Physical exercise and synaptic protection in human and pre-clinical models of multiple sclerosis
Federica Azzolini, Ettore Dolcetti, Antonio Bruno, Valentina Rovella, Diego Centonze,3,*, Fabio Buttari,3
Abstract In multiple sclerosis, only immunomodulatory and immunosuppressive drugs are recognized as disease-modifying therapies.However, in recent years, several data from pre-clinical and clinical studies suggested a possible role of physical exercise as disease-modifying therapy in multiple sclerosis.Current evidence is sparse and often conflicting, and the mechanisms underlying the neuroprotective and antinflammatory role of exercise in multiple sclerosis have not been fully elucidated.Data, mainly derived from pre-clinical studies, suggest that exercise could enhance longterm potentiation and thus neuroplasticity, could reduce neuroinflammation and synaptopathy,and dampen astrogliosis and microgliosis.In humans, most trials focused on direct clinical and MRI outcomes, as investigating synaptic, neuroinflammatory, and pathological changes is not straightforward compared to animal models.The present review analyzed current evidence and limitations in research concerning the potential disease-modifying therapy effects of exercise in multiple sclerosis in animal models and human studies.Key Words: disease-modifying behaviour; endocannabinoid system; long-term potentiation; multiple sclerosis; neuroplasticity; neuroprotection; physical exercise; synaptopathy
Introduction
Multiple Sclerosis (MS) is an autoimmune neuroinflammatory and neurodegenerative disease of the central nervous system (CNS), representing the most frequent cause of non-traumatic disability in young adults (Koch-Henriksen and Sørensen, 2010).
MS therapy is based on a multidisciplinary approach.To date,immunomodulatory and immunosuppressive drugs are the only recognized disease-modifying therapies (DMTs), as they can suppress acute inflammatory activity and prevent or slow down disability progression.In addition to pharmacological DMTs, symptomatic drugs and physical rehabilitation(hereafter defined as “exercise”) are generally considered only as supportive tools, able to improve quality of life and specific symptoms in people with MS (pwMS).However, in the last years, several experimental and clinical data suggested that exercise in pwMS could exert not only symptomatic effects, but also contribute to ameliorating disease course, cognitive and motor deficits, mood disorders and reducing the accumulation of disability,thus expressing a potential DMT activity (Dalgas et al., 2019; Centonze et al.,2020).
Many proposed mechanisms can support the disease-modifying activity of exercise, most of them derived from pre-clinical models.For example,exercise correlates with the reduction of CNS proinflammatory cytokines,brain-blood barrier permeability, and elevation of brain-derived neurotrophic factor (BDNF) as reported by Prosperini and Di Filippo (2019) and Diechmann et al.(2021) and modulates microgliosis and astrogliosis (Centonze et al.,2020).Moreover, consistent data suggest that exercise could promote clinical recovery by enhancing synaptic plasticity, in particular long-term potentiation (LTP) (Stampanoni Bassi et al., 2018; Prosperini and Di Filippo,2019).Repetitive and structured exercise improves brain health facilitating the formation of new and the reorganization of existing synaptic connections,probably in proportion to the intensity of the exercise (Schmolesky et al.,2013; Hortobágyi et al., 2022).
Neuroplasticity is a characteristic of the CNS based on the ability of the brain to adapt to environmental and behavioral changes, during an individual lifespan, through remodeling of neural networks.This assumption is crucial to explain the effects of exercise on functional adaptations in MS through neuroplasticity.A single bout of exercise, indeed, induces repetitive stimulation of the synapses organizing those neural networks responsible for regulating a given task throughout exercise as reported by Sandroff et al.(2018).Accordingly, a study by Chaves et al.(2020) showed that exercise induces brain excitability changes, resulting in LTP (as demonstrated by an increased motor-evoked potential amplitude and decreased intracortical inhibition).The effects of exercise on neuroplasticity are particularly relevant considering that MS is characterized by synaptopathy, a severe inflammationdriven synaptic dysfunction, and loss, observed since the early stages of the disease, which represents a key link between neuroinflammation and neurodegeneration.Briefly, neuroinflammation enhances excitatory(glutamatergic) and reduces inhibitory (GABAergic) transmission, in turn causing excitotoxic damage and neuronal death.The dysregulation of the endocannabinoid system (ECS) plays a pivotal role in MS synaptopathy as previously described by Centonze et al.(2007) and Pryce et al.(2003): under physiological conditions, cannabinoid receptor type 1 (CB1R) stimulation exerts protective effects by limiting the inflammation-induced enhancement of excitatory glutamate transmission.In MS, a dysfunction of CB1R activity has been observed, and it is linked to neurodegenerative damage both in humans and in experimental autoimmune encephalomyelitis (EAE) (Baker and Pryce, 2008; Rossi et al., 2009).Exercise can indirectly reduce synaptopathy in relation to the aforementioned anti-inflammatory activity, but also acts centrally by promoting neuroplasticity.For example, exercise enhances the secretion of neurotrophic factors or restores the protective activity of the ECS as mentioned in earlier reports (Rossi et al., 2009; Schmolesky et al., 2013;Prosperini and Di Filippo, 2019).Thus, as inflammatory synaptopathy is a determinant pathological process in MS and is at least partially reversible, it could represent a novel therapeutic target in MS.
Figure 1 summarizes the main effects of exercise on peripheral immune system and in CNS in animal models of MS and pwMS.
Search Strategy
The present mini-review encompasses the potential disease-modifying activity of exercise in MS, both in preclinical and clinical studies.Reviewers searched relevant papers in NCBI (PubMed), Scopus, and Cochrane Library until May 2023.The search was restricted to English-language papers that focused on exercise training studies in preclinical models and clinical studies.The search was conducted using the following key words and MeSH headings: multiple sclerosis, MS, physical exercise, exercise training, physical rehabilitation,synaptopathy, neuroplasticity, neuroprotection.
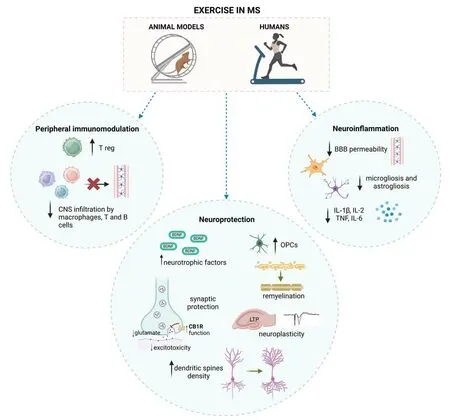
Figure 1 |Proposed effects of exercise in animal models of MS and pwMS.
Pre-Clinical Studies
Although with several limitations, animal models represent useful tools to investigate the neurobiological mechanisms and clinical effects of exercise in MS.In EAE, it is possible to partially reproduce human physical interventions and motor activities and to simplify and control a priori the variables to analyze, for example by commencing preventive exercise before the induction of EAE.Animal models also allow the evaluation of the long-term effects of exercise in a relatively narrow time window, while a considerably longer follow-up is required in humans.
In EAE, several data suggest that exercise exerts mainly neuroprotective functions and, to a lesser extent, anti-inflammatory effects.In one study comparing mice randomly assigned to empty or running wheel-equipped cages, a reduction in synaptic dysfunction was observed in the hippocampal CA1 area of voluntary exercising mice compared to sedentary mice.This effect was associated with an increased survival of GABAergic parvalbuminpositive (PV+) interneurons in a study by Rizzo et al.(2021).In MS, the loss of PV+ interneurons, a well-known pathological hallmark of the disease, causes a reduction in inhibitory GABAergic transmission and is therefore involved in synaptic dysfunction as demonstrated in previous studies (Clements et al.,2008; Dutta et al., 2011).The same study by Rizzo et al.(2021) also showed direct anti-inflammatory effects of exercise in EAE mice: a reduction of the transcriptional expression of the microglial marker IBA1, of hippocampal concentration of pro-inflammatory cytokines (tumor necrosis factor,interleukin [IL]-1β, and IL-6) and CA1 microgliosis was observed in exercising vs.sedentary mice.Moreover, exercise may probably reduce synaptopathy through indirect effects on the ECS.EAE, indeed, causes severe ECS dysfunction, including a loss of sensitivity to stimulation of CB1 cannabinoid receptors, which are central in the presynaptic control of neurotransmitter release as previously reported by Rossi et al.(2009).In EAE mice, indeed,voluntary exercise induces upregulation of the striatal dopaminergic system according to Werme et al.(2000, 2002) and El Rawas et al.(2009), which in turn allows the restoration of striatal GABAergic synapse sensitivity to CB1R through the stimulation of dopaminergic D2-like receptors, with consequent reduction in excitotoxicity as described by Centonze et al.(2004) and Giuffrida et al.(1999).In one study by Rossi et al (2009), some mice were housed in cages equipped with running wheels from the day of immunization.Voluntary exercising mice showed fewer motor deficits and increased density of striatal dendritic spines compared to sedentary mice, further supporting the beneficial role of exercise on synaptic integrity.No differences emerged in the two groups of mice regarding the extent of glutamatergic transmission alterations, nor of inflammatory infiltrates, demyelination, and axonal loss in the spinal cord, suggesting that exercise played primarily neuroprotective and not direct anti-inflammatory effects (Rossi et al., 2009).In a later EAE study by Pryor et al.(2015), however, exercise-trained mice showed less disability, later disease onset, and lower inflammatory infiltrate and demyelination in the spinal cord.
A combined EAE and clinical study by Gilio et al.(2022) investigated the relationship between preventive exercise and IL-2-driven mood disorders.PwMS were divided into subgroups according to the levels of physical activity in the 6 months before MS diagnosis: they were assigned to the “exercise group” if they performed > 150 minutes/week of sport activity, to the “lifestyle physical activity group” if they performed > 150 minutes/week of moderate physical activity or in the “sedentary group” if they did not perform physical activity at all.In the animal model, on the day of EAE immunization mice were randomly assigned to empty or running-wheel enriched cages (Gilio et al., 2022).Preventive exercise correlated with lower levels of anxiety and depression, and with lower cerebrospinal fluid (CSF) of IL-2 concentrations in newly diagnosed MS patients.In line with these results, voluntary exercise started from the day of immunization in EAE, reduced mood abnormalities and intracerebral levels of IL-2.Furthermore, intracerebral injection of IL-2 in healthy mice induced depression and anxiety-like behaviors by altering CB1R-mediated control of GABAergic transmission, thus suggesting that the positive effects of exercise on mood disturbances are mediated through synaptic protection and reduction of neuroinflammation, specifically of CSF IL-2 concentrations (Gilio et al., 2022).Other effects of exercise were investigated in non-inflammatory demyelinating models of MS.In one model of cuprizone-exposed mice, Mandolesi et al.(2019) demonstrated that 6-week voluntary running wheel exercise, started from the day of disease induction,dampened microgliosis, astrogliosis, and demyelination both in the corpus callosum and the striatum.In one lysolecithin demyelinating model by Jensen et al.(2018), voluntary running wheel exercise promoted the proliferation of oligodendrocyte precursor cells and axonal remyelination.Lastly, exercise also exerts effects on peripheral immunoregulation.Einstein et al.(2018)have found significantly higher biomarkers of T regulatory cells in mice during forced resistance training.Moreover, in one study by Fainstein et al.(2019),the injection of T lymphocytes extracted from 6-week forced-treadmill exercise-preconditioned EAE donor mice but not from sedentary donor mice induced disability reduction and promoted remyelination in recipient sedentary EAE mice.
Clinical Studies
Several clinical studies demonstrated that exercise programs in pwMS are associated with a number of favorable clinical outcomes such as reduced relapse rate and disability progression (Motl, 2020), reduced spasticity,fatigue, depression, and anxiety, and improvement in walking and balance abilities as well as in quality of life (Motl and Pilutti, 2012; Amatya et al.,2019) suggesting a potential DMT effect of exercise in MS.However, although direct neuroprotective and anti-inflammatory effects of exercise emerged in several animal models, the clinical translation of these data in pwMS is not straightforward: systematic data demonstrating the neurobiological mechanism that allows exercise to act as a DMT and in particular as a synaptic protector in humans are still lacking or sparse (Centonze et al., 2020; Motl,2020; Diechmann et al., 2021).Interesting data supporting the link between exercise and the endocannabinoid system in humans derives from a study by Mori et al.(2014) in which MS patients carrying certain genetic variants of CB1Rs causing reduced protein expression of the receptor did not express transcranial magnetic theta burst stimulation-induced LTP-like cortical plasticity, and showed poor clinical benefits after a 2-week daily aerobic exercise therapy.
就在柯达DCS Pro 14n发布的同一天,佳能也推出了属于EOS系统的首台全画幅数码单反产品EOS-1Ds。相对于康泰时和柯达的那两台相机,EOS-1Ds全画幅数码单反相机已经进入了另一个新的时代,从极其现代化的造型就可以看出端倪,这与我们现在使用的相机已经非常接近了。
Another limited pilot study (n= 10 patients) by Chaves et al.(2020)investigated the effects of a single bout of aerobic training in Progressive MS.Corticospinal excitability was measured with transcranial magnetic stimulation before and after exercise (40-minute treadmill training).The variables analyzed were resting and active motor thresholds, motor-evoked potential amplitudes, recruitment curves, and length of the cortical silent period.Aerobic training reduced inhibition (shorter cortical silent period) and increased excitation (increased motor-evoked potential amplitude) in the hemisphere corresponding to the stronger hand, demonstrating exerciseinduced neuroplasticity.Other studies investigated the association between exercise and neurotrophic factors.In particular, BDNF was increased in 6 of 14 randomized clinical trials analyzed in a meta-analysis, although no clear direct clinical neuroprotective effects of BDNF elevation have clearly emerged so far as reported by Diechmann et al.(2021).
Most clinical studies evaluated the potential role of exercise as DMT only with clinical and MRI outcomes.For example, one study by Riemenschneider et al.(2022) investigated the potential DMT activity of exercise in early MS.Patients diagnosed with MS within the previous 2 years underwent an exercise program conducted twice weekly for 48 weeks.Patients underwent high-intense aerobic exercise, monitored relative to their individual maximal heart rate (HRmax), with continuous sessions lasting 30–60 minutes with an intensity of 60–80% HRmax and interval training sessions lasting 30–60 minutes with an interval duration of 1–10 minutes and intensities varying from 65–95% HRmax.Relapse rate, global brain atrophy, and other MRI measures represented the primary outcome of the study and did not differ in exercising compared to sedentary patients.However, the microstructural integrity of motor-related tracts was higher in exercise-trained patients.
MRI-based evidence of functional or structural plasticity occurring following motor (or cognitive) rehabilitation in pwMS was also investigated in a systematic review analyzing 16 studies by Prosperini et al.(2015).Despite heterogeneity, all studies documented changes in white matter microarchitecture, task-related activation, and/or functional connectivity following both task-oriented and selective training, assessed with advanced MRI measures, such as functional MRI and diffusion tensor imaging.
Only one randomized clinical trial by Gravesteijn et al.(2023) investigated the relationship between exercise and serum neurofilament light (NFl) and glial fibrillary acidic protein (GFAP) levels, biomarkers of axonal damage and astrogliosis, respectively.Patients with RRMS and PMS (n= 89) were randomized to aerobic training (n= 43) or MS nurse controls (n= 46).Serum concentrations of BDNF, NfL, and GFAP were measured at baseline and week 16 post-treatment.Although in the original study exercise-trained patients showed a reduction in serum NFl and GFAP levels compared to the control group, a secondary analysis rejected this finding (Gravesteijn et al., 2023).Obviously, further data are needed to obtain conclusive evidence.Importantly,it could be assumed that the neuroprotective effects of exercise should be monitored with other biomarkers, specifically linked to the neurobiological effects of exercise in MS pathophysiology.The identification of CSF biomarkers of synaptic dysfunction, indeed, represents an intriguing field of interest,considering the robust evidence for a synaptic protective effect of exercise derived from pre-clinical studies (Rossi et al., 2009; Pryor et al., 2015; Rizzo et al., 2021; Gilio et al., 2022).In the last years, several synaptic proteins were identified as potential markers of synaptic integrity, both pre-synaptic(synaptotagmin-1, synaptophysin, synaptosomal-associated protein 25 and growth-associated protein 43) and post-synaptic (neurogranin, neuronal pentraxins) (Camporesi et al., 2020).Extracellular vesicles (EVs) represent promising biomarkers to detect and measure synaptic loss.In one study,Bhargava et al.(2021) measured pre and post-synaptic proteins synaptopodin and synaptophysin in neuronal-enriched EVs (NEV) from plasma of relapsingremitting and progressive MS patients.They found significantly lower levels of NEV synaptopodin and synaptophysin in MS patients compared to healthy controls.Thus, even if currently synaptic biomarkers are still in an exploratory phase of validation, they will probably provide a relevant contribution in the near future.
Discussion and Future Perspectives
Consistent data, derived from preclinical and clinical studies, support the neuroprotective role of exercise in MS.Animal studies allow for a more direct analysis of the possible neurobiological mechanisms underlying the potential disease-modifying activity of exercise, in particular of its action on LTP and ECS and the associated synaptic protection, but also of exercise-mediated peripheral immunomodulation (Rossi et al., 2009; Einstein et al., 2018;Fainstein et al., 2019; Rizzo et al., 2021).Overall, the data currently available show a beneficial effect of physical exercise in pwMS (Motl and Pilutti, 2012;Stampanoni Bassi et al., 2018; Amatya et al., 2019; Centonze et al., 2020;Proschinger et al., 2022).However, clear evidence of a disease-modifying effect of exercise in MS is still under investigation, as available data are sparse and often conflicting (Dalgas et al., 2022; Heesen and Rosenkranz, 2022;Motl and Sandroff, 2022).Main limits in human studies are represented,on one hand, by the difficulty to access tissue pathological markers and to easily assess synaptic function and exercise-induced neuroplasticity; on the other hand, by the lack of standardized exercise protocols (i.e., type, timing,duration, intensity, etc.), of clinical and paraclinical markers, as well as of definite timepoints of biomarker assessment as pointed by Dalgas et al.(2020).At present, reviews and meta-analyses included studies with highly heterogeneous exercise programs (such as aerobicvs.progressive resistance training, balance training, etc.), different patients’ populations (i.e., relapsingvs.progressive), or disease phases (i.e., relapsevs.stability), and variable clinical or paraclinical outcomes (Dalgas et al., 2020; Diechmann et al., 2021;Proschinger et al., 2022; Riemenschneider et al., 2022).Importantly, clinical studies investigating neurophysiological modifications of neuroplasticity before and after exercise are very limited in number and characterized by small sample size (Nielsen and Norgaard, 2002; Thickbroom et al., 2008;Chaves et al., 2019, 2020; Hortobágyi et al., 2022).Defining and applying a systematic approach in clinical trials is therefore necessary to evaluate the impact of exercise on MS and its potential underlying neurobiological effects as suggested by Dalgas et al.(2020).The first step to improve the methodological bases of research in this field is to provide a priori study designs, possibly randomized and controlled trials, with adequate sample size (i.e., with multicentric enrolment) and adequate follow-up duration to assess long-term effects of exercise, and to identify appropriate biomarkers and a precise timing to collect results.Namely, it would be useful to define biomarkers able to: (1) address the neuroprotective and anti-inflammatory disease-modifying effects of exercise (specific structural and functional MRI parameters, NFl levels, etc.); (2) measure the changes in the biological effects of exercise (synaptic biomarkers, serum, and CSF cytokine levels, immune cell sub-populations, etc); (3) assess exercise intensity and effects on fitness(increase in muscle strength, change in cardiorespiratory measures, lactate and creatin-phospho-kinase levels, etc.).Moreover, it would be appropriate to set time points before, immediately after the intervention, and in the long term.Finally, it must be considered that animal models, although simplistic in comparison to human pathophysiology, allow for a uniform distribution and control of variables, consistent and rigorous results, and an understanding of the mechanistic effects of exercise on the neurobiology of MS.A translational approach, based on the use of similar exercise regimens and biomarkers in animals and humans is therefore desirable, to infer neurobiological data that can potentially be applied to humans.
Conclusions
In summary, exercise in MS exerts beneficial effects against fatigue and spasticity as well as motor, cognitive, and mood functions and, importantly,it could probably play a disease-modifying activity through the reduction of inflammatory synaptopathy, neuro-inflammation, and systemic immune dysregulation.Although definitive evidence demonstrating the neurobiological bases of the putative disease-modifying effect of exercise in MS is still lacking,the concept of exercise as a disease-modifying behavior should be cautiously introduced.
The importance of add-on therapies to pharmacological DMTs is indeed crucial, as current drugs act on a purely immunological side, often have no impact on symptoms such as fatigue, cognition, or mood, and their direct effect on neurodegenerative mechanisms is often limited.
Systematic and larger studies, possibly with a translational design and the use of specific biomarkers, are needed to rigorously assess clinical and neurobiological mechanisms and outcomes of exercise in MS.
Author contributions:Conceptualization:FA and DC;methodology:FA,FB;resources:AB;data curation:ED;writing-original draftpreparation,image production:FA;writing-review and editing:DC,VR;visualization:ED,AB.All authors have read and agreed to the published version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:Not applicable.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Lindsey Wooliscroft,Oregon Health & Science University,USA.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- The big data challenge – and how polypharmacology supports the translation from pre-clinical research into clinical use against neurodegenerative diseases and beyond
- P-aminobenzoic acid promotes retinal regeneration through activation of Ascl1a in zebrafish
- Lupenone improves motor dysfunction in spinal cord injury mice through inhibiting the inflammasome activation and pyroptosis in microglia via the nuclear factor kappa B pathway
- Two-photon live imaging of direct glia-to-neuron conversion in the mouse cortex
- Ferroptosis mechanism and Alzheimer’s disease
- Neutrophil extracellular traps mediate neuroimmunothrombosis

