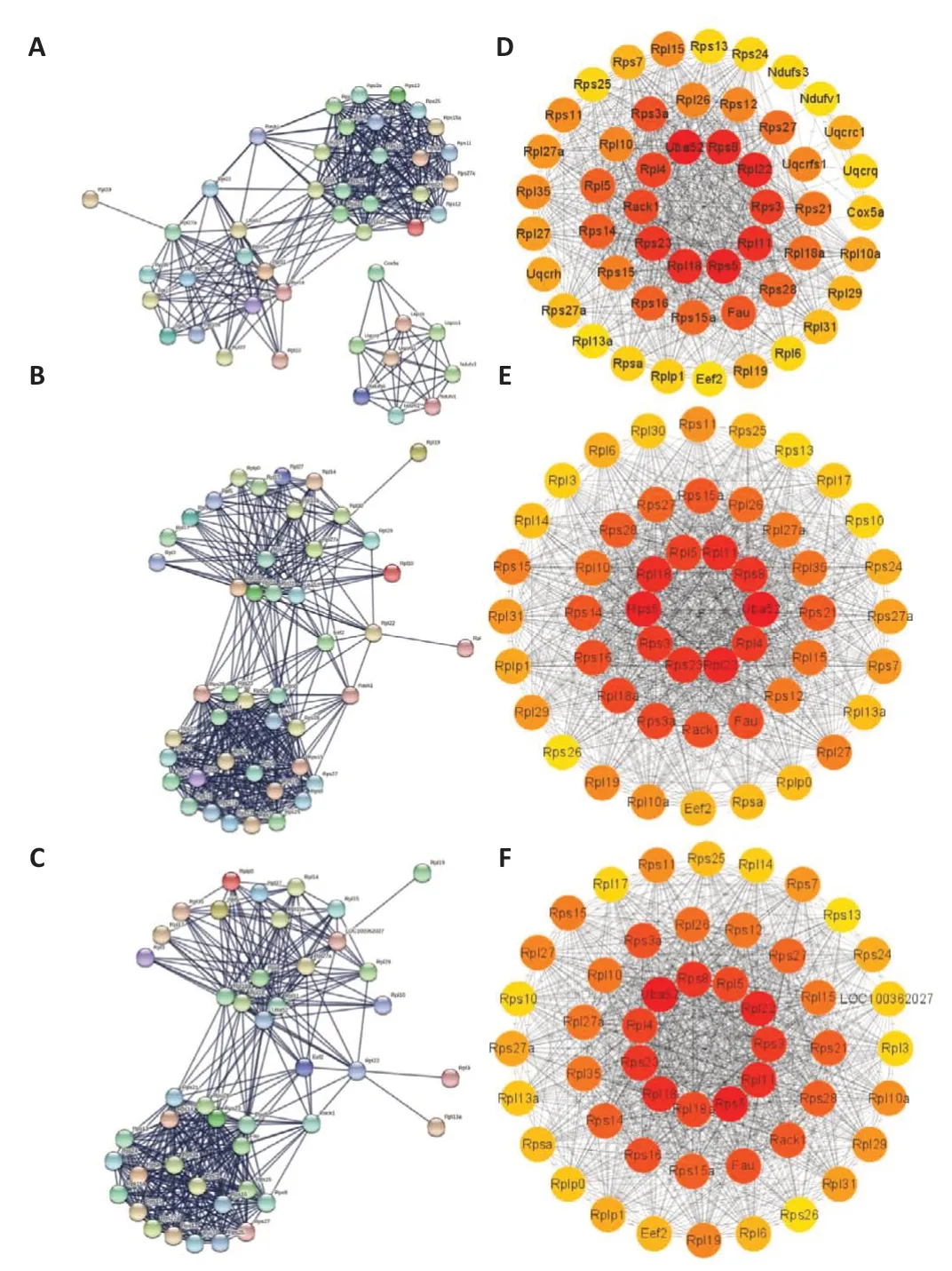
Figure 6 | Significant genes identified from protein-protein interaction network (PPI)networks constructed based on Degree topology.
Identification of FC-EXO and SC-EXO hub genes
Taking into account that exosomes from different sources not only perform their primary functions first but also affect each other, we next constructed a PPL interaction network incorporating the top 50 genes from both the Fibroblast and the Schwann cell groups to identify a common hub gene(Figure 7A).According to the degree score in cytohubba, the reddest and largest dot in Figure 7A, which represents rps5, is the hub gene; and Rps5 was also identified as an important candidate gene in various groups.To perform a preliminarily exploration of the main functions of these ribosomal genes, we applied MCODE in Cytoscape to identify the biofunction module(Figure 7B).The pink dots indicate genes that were both expressed and functional in FC-EXOs and SC-EXOs.In addition, the network contained genes from the RPL and RPS families, which encode ribosomal proteins, and the genes from the Cox family, such as cox1 and cox5b, which are associated with promoting proliferation and inhibiting apoptosis.Interestingly, both ofthe genes that were only expressed in FC-EXOs were ribosome-associated genes.For example, the Gnb2l1 gene that we identified in FC-EXOs drew our attention because it is a highly conserved gene that encodes a protein that binds to the 40S ribosome subunit, suggesting that it links cell regulation and translation (Gibson, 2012).It has been suggested that Gnb2l1 can transport proteins to their sites of action, promote crosstalk between different signaling pathways, or recruit other signaling proteins into complexes (Buoso et al.,2017).As such, it is a key mediator in a variety of pathways and is involved in a variety of biological events, including brain activity and abnormal apoptosis(Calura et al., 2008).These reports further validate our hypothesis that FCEXOs primarily function in axon regeneration by promoting cell proliferation and differentiation, regulating abnormal apoptosis, and inhibiting scarring.The green dots at the bottom of the network diagram account for most of the modules, suggesting that the network adequately represents the SCEXO functional modules.In addition to ribosome-related genes, this module contains a large number of mitochondria-related genes, such as Ndufs8,Ndufa4, and Ndufv3, which are involved in formation of the mitochondrial complex.Because mitochondria provide energy for the cell, and ribosomes synthesize proteins, after mitochondria oxidize and break down organic matter for energy, a part of this energy will be supplied to the ribosomes for protein synthesis and exosome delivery.This also supports our hypothesis that Schwann cells provide ribosomes and energy to damaged axons through exosomes, promoting protein synthesis and accelerating axon regeneration.Notably, RPS5 remains the hub gene with the highest degree interaction score, suggesting that rps5 may regulate the functions of exosomes derived from different cellular sources to promote axon regeneration.Rps5 is closely related to ribosome formation, effective protein translation, and cell growth and proliferation (Ghosh et al., 2014; Vizirianakis et al., 2015; Tomioka et al.,2018).Interestingly, Rps5 also plays a significant role in suppressing abnormal fibrosis (Xu et al., 2014; Li et al., 2021), which is consistent with the multiple functions identified in the functional module analysis.All of these reports strongly support our finding that rps5 is a common link among exosomes from different cellular sources that promotes axon regeneration by providing ribosomes, promoting energy metabolism, and assisting protein synthesis.These findings may lead to a breakthrough in resolving scar hyperplasiamediated restriction of axon regeneration.Therefore, we next explored the effect of FC-EXO- and SC-EXO-derived Rps5 on axons, as well as the upstream regulatory mechanism of Rps5.
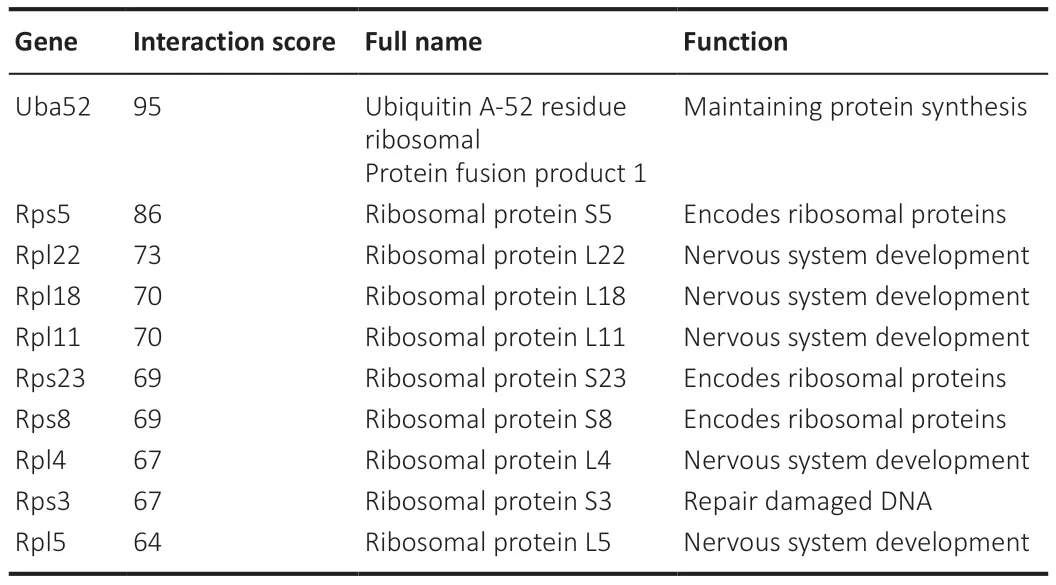
Table 1 | Functional roles of the top 10 hub genes in the Fibroblast group, as ranked by the Degree method
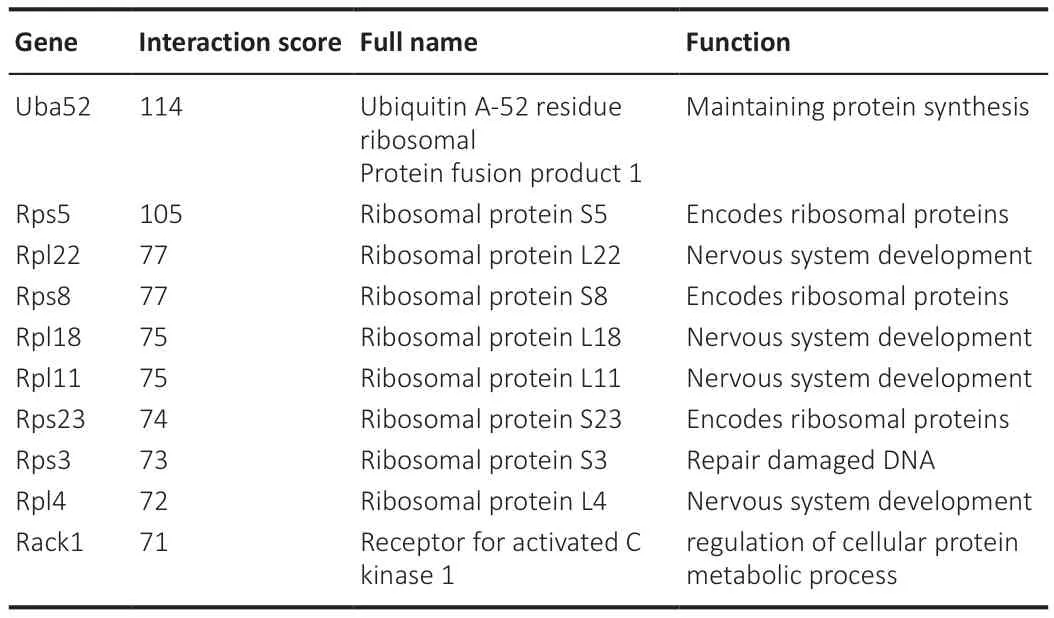
Table 2 |Functional roles of the top 10 hub genes in the Schwann cell group, as ranked by the Degree method

Table 3 |Functional roles of the top 10 hub genes in the unique FC∩SC (theintersection of the Fibroblast group and the Schwann cell group) group, as ranked by the Degree method
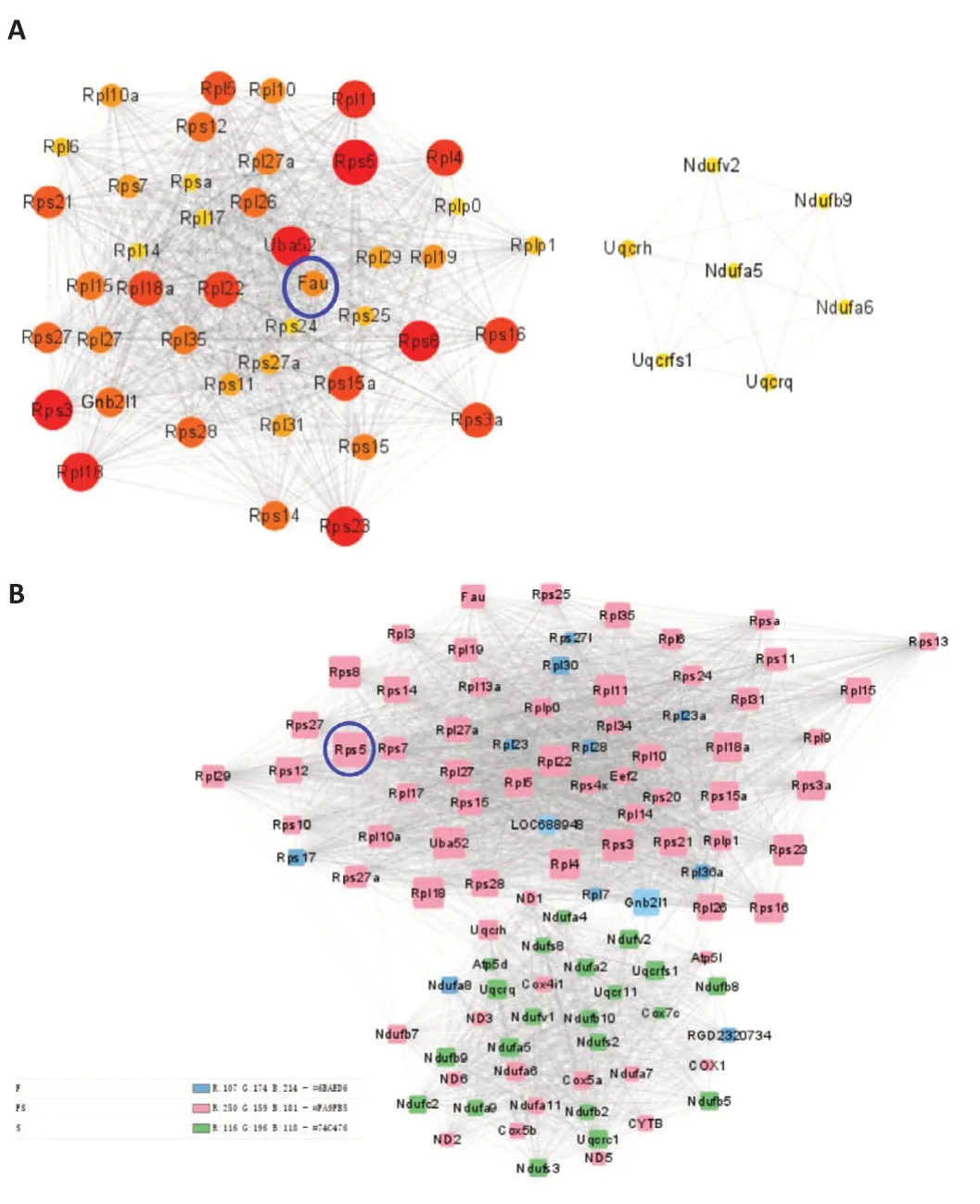
Figure 7 | Identification of the hub gene Rps5 based on Degree topology and module analysis.
Regulating Rps5 expression in fibroblasts and Schwann cells in vitro affects DRG neuron axon growth
To determine the effect of regulating Rps5 expression in fibroblasts and Schwann cells on axon regeneration, we established an indirect contact coculture model of fibroblasts or Schwann cells with DRG neurons (Figure 8A and B).First, we transfected fibroblasts and Schwann cells with a genespecific siRNA to down-regulate Rps5 expression.qRT-PCR analysis showed that, compared with the control siRNA group, the gene-specific siRNA effectively reduced Rps5 expression in fibroblasts and Schwann cells.On the basis of these results, we selected siRps5-3 for further co-culture experiments(Figure 8C).In the co-culture experiment, we measured the axon length of DRG neurons by immunofluorescence 24 hours after indirect contact with fibroblasts or Schwann cells in which Rps5 expression had been knocked down (Figure 8D and E).The average total length of DRG neuron axons in the experimental group co-cultured with fibroblasts was 450.77 µm, and the average of the longest axons on each cellwas 193.42 µm; the average total axon length of DRG neurons in the control group was 711.65 µm, and the average length of the longest axon was 299.22 µm (Figure 8F).The average length of DRG neuron axons in the experimental group co-cultured with Schwann cells was 298.40 µm, and the average length of the longest axons was 123.04 µm; the average total axon length of DRG neurons in the control group was 898.18 µm, and the average longest axon length was 231.76 µm(Figure 8G).In summary, down-regulation of Rps5 expression in fibroblasts or Schwann cells significantly inhibits axon growth in co-cultured DRG neurons.
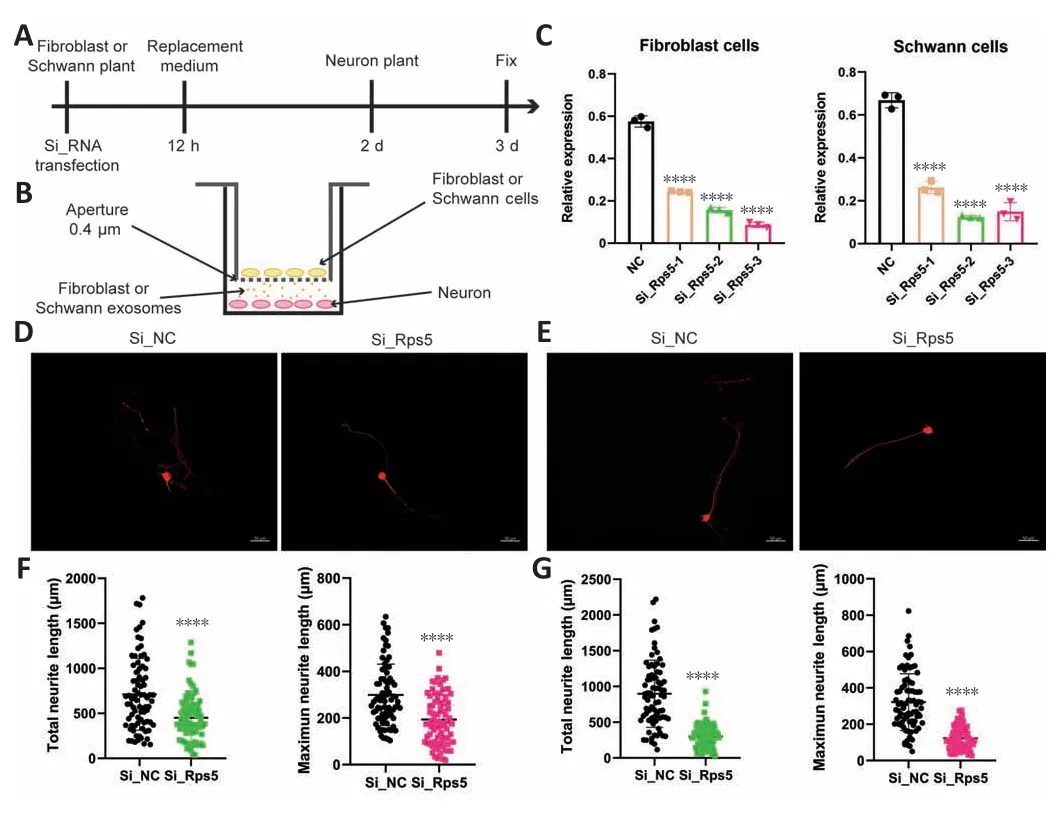
Figure 8 |Rps5 expression in exosomes derived from fibroblasts and Schwann cells in vitro can effectively promote axon regeneration in dorsal root ganglion (DRG)neurons.
Database prediction identified five lncRNA-miRNA-Rps5 axes
Recently, a growing body of evidence has revealed the importance of ceRNA networks in biological processes, and numerous studies have emphasized that lncRNAs can act as ceRNAs by binding to miRNAs, thereby influencing and regulating the expression of the target mRNAs, enabling large-scale control of the transcriptome.However, our current understanding of lncRNA-miRNAmRNA regulatory networks during axon regeneration after peripheral nerve injury is not complete.Given that we identified rps5 as a hub gene in FC-EXOs and SC-EXOs, we therefore sought to identify miRNAs that regulate rps5.As shown in Figure 9A, a total of 40 miRNAs that were the most likely to target Rps5 were identified with the help of the miRWalk prediction database.The miRNA-mRNA (Rps5) regulatory network is shown in Figure 9B: the blue nodes represent miRNAs, and the pink nodes represent Rps5.Next, using the starbase database, we identified 192 lncRNAs that were predicted to be upstream of the 40 miRNAs predicted to target Rps5.Comparing this list of lncRNAs with the list of 6941 lncRNAs obtained from the exosome sequencing results, we identified five lncRNAs that were expected to competitively bind Rps5-targeting miRNAs, including Jpx, Ftx, Pvt1, H19, and Miat.For better visualization, Jpx-miRNA, Ftx-miRNA Pvt1-miRNA, H19-miRNA, and MiatmiRNA sub-networks were constructed (Figure 9C–G, green represents miRNAs, and blue represents lncRNAs).These lncRNAs are expected to regulate Rps5 function and could become new targets for multi-exosome combination therapy.
Construction of a ceRNA regulatory network targeting Rps5
Next, we generated pie charts based on the expression of the five lncRNAs identified as described above––Jpx, Ftx, Pvt1, H19, and Miat––showing their respective proportions in FC-EXOs and SC-EXOs (Figure 10A and B).The lncRNA-miRNA-Rps5-ribosome network (Figure 10C) established based on possible upstream regulatory mechanisms and downstream regulatory pathways includes five lncRNAs, 27 miRNAs, and one hub mRNA; in the figure, the blue dots represent lncRNAs, the plum red dots represent miRNAs,the gold dots represent the hub gene Rps5, and the green dots represent significantly enriched functional pathways and that is the ribosomes functional pathway.This network may provide effective combinatorial targets to optimize the functions of exosomes from different sources, promote sustained axonal regeneration, and provide new insights into the molecular mechanisms of axonal regeneration after peripheral nerve injury.However,whether each ceRNA axis plays a role in promoting axonal regeneration and inhibiting scar formation remains to be verified.
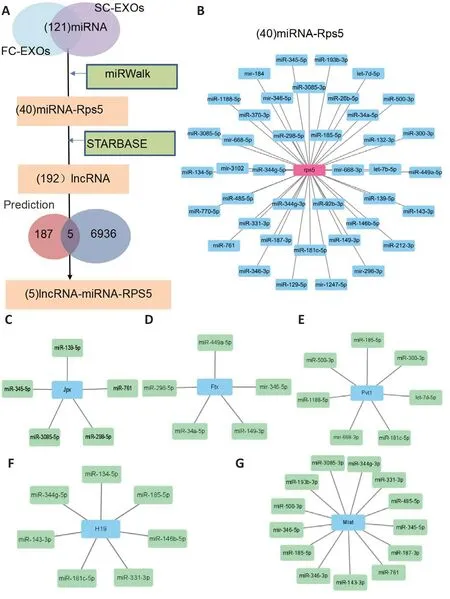
Figure 9 |Prediction of the lncRNA-miRNA-Rps5 regulatory network.
Functional verification of lncRNA-Fxt- and lncRNA-Miat-mediated regulation of target gene expression
lncRNA-Miat overexpression has been reported to improve cell viability,inhibit cell apoptosis (Li et al., 2019a) and oxidative stress (Xu et al., 2021),and promote axonal regeneration through sponge adsorption, thus playing a neuroprotective role (Qi et al., 2021; Shen et al., 2021).In addition, lncRNAMiat may help inhibit fibrosis (Shao et al., 2021).However, few studies have reported on changes in lncRNA-Miat expression in exosomes.In addition,we were surprised to find that lncRNA-Ftx has been reported to play a particularly significant role in promoting axonal growth (Zuo et al., 2020).For example, the lncRNA-Ftx ceRNA axis significantly inhibits neuronal apoptosis(Li et al., 2019b), regulates astrocyte proliferation, and inhibits the formation of glial scars in the damaged spinal cord (Xiang et al., 2021).Therefore, we speculated that the Ftx-miRNA-Rps5 and Miat-miRNA-Rps5 axes mediate FCEXO- and SC-EXO-induced promotion of axonal regeneration by promoting cell proliferation, targeting the delivery of ribosomes, promoting energy metabolism, inhibiting scar formation, and promoting targeted differentiation of neurons.To explore this possibility, we analyzed the miRNAs by Ftx and Mia in FC-EXOs and SC-EXOs using R software.As shown in Figure 11A,Fxt regulates 9733 genes, including Rps5, through miR-449a-5p, miR-346-5p, miR-149-3p, miR-34a-3p, and miR-298-5P.GO analysis showed these genes participated in dendrite development, axonogenesis, and dendrite morphogenesis.As Figure 11B shows, Mait regulates 2374 genes, including the hub gene Rps5, through miR-344g-3P, miR-331-3P, miR-485-5p, miR-345-5p, miR-187-3P, miR -761, miR-143-3P, miR-346-3p, miR-185-5p, miR-346-5p, miR-500-3p, miR-193b-3p, and miR-3085-3p.In addition, Mait targets are enriched in GO terms like dendrite development, dendrite morphogenesis,and axonogenesis.Thus, our findings suggest that both Fxt and Miat can be secreted via exosomes and promote axonal regeneration.KEGG pathway analysis showed that Fxt (Figure 11C) and Miat (Figure 11D) are closely related to axon guidance.The relationship between these two lncRNAs and a single function is likely because the regulatory role of a single ceRNA axis is limited and does not fully exploit the potential of FC-EXOs and SC-EXOs(Gallaher and Steward, 2018).Therefore, the lncRNA-miRNA-mRNA ceRNA(Ftx and Miat) network can more fully exploit the potential of FC-EXOs and SC-EXOs than a single ceRNA axis to transport ribosomes to damaged axons,promote protein synthesis, accelerate axon regeneration, inhibit scarring, and promote directed differentiation of nerve fibers.
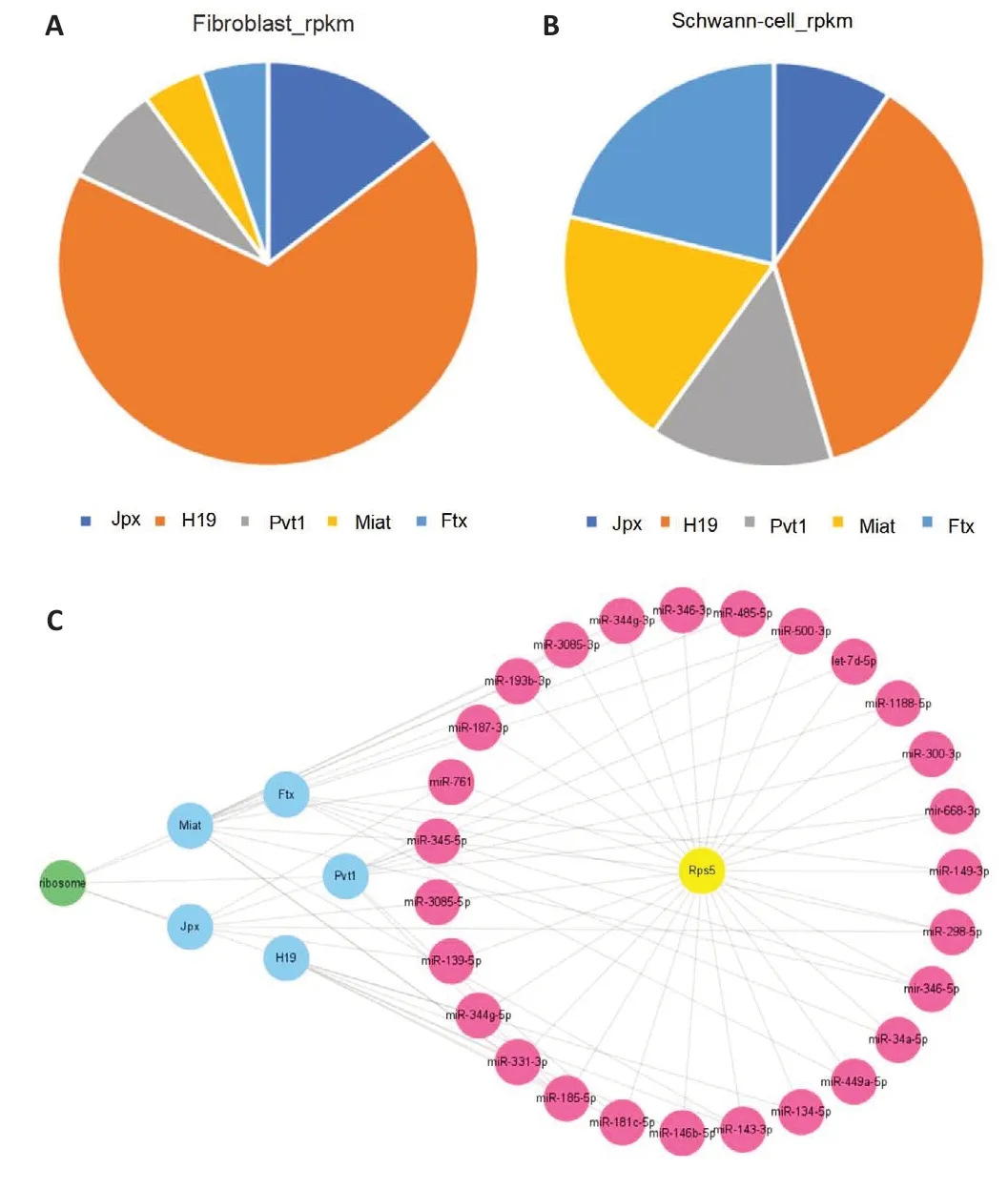
Figure 10 |Construction of a ceRNA regulatory network.
Discussion
Peripheral nerve injury is defined as temporary or permanent damage that result in functional deficits, and impaired axon regeneration is a major challenge in clinical practice.The ceRNA hypothesis proposes a novel regulatory mechanism in which lncRNAs act as endogenous miRNA sponges that competitively bind miRNAs to release the corresponding mRNAs,enabling multiple pathways to influence and regulate the expression of target genes (Yao et al., 2021; Hu et al., 2022).In this study, mRNAs with greater than 20 counts were grouped, and differentially expressed mRNAs between FC-EXOs and SC-EXOs were identified.Subsequently, the pathways and functions associated with each group were explored through GO and KEGG analyses, which showed that FC-EXOs promote cell proliferation and inhibit scar formation through certain ribosomal protein genes, while SC-EXOs regulate energy metabolism to promote protein synthesis, mainly through ribosome translocation.Constructing a PPI interaction network identified Rsp5 as a hub mRNA for both exosome types.Five lncRNAs and 27 miRNAs were identified as upstream regulators of Rps5 using the mirwalk and starbase prediction databases, and a ceRNA network containing Rps5 was constructed using Cytoscape.This ceRNA network is expected to regulate exosomes produced by fibroblasts and Schwann cells, promoting axon regeneration through up-regulation of Rps5 expression.
The functional and structural integrity of the nervous system depend on the coordinated action of neuronal and non-neuronal cells (Beh et al.,2020).Peripheral nerve regeneration involves a series of steps, including axon sprouting, elongation, and maturation (O’Brien et al., 2022).Schwann cells are the main glial cells of the PNS and play a key role in regeneration of injured nerves, as they are the main cell type responsible for axonal wrapping, myelination, and nerve repair (Yao et al., 2021).In recent years,the mechanism of axonal synthesis has become increasingly clear.A large number of axon-localized mRNAs have been identified that encode a broad range of proteins, and labeled ribosomes have been detected, although their origin remains controversial.Studies have shown that Schwann cells in the PNS provide ribosomes to axons, and that ribosomal proteins are the basis of cellular programming or function (Court et al., 2011; Müller et al., 2018).Furthermore, human fibroblasts can be converted directly into neuronal cells by treating them with a mixture of seven small molecules,bypassing the neural progenitor cell stage, which enables them to occupy the site of glial scars, reducing the formation of nerve scars (Hu et al., 2015).This dependence of axon regeneration on non-neuronal cells, especially the integration of different functions of non-neuronal exosomes on axon regeneration, is a relatively unexplored dimension of the nervous system.This evolving field may influence our understanding of axonal cell biology under normal and pathological conditions.
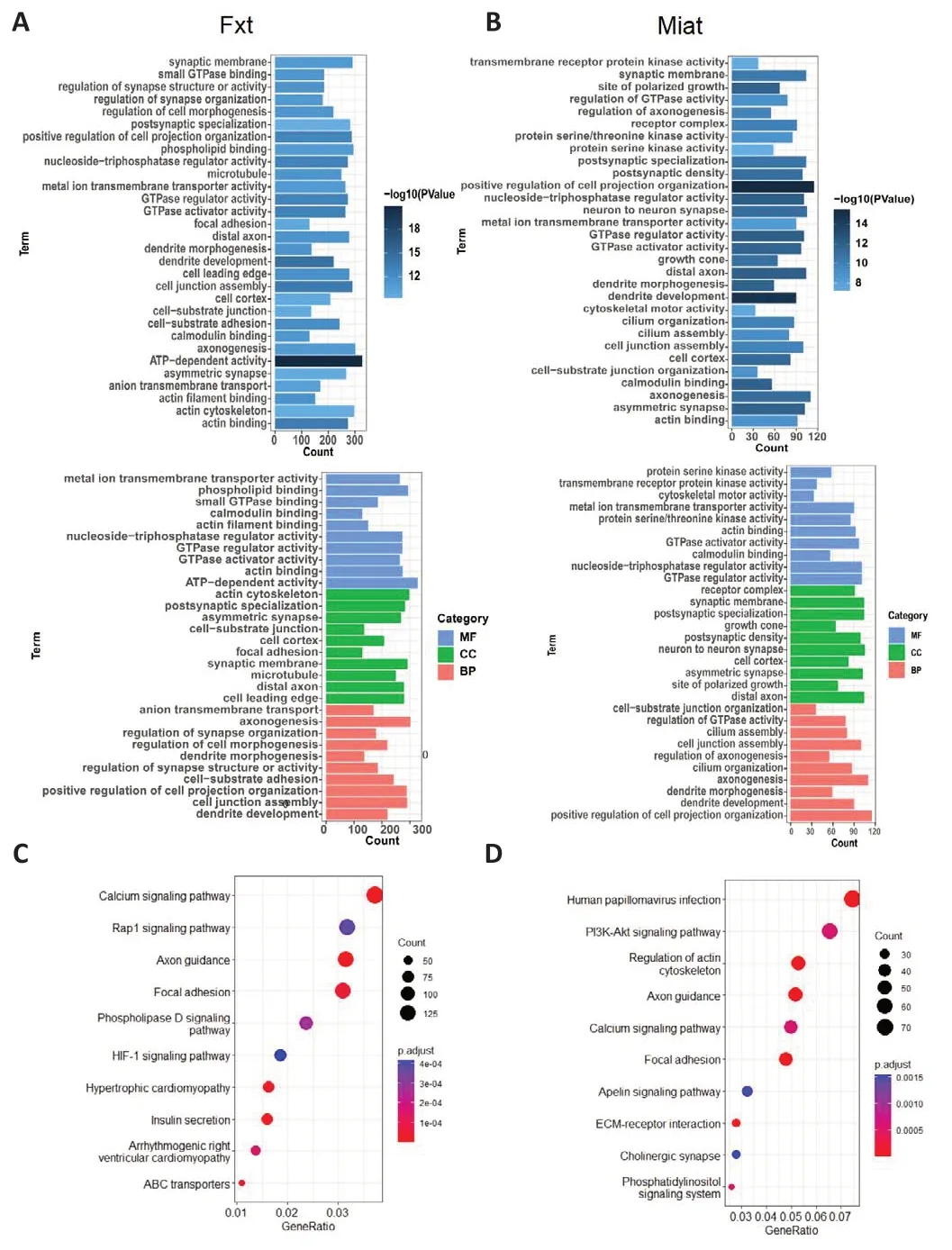
Figure 11 | Confirmatory enrichment analyses of Fxt- and Miat-targeted genes.
In this study, a series of bioinformatics analyses were performed to assess mRNAs with greater than 20 counts, and a hub mRNA (Rps5) regulating exosomes produced by fibroblasts and Schwann cells was identified.On the one hand, this suggests that the up-regulation of ribosomal protein S5 (RPS5)is the mechanism underlying exosomes -mediated inhibition of fibrogenesis.On the other hand, siRNA-mediated RPS5 gene silencing has been shown to lead to the failure of MEL cell differentiation (Vizirianakis et al., 1999).Moreover, researchers have reported that Rps5 gene inhibition is likely to play a role in inducing cell differentiation (Fortier et al., 2015).These data are valuable for understanding the role of ribosomal proteins in the differentiation and apoptosis of damaged neurons.The results from our indirect co-culture experiments showed that down-regulation of Rps5 in fibroblasts and Schwann cells effectively inhibited DRG neuron axon growth, indicating that Rps5 in FC-EXOs and SC-EXOs is closely related to neuronal axon growth.Rps5 is expected to be a hub gene regulating exosomes produced by different cell types; however, its upstream and downstream regulatory mechanisms remain unclear.
LncRNAs are molecules of more than 200 nucleotides in length that are transcribed by RNA polymerase.LncRNAs not only affect the development of a variety of systems including the nervous system, but also play important roles in cellular processes such as epigenetic regulation, cell cycle regulation,cell differentiation regulation, and post-transcriptional regulation.In vitro,down-regulation of LINC00324 reduced proliferation, colony formation,migration, and invasion of retinoblastoma, while promoting apoptosis and cell cycle arrest (Dong et al., 2020).In the current study, we identified some poorly characterized lncRNAs as being involved in peripheral nerve regeneration, including Jpx, Ftx, Pvt1, H19, and Miat.Furthermore, we constructed a lncRNA-miRNA-mRNA ceRNA regulatory network to provide an important starting point for subsequent studies, which could focus on verifying the influence of RNA modification on the expression of target genes and proteins in damaged neurons (Wan et al., 2020; Xu et al., 2020).Two of the lncRNAs identified in non-neuronal cell vesicles in this study—Fxt and Miat—may provide new insights into the search for hub genes that regulate and integrate multi-source exosomes and provide therapeutic targets for nerve regeneration.They will also play a role in the future development of RNA technologies, targeted therapies, and precision medicines.
Our study had several limitations, such as the lack of cellular or animal experiments to validate biological functions and the regulatory network.These points will be addressed in our future studies.
In conclusion, we performed RNA transcriptome sequencing of FC-EXOs and SC-EXOs.Through DEG, GO, KEGG, and PPI analyses, as well asin vitroco-culture experiments, we found that the ribosome-related gene Rps5 is enriched in FC-EXOs and SC-EXOs and may be a useful target for promoting axonal regeneration.The ceRNA regulatory network targeting Rps5 that we constructed lays a foundation for further exploring the mechanisms by which FC-EXOs and SC-EXOs inhibit scar formation and promote axon regeneration and functional recovery after nerve injury, and could be leveraged to promote peripheral nerve injury repair.
Author contributions:Conceived and designed the experiments:SZ,JL,DM.Performed the experiments:XZ,YLv,HX,RW.Analyzed the data:XZ,YLv,HX,SZ.Contributed reagents/materials/analysis tools:XZ,YLi,CL,MZ,XG,JL,DM.Wrote the manuscript:XZ,HX,SZ,DM.All authors have read and approved the final manuscript.
Conflicts of interest:The authors declare that they have no conflict of interest.
Data availability statement:No additional data are available.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- The big data challenge – and how polypharmacology supports the translation from pre-clinical research into clinical use against neurodegenerative diseases and beyond
- P-aminobenzoic acid promotes retinal regeneration through activation of Ascl1a in zebrafish
- Lupenone improves motor dysfunction in spinal cord injury mice through inhibiting the inflammasome activation and pyroptosis in microglia via the nuclear factor kappa B pathway
- Two-photon live imaging of direct glia-to-neuron conversion in the mouse cortex
- Ferroptosis mechanism and Alzheimer’s disease
- Neutrophil extracellular traps mediate neuroimmunothrombosis

