Lutein-stevioside nanoparticle attenuates H2O2-induced oxidative damage in ARPE cells
Zhuqing Di, Meimei Nie, Ye Chen, Jingfeng Song, Yyun Xu,Zhongyun Zhng,b,, Guoong Zhng, Shumo Yn, Xing Zhng, Djing Li,
a Institute of Agro-Product Processing, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, China
b Jiangsu Key Laboratory for Food Quality and Safety-State Key Laboratory Cultivation Base, Ministry of Science and Technology, Nanjing 210014, China
c Jiangsu Aland Nutrition Co., Ltd., Taizhou 214500, China
d Aland Nutrition Taizhou Co.,Ltd., Taizhou 225300, China
Keywords: Lutein Stevioside Antioxidant Human retinal pigment epithelial cell Mechanism
ABSTRACT In order to improve the bioavailability of lutein (LUT), a novel lutein-stevioside nanoparticle (LUT-STE) were prepared previously, but the information about LUT-STE on protecting of eye health was limited. This study investigated the effect of LUT-STE on antioxidant activity of H2O2-induced human retinal pigment epithelial(ARPE) cells. LUT and LUT-STE (f inal concentration of 5 μg/mL) signif icantly enhanced cell viability from(74.84 ± 5.10)% to (81.92 ± 10.01)% (LUT) and (89.33 ± 4.34)% (LUT-STE), and inhibited the cell apoptosis(P < 0.05). After pretreatment with LUT-STE in ARPE cells, the levels of superoxide dismutase (SOD),catalase (CAT) and glutathion peroxidase (GSH-Px) in ARPE cells were signif icantly increased (P < 0.05),the contents of reactive oxygen species (ROS) and malondialdehyde (MDA) were decreased. In addition,the vascular endothelial growth factor (VEGF) levels were inhibited by 13.61% and 17.39%, respectively,pretreatment with LUT and LUT-STE. Western blotting results showed that the pretreatment with LUT-STE inhibited the expression of caspase-9 and caspase-3 and up-regulated Bcl-2/Bax pathway to inhibit H2O2-induced apoptosis. In summary, the novel delivery LUT-STE had more pronounced inhibitory effect on H2O2-induced damage in human ARPE cells.
1. Introduction
Lutein (3,3-dihydroxy-α-carotene, LUT), a natural carotenoid containing two different ionone rings, is widely found in vegetables,fruit and f lowers[1]. Current evidence suggests lutein and its isomers play important roles in ocular development and in lowering risk for the development of common age-related eye diseases in older age[2-3].The retinal pigment epithelial (RPE) cells, an integral part of the retina, located in the outermost layer of the retina, contain vital macular carotenoids such as zeaxanthin and LUT[4]. Depending on the concentration, macular carotenoids absorb 40%–90% of incoming blue light[5]. This absorption protects the retina from photochemical damage and reduces light scattering[6]. Studies have found that a negative correlation between serum LUT concentration and eye diseases such as cataract and age-related macular degeneration (AMD)[7].Oxidative stress is a well-established factor that contributes to the pathogenesis of several age-related diseases, including AMD[8].Hydrogen peroxide (H2O2) is one of the physiologically relevant oxidants in ocular tissue and one of the major factors leading to aging. Numerous studies have shown that H2O2can induce oxidative damage and apoptosis in a variety of cellsin vitro[9-10]. More and more evidence showed that RPE cells are vulnerable to oxidative stress,especially from reactive oxygen species (ROS)[11], and the damage is thought to be an early event in AMD. In addition, during the early period of AMD, RPE cells gradually became dysfunctional and died due to apoptosis due to insufficient cell protection reaction and oxidative stress[12]. Therefore, protecting RPE cells from oxidative damage may be an effective strategy to improve AMD.
Currently, it is speculated that the protection mechanism of LUT against eye diseases is strong related to its antioxidant properties[13-14].Due to its unique ionone ring dihydroxy structure, LUT can act as a strong antioxidant to quench singlet oxygen and scavenge other ROS. Studies have found that when C4 atom on LUT conjugated polyene chain is deprotonated, ROS activity can be inhibited and damage to normal cells can be prevented[15]. Schaffer et al.[16]found that the spleen of mice contains a high concentration of LUT, and the accumulated LUT may play an important role in the capture and destruction of pathogens and the elimination of senescent cells, so as to protect the spleen from oxidative stress[16]. Bhattacharyya et al.[17]found that LUT can protect liver cell DNA from oxidative damage by inhibiting the levels of Fe2+and H2O2and reducing the generation of hydroxyl radicals. LUT can also bind with lipids to inhibit lipid peroxidation caused by free radicals and resist cell damage caused by free radicals in the human body[18].
However, the multiple conjugate double bonds in LUT structure makes it extremely unstable and prone to isomerization, oxidation and degradation[19]. At the same time, the high hydrophobicity of LUT severely affects its absorption and bioavailability in the human body. Manufacturing suitable carrier is one of the effective ways to solve these problems. As reported, nanocarrier system, liposomes and emulsion-based delivery systems are effective approaches to enhance the bioavailability of LUT[20-21]. In addition, to improve stability and extend the storage time, watersoluble nanoparticles is developed to delivery[22]. Our previous study prepared a novel lutein-stevioside(STE) nanoparticle (LUT-STE) by using STE as the wall material.STE is a natural diterpenoid steviol glycoside, which is composed of a hydrophilic glucosyl unit and a rhamnose unit on both sides of a hydrophobic steviol[23]. Recent studies have shown that STE shows a remarkable surface activity and can increase the water solubility of numerous hydrophobic compound[24]. The property of LUT-STE was further evaluated, LUT-STE nanoparticles showed a uniform distribution with an average particle size of (165 ± 2) nm. Multiple spectroscopy and NMR analyses showed LUT and STE could interact through hydrogen bonds, C–H–π interaction and van der Waals forces. LUT was well distributed in the hydrophobic cavity of STE by molecular docking simulation. The bioavailability of LUT-STE was increased by 7.71 and 2.39 times compared with LUT usingin vitrodigestion model and Caco-2 cell model, respectively[25]. Althoughin vivoandin vitroexperiments confirmed that LUT-STE was a better carrier for LUT, our understanding of the mechanisms underlying the benefits of the novel LUT-STE concerning on protecting retinal was still not clear.
Human adult retinal pigment epithelial (ARPE-19) has been widely used as an alternative to primary cultures, which exhibit similar epithelial cell morphology. ARPE-19 cell line was used asin vitromodel of RPE in the present study. The objective was to investigate the protective effects of LUT-STE on H2O2-mediated oxidative stress ARPE-19 cell and evaluate the effects of the novel nanoparticle on AMD.
2. Materials and methods
2.1 Chemicals
ARPE-19 cells were obtained from Cell Centre of the Chinese Academy of Sciences (Shanghai, China). LUT (85%) was purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China).All-trans LUT standard (≥ 97%) was purchased from Sigma Aldrich(St. Louis, USA). STE (≥ 90%) was purchased from Macklin Biochemical Technology Co., Ltd. (Shanghai, China). DMEM/F-12 medium, fetal bovine serum (FBS) and apoptosis (Annexin V-FITC/propidium iodide (PI)) kits were purchased from Thermo Fisher Biochemistry Co., Ltd. (Shanghai, China). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) cell proliferation kit, ROS assay kit, and bicinchoninic acid (BCA)-protein-assay kit were bought from Beyotime Biotechnology Co.,Ltd. (Shanghai, China). Andygene human caspase-3, catalase (CAT),glutathione-peroxidase (GSH-Px), and superoxide dismutase (SOD)activity assay kits and vascular endothelial growth factor (VEGF)enzyme linked immunosorbent assay (ELISA) kit were bought from SenBeiJia Biological Technology Co., Ltd. (Nanjing, China).Antibodies for caspase-3, caspase-9, B-cell lymphoma-2 (Bcl-2), Bcl-2 associated X Protein (Bax) and p53 andβ-actin were obtained from Cell Signaling Technology, Inc., USA.
2.2 Cell culture and treatment
LUT-STE was prepared in our laboratory previously reported[25].The LUT and LUT-STE were dissolved by 0.1% dimethyl sulfoxide(DMSO), STE was dissolved in phosphate buffered saline (PBS). The physical mixture the mixture of LUT and STE is recorded as LUT +STE, dissolved by DMSO to the set concentration, the quality of LUT and STE in LUT + STE is equivalent to that in LUT-STE. ARPE cells were maintained in DMEM/F12 medium supplemented with 10% FBS at 37 °C in a 5% CO2humidified incubator[26]. The cells were used for all the experiments at 80%−90% confluence. The cells at a density of 5.0 × 105cells/mL were seeded in a 96-well or 6-well plate, and pretreated with LUT, STE, LUT + STE, LUT-STE at a final concentration of 5 μg/mL for 4 h. Then, the sample cells were stimulated with 800 μmol/L H2O2for 2 h. Normal-cell without any pretreatment and H2O2stimulation was used as the control, labled as BLK. H2O2-stimulation group (H2O2), without pretreatment, was set as the oxidative model. H2O2was dissolved in DMEM/F12 medium,final concentration remained 800 μmol/L. The 6-well plates were used in the apoptosis analysis, ELISA, and western blotting. The 96-well plates were used in the MTT and ROS assays.
2.3 Cell-viability assays
The determination of cell viability was used by the MTT method[8]. ARPE cells with 1 × 105cells/well were incubated into 96-well plates and cultured for 24 h for pretreated with LUT, STE,LUT + STE and LUT-STE for 4 h and stimulated with 800 μmol/L H2O2for 2 h. The BLK group was without any pretreatment, and the cells stimulated with H2O2only were used as the oxidative model.Then 10 μL MTT (5 mg/mL) solution was added to each well and incubated at 37 °C for 4 h. The supernatant was discarded and 150 μL of DMSO was added to the each well to dissolve formazan crystals. After shaking slowly for 10 min, the well plate was placed on Microplate Reader (Bio-Rad, Hercules, USA). The absorbance of each well was examined at 490 nm.
2.4 Determination of intracellular ROS
Intracellular ROS level was measured by a 2’,7’-dichlorodihydr ofluorescein diacetate (DCFH-DA) kit in ARPE-19 cells. Concisely,the cells were cultured in 6-well or 96-well plates with different treatments mentioned above. After washing the cells by PBS,10 μmol/L DCFH-DA solution was added to each well and incubated for 30 min. The fluorescence intensity was measured by a fluorescence plate at excitation wavelength of 485 nm and emission wavelength of 530 nm. Finally, the cells in 6-well plate were washed with PBS and examined under an inverted fluorescent microscope(Model 1X73, Olympus Corp., Japan).
2.5 Apoptosis assays
ARPE cells with different treatments were collected, washed with PBS for three times, centrifuged (1 000 r/min, 5 min). Then 195 μL 1 × Binding Buffer was added to resuspended the cells,followed by 5 μL of Annexin V-FITC and 10 μL of a PI solution, and gently mixed. The reaction was carried out at room temperature for 15 min, and 400 μL of binding buffer was added. Flow cytometry was done by fluorescence activated cell sorting (FACS) (Beckman Coulter, Fullerton, USA).
2.6 ELISA assay
The levels of CAT, SOD, GSH-Px, MDA and VEGF were determined by ELISA kits[21]. After pretreated with different groups,the supernatants in 6-well plates were collected and determined according to the ELISA kit protocol instructions. The total cell protein was measured with a BCA protein assay kit. The absorbance was measured at 450 nm on Microplate Reader (Bio-Rad, Hercules, USA).
2.7 Western blot
The pretreated ARPE cells were washed with PBS for three times, collected after centrifugation (8 000 ×gat 4 °C for 20 min),and lysed for 1 h on ice. The total cell protein was measured with a BCA kit. A total of 20 μg of the protein sample was separated by 4%−20% sodium dodecyl sulfate polyacrylamide gel electrophoresis(SDS-PAGE) gels, and transferred to a polyvinylidene fluoride(PVDF) membrane (Merck, Germany). The membranes were blocked with 5% nonfat dairy milk in Tris-buffered saline with Tween-20 for 1.5 h, and incubated with a primary antibody at 4 °C overnight,followed by incubation with a horseradish peroxidase(HRP)-conjugated secondary antibody for 2 h. After washing, blots were treated with ECL blotting solution for 10 min and then exposed to film in a dark box. The protein band intensities were measured using the ImageJ software and normalized toβ-actin. Each western blot was performed in triplicate.
2.8 Statistical analysis
Each group was repeated three times, data were expressed as mean values ± SD. Data were assessed by one-way ANOVA followed by Duncan’s multiple-range test or Student’sttest (SPSS 22.0, NC,USA). Statistically significant differences were set atP< 0.05.
3. Results
3.1 Effects on survival rate of ARPE cells
MTT method was used to detect the effects of different treatments on the cell viability of ARPE cells, as shown in Fig. 1. Compared with BLK group, ARPE-19 cells were exposed to 800 μmol/L H2O2for 2 h caused a significant reduction in the cell viability (P< 0.05),indicating that H2O2has a toxic effect on cells and significantly affects the growth of ARPE cells, as 800 μmol/L H2O2induced a 25.16%loss of cell viability (P< 0.05). Pretreatments with 5 μg/mL LUT,STE, LUT + STE and LUT-STE inhibited the loss of cell viability,which were (81.92 ± 10.01)%, (75.22 ± 6.06)%, (85.36 ± 6.85)% and(89.33 ± 4.34)%, respectively (Fig. 1). There was no significant difference between LUT, STE and H2O2model group, LUT + STE and LUT-STE were significantly different from H2O2model group(P< 0.05). Pretreatment with LUT-STE significantly enhanced the cell viability by 19.36%. Chong et al.[27]found that when the concentration of LUT was below 100 μmol/L (568.85 μg/mL), no significant difference in cell survival rate was observed after 24 h treatment of ARPE cells. Therefore, the concentration of 5 μg/mL LUT has no toxicity to ARPE cells in the present study, and even being able to protect ARPE cells against H2O2-induced cytotoxicity.
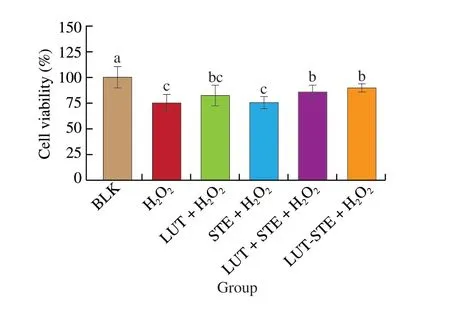
Fig. 1 Effects of LUT, STE, LUT+STE and LUT-STE on ARPE-19 cell viability. ARPE-19 cells were pretreated with 5 μg/mL LUT, STE, LUT + STE and LUT-STE aqueous solution for 4 h prior to an incubation with 800 μmol/L H2O2 for 2 h. Values are shown as the means ± SD. Data with different letters display significant differences (P < 0.05).
3.2 Effects on ROS level in ARPE cells
The effects of different treatments on H2O2induced ROS level in ARPE cells were shown in Fig. 2. ARPE-19 cells exposed to 800 μmol/L H2O2had a significantly higher ROS level than the BLK group (P< 0.05). The addition of LUT and/or STE could able to protect ARPE-19 cells against H2O2-induced ROS. Pretreatments with 5 μg/mL LUT, STE, LUT + STE and LUT-STE significantly reduced levels of H2O2-induced ROS by (25.64 ± 2.15)%,(29.57 ± 4.15)%, (56.24 ± 3.64)%, and (83.15 ± 2.57)%, respectively(P< 0.05), compared with the oxidative model. LUT-STE, the novel nanoparticle we prepared, exhibited the strongest antioxidant capacity with a ROS level even less than that of the BLK group (P< 0.05),indicating that LUT-STE has better antioxidant capacity.
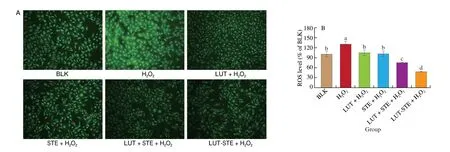
Fig. 2 Effects of LUT, STE, LUT + STE and LUT-STE on ROS levels in ARPE-19 cells. (A) ROS representative images in ARPE-19 cells (10 ×). (B) ROScontent. ARPE-19 cells were pretreated with 5 μg/mL LUT, STE, LUT + STE and LUT-STE aqueous solution for 4 h prior to incubation with 800 μmol/L H2O2 for 2 h. Values are shown as the means ± SD. Data with different letters display significant differences (P < 0.05).
3.3 Protective effects against H2O2-induced cell apoptosis in ARPE-19 cells
The effects of different treatment on apoptosis of ARPE cells were shown in Fig. 3. H2O2-induced apoptosis increased 1.12 times higher than BLK group, and pretreatment of LUT, STE, LUT + STE and LUT-STE could protect ARPE cells from H2O2-induced apoptosis.The total apoptosis rates of BLK, H2O2, LUT, STE, LUT + STE and LUT-STE groups were (8.46 ± 0.59)%, (17.94 ± 1.01)%,(12.01 ± 1.20)%, (12.07 ± 2.02)%, (10.85 ± 0.95)% and (5.76 ± 1.24)%,respectively. Compared with H2O2model group, pretreatment of LUT,STE, LUT + STE and LUT-STE could inhibit cell apoptosis induced by H2O2by 33.05%, 32.72%, 39.52% and 67.89%, respectively.LUT-STE showed the best inhibitory effect on apoptosis.
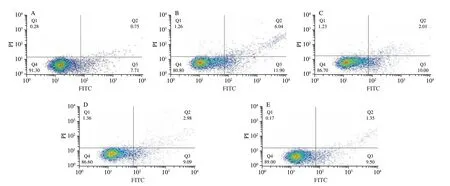
Fig. 3 Effects of LUT, STE, LUT + STE and LUT-STE on ARPE cell apoptosis. (A) BLK; (B) H2O2; (C) LUT; (D) STE; (E) LUT + STE; (F) LUT-STE.ARPE-19 cells were pretreated with 5 μg/mL LUT, STE, LUT + STE and LUT-STE aqueous solution for 4 h prior to a incubation with 800 μmol/L H2O2 for 2 h.Apoptosis was measured by flow cytometry using Annexin V-PI double staining. Graphs represent the combined percentages of early (Q2) and late (Q3) apoptosis relative to all the cells. Values are shown as the means ± SD. Data with different letters display significant differences (P < 0.05).
3.4 Effects of different treatment groups on MDA content of ARPE cells induced by H2O2
The effect of different treatment groups on MDA content of ARPE cells induced by H2O2is shown in Fig. 4. ARPE-19 cells exposed to 800 μmol/L H2O2had a significantly higher MDA content than the BLK group, but there was no significant difference. Pretreatment with LUT and STE decreased the level of MDA, but its MDA content was not significantly different from that of the oxidative model.Compared to the oxidative model, LUT + STE and LUT-STE were able to significantly attenuate the MDA contents in ARPE-19 cells(P< 0.05) by 23.81% and 17.40%, respectively. In general, LUT + STE exhibited the strongest inhibitory effect.
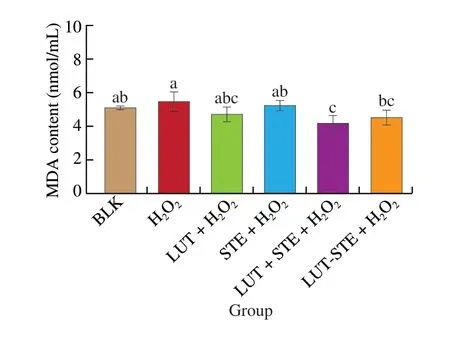
Fig. 4 Effects of LUT, STE, LUT + STE and LUT-STE on MDA levels in ARPE-19 supernatants. ARPE-19 cells were pretreated with 5 μg/mL LUT,STE, LUT + STE and LUT-STE aqueous solution for 4 h prior to a incubation with 800 μmol/L H2O2 for 2 h. Values are shown as the means ± SD. Data with different letters display significant differences (P < 0.05).
3.5 Effects on levels of extracellular antioxidant enzymes CAT, SOD and GSH-Px in ARPE cells
The effects of different treatment groups on the levels of extracellular antioxidant enzymes in ARPE cells are shown in Fig. 5.After 800 μmol/L H2O2treatment, the SOD content reduced from(125.91 ± 4.39) pg/mL to (110.69 ± 7.56) pg/mL (P< 0.05),and GSH-Px content reduced from (43.48 ± 3.56) pmol/mL to(32.95 ± 2.80) pmol/mL (P< 0.05), but the CAT content had no significant difference compared to the BLK group. ARPE cells pretreatment with LUT, STE, LUT + STE and LUT-STE could increase the contents of these antioxidant enzymes to a certain extent.Compared with H2O2model group, the contents of SOD and GSH-Px in LUT, STE, LUT + STE and LUT-STE groups were significantly increased (P< 0.05). The content of CAT in LUT-STE and LUT +STE groups increased significantly (P< 0.05), while pretreatment with LUT and STE had no significant effect. The contents of SOD and CAT in LUT-STE group were higher than other pretreatments,suggesting that LUT-STE had better antioxidant activity.
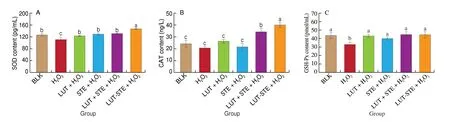
Fig. 5 Effects of LUT, STE, LUE + STE and LUT-STE on (A) SOD, (B) CAT, (C) GSH-Px levels in ARPE-19 supernatants. ARPE-19 cells were pretreated with 5 μg/mL LUT, STE, LUT + STE and LUT-STE aqueous solution for 4 h prior to incubation with 800 μmol/L H2O2 for 2 h. Values are shown as the means ± SD. Data with different letters display significant differences (P < 0.05).
3.6 Effects on the level of VEGF in ARPE cells
The effects of different treatments on the level of VEGF in ARPE cells supernatants were shown in Fig. 6. After 800 μmol/L H2O2incubation, the VEGF content in supernatant of ARPE cells was up-regulated, which was 1.30 times that of BLK group. The addition of LUT, LUT-STE and LUT + STE were able to decrease VEGF production to some extent. After pretreatment with LUT, STE,LUT + STE and LUT-STE, VEGF levels were inhibited by 17.39%,9.01%, 9.4% and 13.61%, respectively. LUT and LUT-STE exhibited significant inhibitory effects (P< 0.05), however, STE and LUT +STE did not show significant inhibition. Compared with LUT-STE,LUT had the best inhibitory effect.
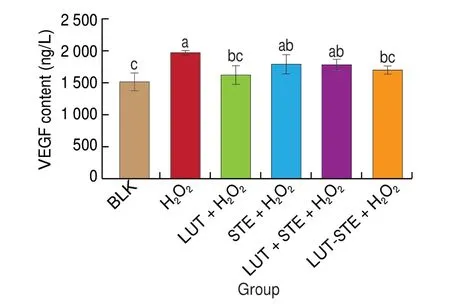
Fig. 6 Effects of LUT, STE, LUT + STE and LUT-STE on VEGF levels in ARPE supernatants. ARPE-19 cells were pretreated with 5 μg/mL LUT, STE,LUT + STE and LUT-STE aqueous solution for 4 h prior to incubation with 800 μmol/L H2O2 for 2 h. Values are shown as the means ± SD. Data with different letters display significant differences (P < 0.05).
3.7 Effects on protein expression of Bcl-2, Bax, caspase-3,caspase-9 and p53 in ARPE cells
The effects of different treatment groups on the protein expressions of Bcl-2, Bax, caspase-3, caspase-9 and p53 in ARPE cells were shown in Fig. 7. Compared with BLK, the expression level of Bcl-2 in ARPE cells was significantly decreased after H2O2induction, while the expression level of Bax was significantly increased (P< 0.05). Pretreatment with LUT, STE, LUT + STE and LUT-STE can effectively inhibit the influence of H2O2. Compared with H2O2model group, pretreatment with LUT, STE, LUT + STE and LUT-STE could increase the expression of Bcl-2. The relative expression levels were 0.86 ± 0.08, 0.80 ± 0.05, 0.88 ± 0.04, and 0.93 ± 0.06, with promotion rate of 62.26%, 48.15%, 62.94%, and 7.22%, respectively (P< 0.05). In contrast, LUT, STE, LUT + STE and LUT-STE decreased Bax expression with inhibitory rates of 25.51%, 30.61%, 40.82%, and 42.86%, respectively. Furthermore,the Bcl-2/Bax ratio was used to evaluate cell apoptosis among different treatment groups. The Bcl-2/Bax ratios of BLK, H2O2, LUT,LUT + STE and LUT-STE were 0.75, 0.56, 1.19, 1.17, 1.52 and 1.64,respectively. It can be seen that LUT-STE had the highest inhibitory effect, indicating that LUT-STE can effectively inhibit cell apoptosis induced by H2O2. This result was consistent with cell apoptosis by flow cytometry (Fig. 3).

Fig. 7 Effects of LUT, STE, LUT + STE and LUT-STE on expressions of apoptosis-related proteins. (A) Immunoblotting; 1, BLK; 2, H2O2; 3, LUT + H2O2;4, STE + H2O2; 5, LUT + STE + H2O2; 6, LUT-STE + H2O2. Expression levels of (B) Bax, (C) Bcl-2, (D) Caspase-9, (E) Caspase-3 and (F) p53 in ARPE cells.ARPE-19 cells were pretreated with 5 μg/mL LUT, STE, LUT+STE and LUT-STE aqueous solution for 4 h prior to incubation with 800 μmol/L H2O2 for 2 h.Values are shown as the means ± SD. Data with different letters display significant differences (P < 0.05).
The p53 protein can specifically inhibit the expression of Bcl-2 protein, but significantly promote the expression of Bax protein. From the results, after H2O2induction, the expression level of p53 was up-regulated significantly. While the pretreatment with LUT,STE, LUT + STE and LUT-STE inhibited p53 protein expression,compared with H2O2group.
In addition, the expression of caspase-3 and caspase-9 proteins was also observed in the present study. After 800 μmol/L H2O2induction, the expression level of caspase-3 and caspase-9 was increased significantly (P< 0.05), compared to the BLK group.Whereas, pretreatment with LUT, STE, LUT + STE and LUT-STE could inhibit the activities of caspase-9 and caspase-3 levels induced by H2O2. The relative expression of caspase-3 levels in the cells pretreated with LUT was lowest, indicating LUT had the best inhibitory effect in this study.
4. Discussion
AMD, causing degeneration of the light sensitive cells of the macula and RPE, is an age-related disease. Intaking natural antioxidants such as LUT and zeaxanthin in diet can prevent retinal damage[28]. Dietary LUT can selectively accumulate in high density in the retina, similarly, enhance its stability in the retina[29]. Our results confirmed the content of LUT in the eyes of mice fed with LUT-STE was higher than that of the LUT group, indicating that STE embedding could promote the content of LUT in eye tissues[25].Oxidative stress is considered an important factor of ARPE cell death during the pathogenetic process of AMD. Therefore, in this study,H2O2was used to induce oxidative stress in ARPE cell to explore the antioxidant effect of LUT-STE and its underlying mechanisms.
Studies have shown that H2O2induced oxidative damage and apoptosis in retinal cells[30]. Oxidative damage can be inhibited through the intervention of the effective antioxidant defense mechanisms, but ROS levels increase and antioxidant levels decrease in aging tissues[31]. The results showed that ARPE cells treated with 800 μmol/L H2O2significantly decreased cell survival rate and increased intracellular ROS levels, indicating that 800 μmol/L H2O2has oxidative damage and cytotoxicity on ARPE cells. LUT, as a powerful antioxidant, inhibits H2O2oxidative stress through oxidative defense mechanisms. After pretreatment with 5 μg/mL LUT, STE,LUT + STE and LUT-STE, ARPE cell could be effectively protected from H2O2induced oxidative damage. From the results, LUT-STE can significantly reduce ROS level in ARPE cells, improve cell survival rate, and protect ARPE cells from H2O2induced oxidative damage,and its effect was significantly better than LUT, STE and LUT + STE.It indicated that LUT-STE, the novel LUT nanoparticles we prepared,not only improved the bioavailability of LUT, but also improved the antioxidation. LUT-STE is a promising source of antioxidants.
To investigate the potential mechanisms of LUT-STE, this study focused on antioxidant defense system and the apoptosis pathway.SOD, CAT and GSH-Px are important endogenous antioxidant enzymes, which can protect cells from oxidative stress damage and inhibit ROS production[32]. The contents change of antioxidant enzymes SOD, CAT and GSH-Px secreted by ARPE cells were further determined. The results showed that after ARPE cells exposed to 800 μmol/L H2O2significantly increased the contend of MDA,but reduced contents of SOD, CAT and GSH-Px. After pretreatment with LUT, STE, LUT + STE and LUT-STE, on the one hand, MDA secretion was significantly reduced and lipid peroxidation was inhibited. On the other hand, the activity of cells was significantly improved, the contents of SOD, CAT and GSH-Px were increased, so that the ROS in the cell was cleaned in time, resulting in a harmonious and dynamic balance state. Pretreatment with LUT-STE increased contents of antioxidant enzymes secreted by ARPE cells significantly higher than LUT, STE and LUT + STE, indicating that LUT-STE have the best inhibitory effect on cell apoptosis induced by H2O2.Probably because LUT-STE pretreatment favored the accumulation of LUT in the retina, which in turn enhanced the antioxidant effect of LUT. Therefore, our results demonstrated that a possible mechanism for the antioxidant activities of the LUT-STE could be that it interferes with the glutathione antioxidant system, which is involved in peroxide scavenging.
VEGF increases endothelial permeability and proliferation, which stimulates vasculogenesis and angiogenesis[26]. Overexpression of VEGF can lead to vascular diseases in the retina and other parts of the body[33]. Karkkainen et al.[34]found that aging ARPE cell could upregulate VEGF expression, thus blocking effect of VEGF, which has become an important way to fight against AMD. This study confirmed that H2O2induced up-regulated the content of VEGF in ARPE cells.Pretreatment with LUT can inhibit extracellular VEGF production to a certain extent, and the inhibitory effect of LUT and LUT-STE was better than that of STE and LUT + STE. Therefore, LUT-STE can be used as an anti-VEGF therapeutic compounds, which can not only prevent AMD disease, but also effectively control the formation of new blood vessels.
When excessive ROS in the body cannot be cleared in time by endogenous antioxidant defense mechanisms, it can lead to cell apoptosis. Apoptosis is not a passive process, but an active one,involving activation, expression and regulation of a series of genes.Caspases play an important role in cell apoptosis. Caspase-9, is an important initiate factor in the mitochondria dependent apoptosis pathway, acting on caspase-3 protein when caspase-9 is activated.Caspase-3 protein is the executor of apoptosis, and its activation marks the irreversible stage of apoptosis[35]. The study investigated the expression of caspase-9 and caspase-3 protein. H2O2induction can activate caspase-9 protein to increase ROS level in ARPE cells and regulate caspase-3 to perform the apoptosis process. While LUT, STE, LUT + STE and LUT-STE can inhibit the expression of caspase-9, decrease ROS level in ARPE cells and inhibit the activation of caspase-3. Murthy et al.[36]reported that lutein pretreatment significantly reduced the level of caspase-3 in ARPE-19 cells. Liu et al.[37]confirmed that LUT pretreatment significantly reduced the total caspases protein content in ARPE cells. The results of this experiment are consistent with theirs, but LUT-STE has a slightly lower inhibitory effect than LUT on caspases protein expression.
The Bcl-2 family is also a key regulator of apoptosis, including activators (e.g. Bax) and inhibitors (e.g. Bcl-2)[38]. Studies have shown that anti-apoptotic protein Bcl-2 can protect the integrity of mitochondrial membrane[39]. Bcl-2/Bax ratio is considered to be an important indicator for evaluating anti-apoptotic ability[40]. The higher of the ratio, the stronger of the anti-apoptotic ability. H2O2decreased the ratio of Bcl-2/Bax, while pretreatment of LUT, STE, LUT + STE and LUT-STE increased the expression of Bcl-2 and the ratio of Bcl-2/Bax, and reduced the expression of Bax. At the same time, p53 protein can inhibit the expression of Bcl-2 and promote the expression of Bax, H2O2inducing significantly increased the expression level of p53. Pretreatment of LUT, STE, LUT + STE and LUT-STE reduced the expression of p53 protein. These results suggested that LUT and LUT-STE may regulated the expression of Bcl-2 and Bax by regulating p53 protein. The Bcl-2/Bax ratio of ARPE cells pretreatment with LUT-STE was 1.38 times of LUT, indicating that the anti-apoptotic ability of LUT-STE is higher than that of LUT. Therefore, LUT-STE protects ARPE cells from apoptosis by regulating the expression of caspase, p53 and Bcl-2/Bax pathways.
In summary, LUT-STE, the novel lutein nanoparticles we prepared, protected ARPE-19 cells against H2O2-induced oxidative damage. LUT-STE regulated antioxidant effects by reducing the ROS level and lipid peroxidation level in ARPE cells, increasing the levels of the antioxidant enzymes including SOD, CAT, and GSH-Px. On the other hand, LUT-STE inhibited cell apoptosis by blocking the caspase and p53 proteins. Therefore, LUT-STE had more significant inhibitory effect on H2O2-induced damage in ARPE cells than LUT,and thus might have the potential application in protecting eye health.
Conflict of interest
The authors declare no competing financial or non-financial interests.
Acknowledgement
The present work received research funding from the National Natural Science Foundation of China (31801541), the Independent Innovation Fund Project of Agricultural Science and Technology in Jiangsu Province (CX(22)3065), Major Scientific and Technological Achievements Transformation Project of Taizhou (SCG 202105), and the Taizhou Science and Technology Support Plan (TN202106).
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

