Emerging role of liquid biopsy in rat sarcoma virus mutated metastatic colorectal cancer: A case report
João Gramaça,Isabel Gomes Fernandes,Carolina Trabulo,Joana Gonçalves,Rita Gameiro dos Santos,Adriano Baptista,Idília Pina
Abstract BACKGROUND In patients with metastatic colorectal cancer (mCRC),the treatment options are limited and have been proved to be affected by rat sarcoma virus (RAS) mutational status.In RAS wild-type (wt) patients,the combination of antiepidermal growth factor receptor (EGFR) monoclonal antibodies with chemotherapy (CT) is more effective than CT alone.On the other hand,RAS-mutated patients are not eligible for treatment with anti-EGFR antibodies.CASE SUMMARY Eleven patients with initially RAS-mutated mCRC were followed from diagnosis to May 2022.At the time of cell-free DNA determination,five patients had undergone one CT line,five patients had undergone two CT lines,and one patient had undergone three CT lines (all in combination with bevacizumab).At the second and third treatment lines [second line (2L),third line (3L)],patients with neo-RAS wt received a combination of CT and cetuximab.In neo-RAS wt patients treated with anti-EGFR,our findings indicated an increase in progression-free survival for both 2L and 3L (14.5 mo,P=0.119 and 3.9 mo,P=0.882,respectively).Regarding 2L overall survival,we registered a slight increase in neo-RAS wt patients treated with anti-EGFR (33.6 mo vs 32.4 mo,P=0.385).At data cut-off,two patients were still alive: A RAS-mutated patient undergoing 3L treatment and a neo-RAS wt patient who received 2L treatment with anti-EGFR (ongoing).CONCLUSION Our case series demonstrated that monitoring RAS mutations in mCRC by liquid biopsy may provide an additional treatment line for neo-RAS wt patients.
Key Words: Metastatic colorectal cancer;Rat sarcoma virus mutational status;Liquid biopsy;Rat sarcoma virus wild-type;Neo-rat sarcoma virus wild-type;Anti-epidermal growth factor receptor therapy;Case report
INTRODUCTION
Data provided by the World Health Organization indicates that colorectal cancer (CRC) was ranked as the third most prevalent cancer globally in 2020,with over two million documented cases,and was the second leading contributor to cancer-related fatalities,resulting in approximately one million deaths annually[1,2].Approximately 15% to 30% of patients have metastatic disease at presentation and about 20% to 50% will develop metastatic disease in the future,most of them with rat sarcoma virus (RAS)-mutated tumours[3-5].
In general,patients with metastatic CRC (mCRC) have limited treatment options and certain cases are candidates for primary surgery with metastasectomy,with or without systemic treatment.Current evidence demonstrates that the combination of chemotherapy (CT) with anti-epidermal growth factor receptor (EGFR) monoclonal antibodies,such as cetuximab and panitumumab,is more effective than CT alone,in RAS wild-type (wt) mCRC patients that are nonmutated at disease presentation[6].However,RAS-mutated patients are not eligible for treatment with anti-EGFR antibodies[7,8] and,at this point,a systemic molecularly targeted treatment has not been approved for these patients[9].
Considering that the presence of RAS mutations will determine the treatment pathway,enabling more personalized treatments,the European Society for Medical Oncology (ESMO) guidelines recommend RAS gene testing in mCRC tissue or liquid biopsy (LB) as a mandatory prerequisite prior to treatment with anti-EGFR antibodies[3].It has been hypothesized that tumour heterogeneity is related to the limited prognosis of mCRC,presenting a significant obstacle to the efficacy of current cancer therapy[10].After the first cycles of CT treatment of RAS-mutated mCRC patients,RASmutated alleles disappear,with conversion to RAS wt in 20% to 80% of patients[4,6,11].LB with evaluation of cell-free DNA (cfDNA) has shown to be an adequate tool to monitor the conversion of RAS mutant mCRC into RAS wt (Neo-RAS wt)[12];in accordance,ESMO guidelines recommend testing not only prior to anti-EGFR treatment but also after two lines of treatment.Furthermore,even though data are still scarce,Neo-RAS wt patients seem to benefit from anti-EGFR treatment[6,13,14] and,as such,a deeper understanding of the mechanisms and clinical implications of this possibility is warranted.In this case series,we retrospectively analyzed a series of 11 patients with mCRC who were studied in terms of RAS mutational state by LB,aiming to evaluate the impact of the collected data on the therapeutic decision,progression-free survival (PFS),and overall survival (OS).
CASE PRESENTATION
Chief complaints
Eleven patients with mCRC,who were included in the Digestive Tumours consultation of our hospital,were followed since diagnosis to May 2022 (Table 1).All the patients were initially RAS-mutated.The median age was 66 years (50-77) and 18.2% were female.
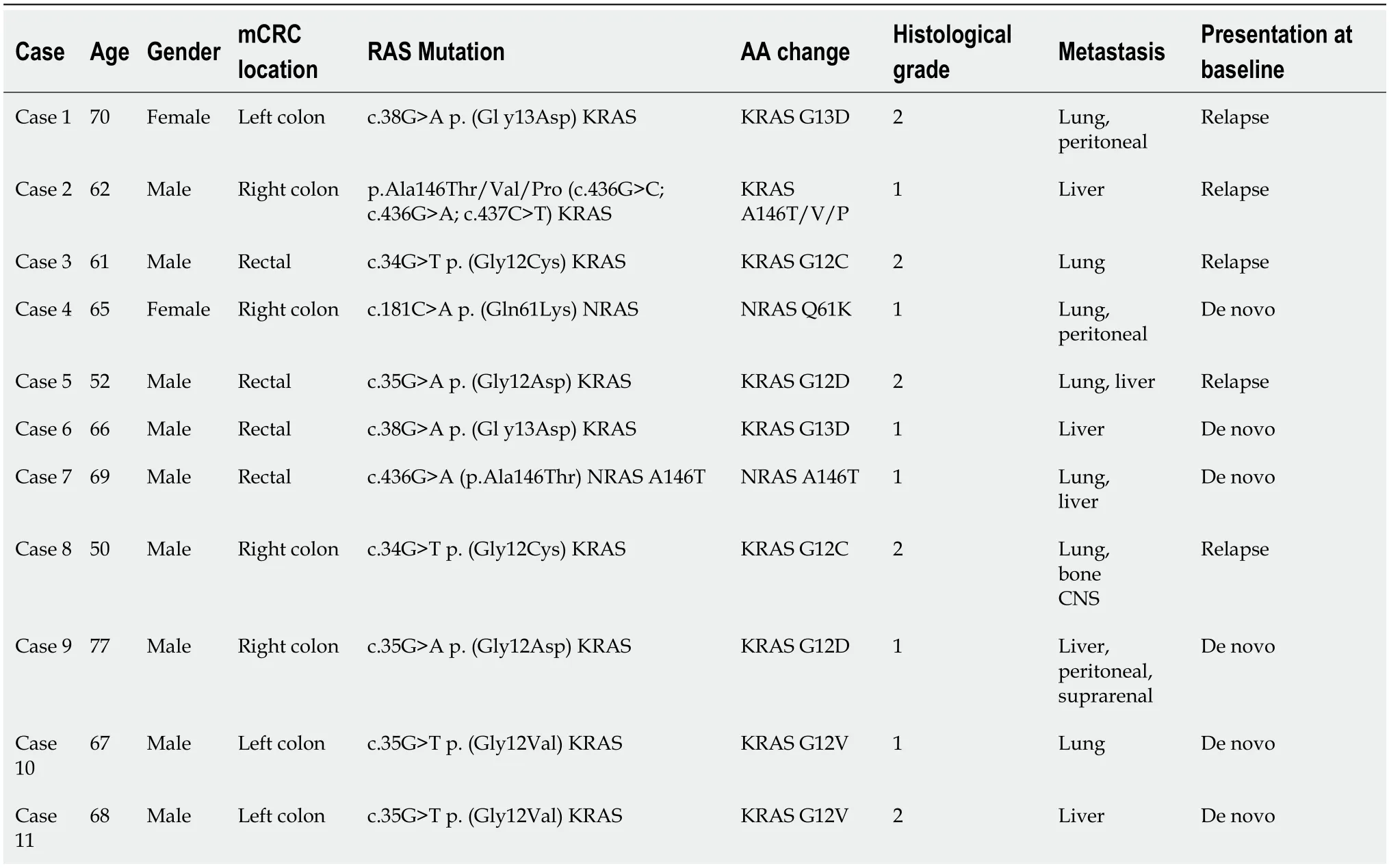
Table 1 Clinical information of the 11 patients at baseline
All the analyzed primary tumours were v-raf murine sarcoma viral oncogene homolog B1 (BRAF) wt and had microsatellite stability;none of them had neurotrophic tropomyosin-receptor kinase (NTRK) rearrangements.Regarding immunochemistry-determined human epidermal growth factor receptor 2 (HER2) overexpression,27.3% had +1 expression,27.3% were non-determined,and 45.5% had 0 expression.In terms of differentiation grade,45.5% were grade 2 and 54.5% were grade 1.
In this series,54.5% of the patients had stage IVde novodisease while 45.5% had stage IV relapsed disease after localized disease treatment.Regarding primary tumour location,36.4% of the patients had right-side disease and 63.6% had left-side disease (including rectal cancer).
History of present illness
The basic clinical characteristics of our patients are outlined in Table 1.Despite the absence of symptoms,Case 1 had relapsed disease,with lung and peritoneal metastasis.The patient had slow progressive disease,with progression under first line (1L) treatment in the lung,peritoneal cavity,and left adrenal gland;at this point,the patient was Neo-RAS wt.In this context,the patient started CT and anti-EGFR therapy;the latter was suspended after an ocular toxicity G3 Common Terminology Criteria of Adverse Events (CTCAE) v5.0 event.Despite this,the patient was kept on maintenance CT and showed a very long and sustained response.Case 2 had right-sided relapsed mCRC with liver metastasis.Although the patient became Neo-RAS wt after two lines of treatment,his response to anti-EGFR therapy and single-drug CT was weak.Case 7 hadde novorectal cancer with lung and liver metastasis,anorexia,fatigue,and bloody stools at presentation.The patient became Neo-RAS wt after one systemic treatment line;despite the very good clinical response,anti-EGFR therapy was suspended after two treatment cycles due to a cutaneous toxicity G2-3 CTCAE v5.0 event.Case 11 also hadde novoleft-sided mCRC and presented with a high tumour burden in the liver,with abdominal pain and hepatomegaly,both at presentation and progression under 1L.
History of past illness
Cases 1 and 9 had a long-standing history of essential hypertension,for over 10 years.Case 1 also had hyperlipidaemia for the same period.Cases 10 and 11 had diabetes mellitus type 2 for over 5 years.In the remaining cases,past history did not present relevant events.
Personal and family history
Personal and family histories did not retrieve significant mCRC related details.
Laboratory examinations
All the analyzed primary tumours were RAS mutated,BRAF wt,and showed microsatellite stability;none of them had NTRK rearrangements.Regarding immunochemistry-determined HER2 overexpression,27.3% of the patients had +1 expression,27.3% were non-determined,and 45.5% had 0 expression.In terms of differentiation grade,45.5% of the patients were grade 2 and 54.5% were grade 1.The LB study was performed on cfDNA from blood plasma collected in Streck tubes,obtained after centrifugation;samples were analyzed at an outside institution.The search for mutations in the Kirsten rat sarcoma viral oncogene homolog (KRAS) (NM_033360.3) (codons 12,13,59 and 61),neuroblastoma rat sarcoma virus viral oncogene homolog (NRAS) (NM_002524.4) (codons 12,13,59 and 61) and BRAF (MN_004333.4) (codon 600) genes was performed through next-generation sequencing using the “Oncomine Lung cfDNA assay” (Ion Torrent™ Oncomine™ assays– Thermo Fisher Scientific).With a molecular coverage of 2500 ×,this method enables the detection of mutations with an allelic fraction of up to 0.1%,with a sensitivity >90% and specificity >98% (i.e.it allows the detection of one copy of mutated DNA in 1000 copies of wt DNA).This method was validated by a third-party laboratory.In LB specimens,quality control values greater than 26 and 35.5 for KRAS and NRAS/BRAF,respectively,suggested low amounts of cfDNA and thus a negative result did not exclude the presence of mutations in these genes.In these cases,a new blood sample was collected.
Imaging examinations
All patients had identifiable metastatic lesions evidenced by computed tomography,magnetic resonance imaging,or positron emission tomography-computed tomography.Regarding patients with Neo-RAS wt status,the best imaging response was partial response in one case,stable disease in two cases,and disease progression in one case.To illustrate,Case 11,who had liver-only metastasis,had a partial response at the first imaging evaluation,which was performed three mo after starting anti-EGFR therapy plus CT (Figure 1),with associated liver function improvement.
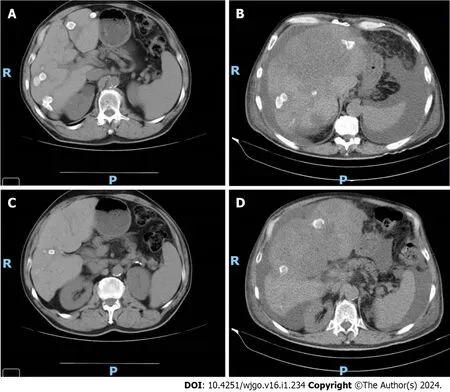
Figure 1 Imaging evaluation of Case 11. A: Imaging evaluation at baseline (July 2021),upper axial plane,[flat chemotherapy (CT) scan with IV contrast];B:Imaging evaluation at 3 mo (October 2021,on the right) upper axial plane,(flat CT scan with IV contrast);C: Imaging evaluation at baseline (July 2021),lower axial plane,(flat CT scan with IV contrast);D: Imaging evaluation at 3 mo (October 2021),lower axial plane,(flat CT scan with IV contrast).
FINAL DIAGNOSIS
In this series,54.5% of the patients had stage IVde novoCRC,while 45.5% had stage IV relapsed CRC after localized disease treatment.All patients had KRAS or NRAS somatic mutation at treatment initiation and all underwent cfDNA determination after one or two lines of systemic treatment.
Regarding metastasis location,at the time of cfDNA determination,63.6% of patients had liver metastasis,27.3% had peritoneal involvement,and 63.6% had lung metastasis;of the latter,five patients had lung involvement without liver disease (Table 1).
TREATMENT
At the time of cfDNA determination,five patients had undergone one CT line,five patients had undergone two CT lines,and one patient had undergone three CT lines.In 1L treatments,90.9% of patients received oxaliplatin+capecitabine/oxaliplatin+folinic acid+fluorouracil and bevacizumab,with a median number of cycles of 9 (4-25);one patient was treated with irinotecan+folinic acid+fluorouracil and bevacizumab (Table 2).
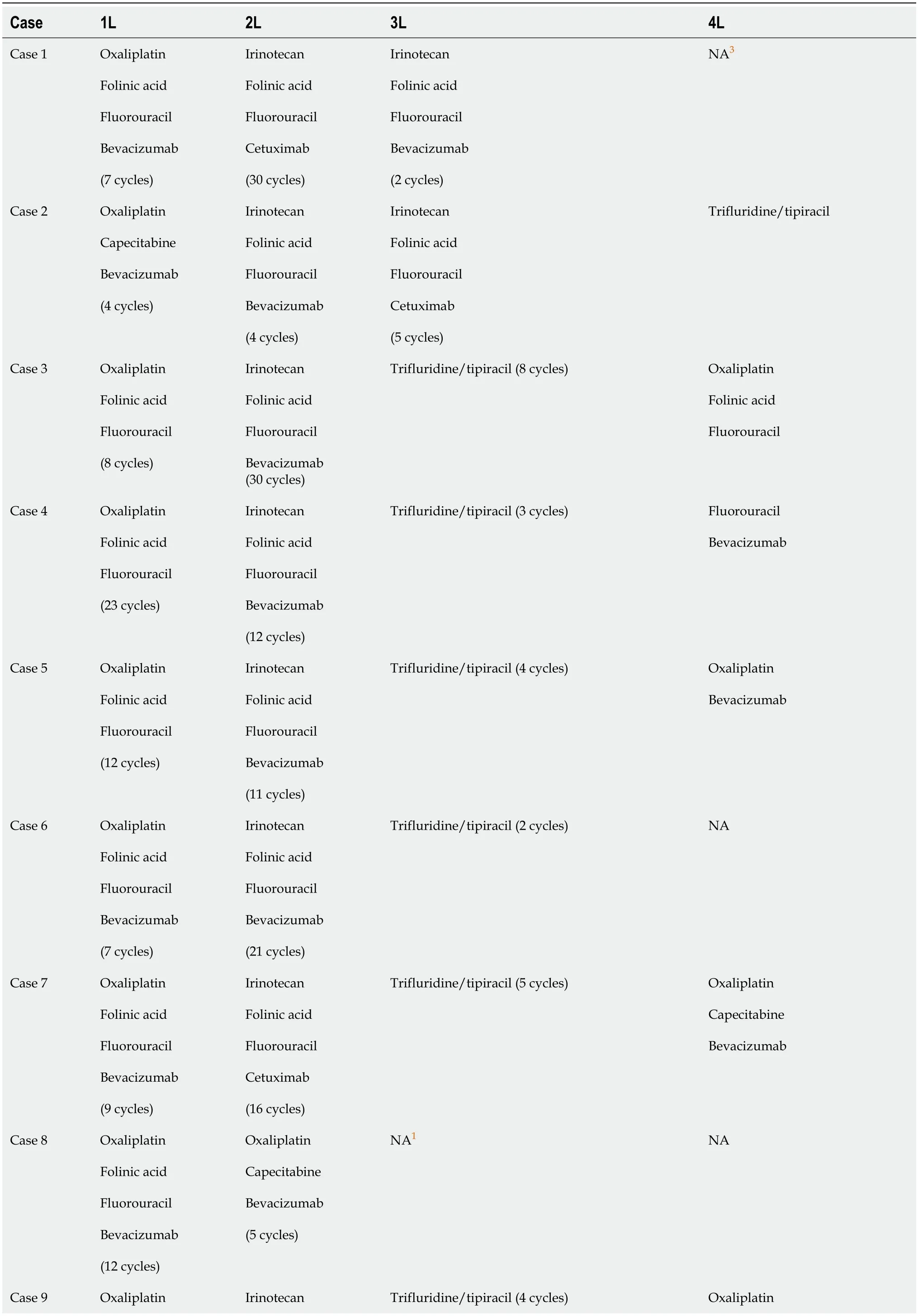
Table 2 Treatment strategy
Regarding treatment sequencing (Figure 2),most patients were treated with folinic acid+fluorouracil+irinotecan and bevacizumab in second line (2L),except for patients with Neo-RAS wt,who were treated with folinic acid+fluorouracil+irinotecan and cetuximab.

Figure 2 Disease progression after second-line treatment. 5-FU: 5-fluorouracil;Ad: Adrenal;B: Bevacizumab;Bo: Bone;C: Cetuximab;CAP:Capecitabine;CAPOX: Capecitabine+oxaliplatin;CNS: Central nervous system;ctDNA: Circulating DNA;DN: De novo disease;FOLFIRI: Folinic acid+fluorouracil+irinotecan;FOLFOX: Folinic acid+fluorouracil,and oxaliplatin;IRI: Irinotecan;Liv: Liver;Loc: Local;LN: Lymph node;Lun: Lung;Oxali: Oxaliplatin;Pat: Patient;Pt:Peritoneal;RASmut: RAS mutated;RASwt: RAS wild-type;RD: Relapsing disease;Rt: Rectum.
A Neo-RAS wt patient,who reached later treatment lines,was treated with folinic acid+fluorouracil+irinotecan and cetuximab in third line (3L).The majority of the remaining patients who received 3L treatment throughout follow-up were administered trifluridine/tipiracil in that setting.
OUTCOME AND FOLLOW-UP
The study was approved by the Ethics Committee of Centro Hospitalar Barreiro Montijo.The study was implemented and conducted in accordance with the principles of the Declaration of Helsinki.Informed consent to publish this paper was obtained from all patients.Follow-up contacts were made during outpatient clinical appointments.When needed,certificates were requested.
We employed SPSS 22.0 software to perform statistical data description and analysis.OS was defined as the duration from the initial diagnosis to either death or loss to follow-up;PFS was defined as the time from CT to the occurrence of relapse,disease progression,death from any cause,or last follow-up assessment.OS and PFS were calculated using the Kaplan-Meier method and survival curves were drawn.To assess the differences in survival times among patients treated with various regimens,the log-rank method was used (P<0.05 was statistically significant).
Survival analysis
Our series had a 1L median PFS of 7.29 mo.Of the 11 patients,4 were Neo-RAS wt in the cfDNA analysis (36.4%),3 of them after one treatment line and the other after two lines (Table 3).Table 4 provides information regarding allele frequencies on cfDNA analysis.
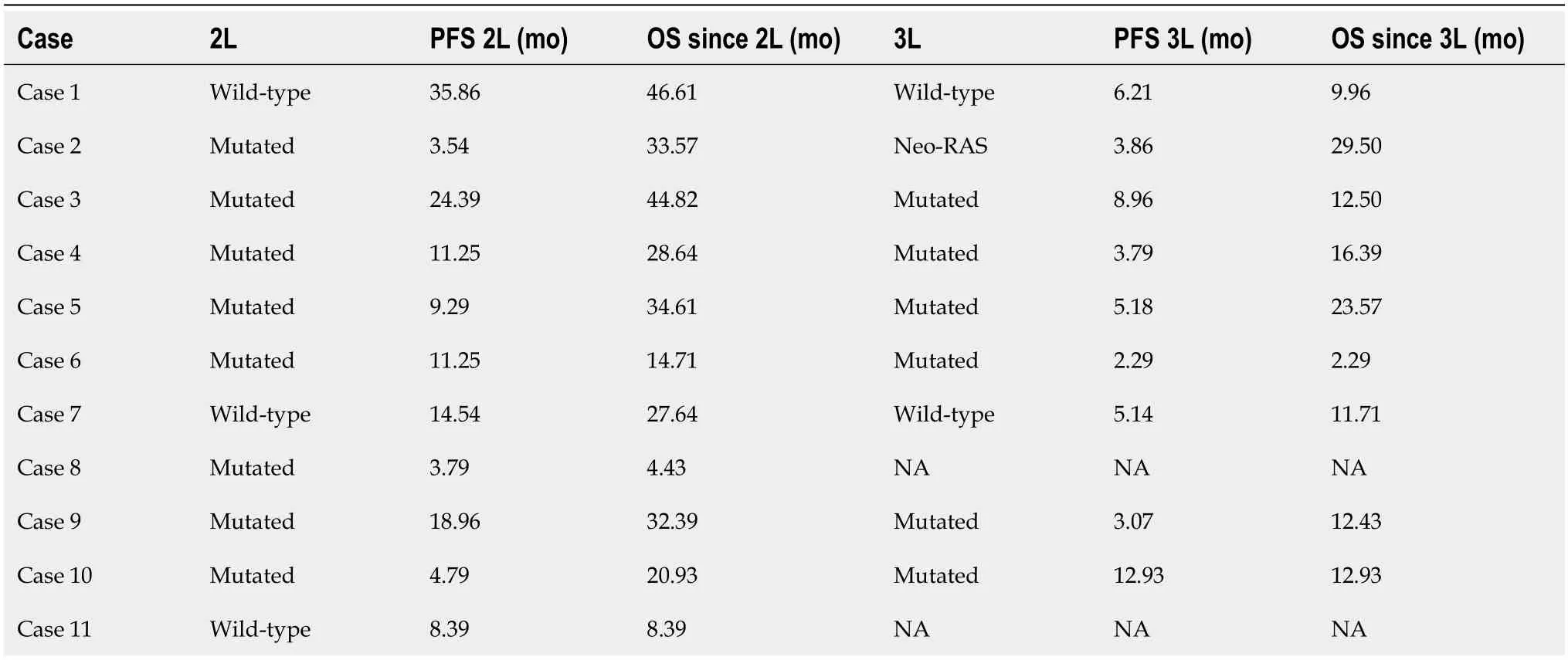
Table 3 Rat sarcoma virus mutation status and survival outcomes after second line and third line treatment

Table 4 Allele frequencies of rat sarcoma virus mutated patients
Focusing on clinical outcomes,despite the reduced number of patients in our series,our results showed a 2L PFS of 14.5 movs9.3 mo (Neo-RAS wt treated with anti-EGFR in 2L linevsall other patients),P=0.119.In the nine patients who received 3L treatment,the data showed a PFS of 3.9 movs3.8vs5.1 (Neo-RAS wt treated with anti-EGFR in 3LvsRAS mutatedvsprevious Neo-RAS wt treated with anti-EGFR),P=0.882.Regarding OS data after 2L line,we saw only a slight trend in favour of the Neo-RAS wt patients treated with anti-EGFR (33.6 movs32.4 mo,P=0.385),statistically limited due to sample size.At data cut-off,two patients were still alive.One was a RAS-mutated patient undergoing 3L treatment and the other was a neo-RAS wt patient who received 2L treatment with anti-EGFR (ongoing).
DISCUSSION
Herein,we describe a series of 11 RAS-mutated mCRC patients who were treated with a combination of CT and bevacizumab at 1L.Four patients became neo-RAS wt after 1L or 2L treatment and were treated with cetuximab,with advantages in terms of survival and disease progression.
Detection of RAS mutations is essential for selection of the best treatment option for mCRC patients.These mutations lead to constitutive activation of GTPase KRAS,resulting in permanent activation of downstream signalling of EGFR.Recent genomic studies performed through LB in mCRC patients have revealed the disappearance of RAS mutant clones in plasma.The results of these studies provided the first evidence of an unforeseen negative selection of RAS mutations during the clonal evolution of mCRC,even early in mCRC treatment.As a proof-of-concept,Gazzanigaet al[13] described the case where a patient initially diagnosed with a primary RAS mutant mCRC switched to wt RAS status in plasma,following failure of 1L therapy;the patient underwent an anti-EGFR 2L treatment,which led to a sustained clinical improvement.Later,the analysis of a small series of patients also showed a degree of benefit with anti-EGFR treatment inmCRC patients who were initially RAS-mutated and became RAS wt on LB,after one or more lines of treatment.For example,Raimondiet al[14] reported PFS of 12,10,and 6 mo in three patients treated with 2L anti-EGFR;a patient treated with fourth line anti-EGFR showed a PFS of 4 mo.Bouchahdaet al[6] reported nine neo-RAS wt patients (out of 16) who underwent treatment with anti-EGFR and CT,after a median of three CT protocols;the patients showed an objective tumour response rate of 50%,including one complete response and four partial responses,and a 1-year survival rate of 60%.
The proposed mechanism for the variable proportions of mutant and wt RAS clones throughout the clinical course of mCRC involves the existence of molecularly distinct subclones within the tumours.Under the stress applied by both the tumour microenvironment and therapies,these subclones continuously compete for space and resources[15].In this scenario,a dynamic evaluation of the clonal evolution in blood may open new opportunities for treatment[4,16].In fact,some patients may experience advantages from the use of cetuximab,after disease progression with treatment schemes with the same drug in previous treatment lines,as shown in RAS wt mCRC[17].In our case series,we observed a lower incidence of Neo-RAS wt (36.4%) but encouraging results with the combination of anti-EGFR therapy and CT treatment,with a 2L PFS of 14.5 mo in Neo-RAS wt patients after one line of treatment.These patients presented with KRAS mutation and lung and/or liver metastasis at the start of treatment,of note,the patient without liver involvement had an adrenal lesion.The best outcomes were obtained in left-sided primary tumours;however,this shall be analyzed considering that the only right-sided tumour had the LB only after two lines of treatment.Even though in our series we observed limiting skin and ocular toxicity secondary to anti-EGFR treatment in half of the patients,this strategy appears to be effective and is in line with the results of other reports and with historical PFS values regarding 2L line treatment in mCRC[11].
CONCLUSION
In RAS mutated mCRC patients,retesting RAS mutational status may be considered upon evident failure of standard-ofcare regimens.To date,the ideal point in time to perform RAS retesting is unclear,but data regarding testing in earlier lines is promising.This strategy would allow different types of treatment in the continuum of care of a mCRC patient,as evidenced in our series.For further elucidation,prospective clinical trials are warranted.At this point,there are four prospective phase II trials ongoing;three of them are studying the efficacy of anti-EGFR therapy in Neo-RAS wt mCRC patients in earlier lines of treatment (the KAIROS trial[13],the ConVertix study[18] and the MoLiMoR study[11]).Before the publication of these results,our study evidenced the prevalence of Neo-RAS wt tumours in initially RAS-mutated mCRC,in a small subset of patients.It also validated the efficacy and safety of 2L anti-EGFR plus CT treatment in this subset of mCRC patients who converted to RAS wt at the time of first progression,translating a personalized and dynamic strategy.
In conclusion,our case series showed that guiding the evolution of RAS mutations in mCRC by LB may provide an additional treatment line for Neo-RAS wt patients in advanced disease phases,in which the available therapeutic options are limited.
ACKNOWLEDGEMENTS
The authors would like to thank Paula Pinto,PharmD,PhD (PMA-Pharmaceutical Medicine Academy) for providing medical writing and editorial assistance.
FOOTNOTES
Author contributions:Gramaça J and Pina I contributed to conceptualization and manuscript design;Gramaça J,Fernandes IG,Trabulo C,Gonçalves J,dos Santos RG and Baptista A contributed to data collection and interpretation;Gramaça J contributed to manuscript drafting;Gramaça J contributed to critical revision of the article.
Informed consent statement:Informed written consent was obtained from the patients for publication of this report and any accompanying images.
Conflict-of-interest statement:Dr.Gramaça reports non-financial support from Merck KGaA,Darmstadt,during the conduct of the study.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016),and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Portugal
ORCID number:João Gramaça 0000-0001-6968-0436.
S-Editor:Qu XL
L-Editor:Webster JR
P-Editor:Zhang XD
 World Journal of Gastrointestinal Oncology2024年1期
World Journal of Gastrointestinal Oncology2024年1期
- World Journal of Gastrointestinal Oncology的其它文章
- Early gastric cancer recurrence after endoscopic submucosal dissection: Not to be ignored!
- Present situation and prospect of immunotherapy for unresectable locally advanced esophageal cancer during peri-radiotherapy
- Comprehensive evaluation of rare case: From diagnosis to treatment of a sigmoid Schwannoma: A case report
- Application of neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy in curative surgery for esophageal cancer: A metaanalysis
- Comprehensive analysis of the role of ubiquitin-specific peptidases in colorectal cancer: A systematic review
- Colorectal cancer’s burden attributable to a diet high in processed meat in the Belt and Road Initiative countries
