具有红色荧光性质的掺硒碳点在生物传感和抗菌中的多功能应用
厉圆圆 卢修联 刘欣雨 张 磊 景 苏
(南京工业大学化学与分子工程学院,南京 211816)
0 Introduction
Over the past decade,there has been a noteworthy rise in the utilization of carbon dots (CDs) in various biological and biomedical applications[1].This trend can be attributed primarily to their small size (1-20 nm), distinct optical characteristics, ease of functionalization, and biocompatibility[2].The ability to tune their functional properties gives CDs unique advantages in bioimaging, making them highly effective as nanocarriers for delivering various cargos in biosensing and tumor therapy applications[3].Among these properties,the fluorescence properties of CDs have sparked interest in their use as a replacement for semiconductor quantum dots[4].However, there are certain challenges associated with CDs that are composed exclusively of carbon atoms, such as poor solubility in water, low quantum yields, and mostly blue - wavelength emission[5].To address these challenges, doping carbon quantum dots with heteroatoms is a highly effective strategy to manipulate fluorescent properties[6].Interestingly,selenium is known to have semiconductor properties and can readily participate in redox reactions for reactive oxygen species regulation[7].Despite the crucial role of selenium in various biological processes,the application of selenium -doped carbon quantum dots in bioanalysis has not been extensively investigated.Xu group developed green-fluorescent seleniumdoped carbon quantum dots through the hydrothermal treatment of high-cost selenocystine under mild conditions[5].Su et al.reported ultrasmall selenium-doped carbon dots (Se-CDs) by using a reactor of internal circulation rotating packed bed, which emitted bright green luminescence under excitation of UV light[8].However, the significant background interference from fluorescent emissions in the green wavelength range hampers efficient biological imaging and other analytical processes[9].
Herein, we have developed red-emission Se-CDs using a one-step hydrothermal method.Utilizing benzeneseleninic acid (BA) ando-phenylenediamine(o-PDA) as starting materials, the prepared Se-CDs have demonstrated three significant advantages(Scheme 1).Firstly, the presence of selenium has resulted in a red-shift in the fluorescence emission of CDs, shifting towards 675 nm.Secondly, the positively charged Se-CDs can effectively load negatively charged single-strand DNA (P1), showing an off-on sensing fluorescence signal to target molecule microRNA-21 (miR-21).Lastly, the redox activity of selenium has rendered Se-CDs effective in phototoxicity againstEscherichiacoli(E.coli)bacteria.
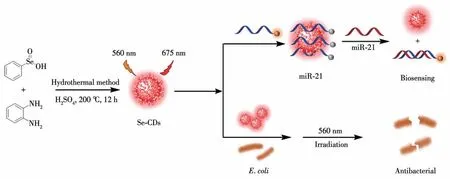
Scheme 1 Schematic illustration of the synthesis of red-emission fluorescent Se-CDs and their applications in biosensing and antibacterial activities
1 Experimental
1.1 Reagent
BA was purchased from energy chemical.o-PDA was purchased from Sinopharm Chemical Reagent Co.,Ltd.Phosphate - buffered saline (PBS, pH=7.4) and annealing buffer for DNA oligos (5X) were purchased from Beyotime Biotechnology.Deoxyribonuclease Ⅰ(DNase Ⅰ) was purchased from Beijing Solarbio Science&Technology Co.,Ltd.
1.2 Synthesis of Se-CDs
Se-CDs were synthesized through a hydrothermal method using BA ando-PDA as starting materials.In this process, a mixture of BA ando-PDA with a molar ratio of 2∶1 was dissolved in 10 mL of distilled water and sonicated until fully dissolved.Subsequently, the solution was transferred to a 15 mL polytetrafluoroethylene reaction vessel, and 5-6 drops of concentrated sulfuric acid were added.The mixture was then heated at 200 ℃for 12 h.After cooling to room temperature,the reaction mixture was transferred to a centrifuge tube and centrifuged at 10 000 r·min-1for 10 min to collect the supernatant.The supernatant was filtered through a 0.22 µm membrane and dialyzed against distilled water using a 500 Da dialysis bag for 6 h,with the dialysis solution changed every 2 h to remove any unreacted precursors.The Se-CDs were obtained by freeze-drying and stored in a refrigerator at 4 ℃until further use.DNA sequences and miR-21 (Table 1)were purchased from Sangon Biological Engineering Technology Co., Ltd.and Shanghai GenePharma Co.,Ltd.respectively.
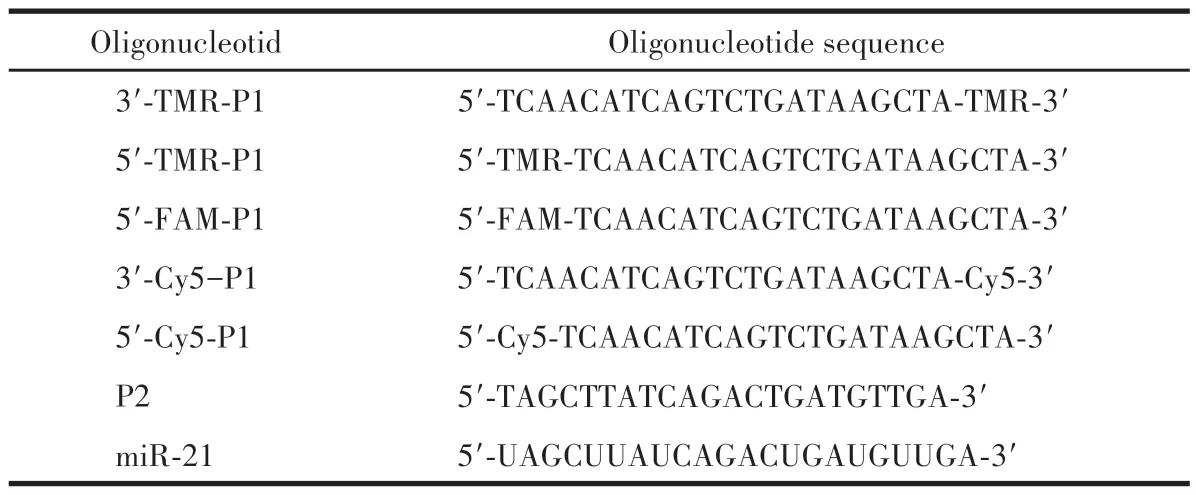
Table 1 Oligonucleotides used in this work
1.3 Characterizations
Transmission electron microscopic (TEM) images of Se-CDs were captured using a JEM-1400 (JEOL)operated at 100 kV.X-ray photoelectron spectroscopy(XPS) experiments were conducted using an ESCALAB 250 spectrometer (Thermo-VG Scientific Co., USA)equipped with an ultrahigh vacuum system.Theζpotentials and hydrodynamic diameters were determined using a Malvern Zetasizer Nano ZS90 instrument.Ultraviolet-visible-near infrared (UV-Vis-NIR)absorption spectra were measured using a UV-Vis spectrometer LAMBDA950 (PerkinElmer, Germany).Fourier transform infrared (FTIR) measurements were performed using a Nicolet iS50 spectrometer.Fluorescent spectra were recorded using an Edinburgh FLS1000 luminescence spectrometer.
1.4 Preparation of P1-functionalized Se-CDs (Se-CDs-P1)
34 µL of Se-CDs solution with varying concentrations (0, 50, 100, 200, 500, 800, 1 000 µg·mL-1) was combined with 200 µL of the dye-labeled recognition DNA(P1)at a concentration of 100µmol·L-1.The mixture was incubated in a dark environment with gentle shaking for 12 h while maintaining the appropriate temperature conditions.After incubation, the mixture was centrifuged at 15 000 r·min-1for 20 min.Subsequently,the resulting Se-CDs-P1 probe could be redispersed in PBS and stored at 4 ℃.
1.5 Detection of miR-21
200 µL of the Se-CDs-P1 solution (1.0 µmol·L-1equitant P1) was mixed with different concentrations of miR-21.The mixtures were incubated in the dark on a shaker at 37 ℃for 3 h while monitoring the changes in fluorescence using an excitation wavelength of 565 nm.Then, the DNase Ⅰsystem was introduced by adding 1.5 µL of DNase Ⅰ(10 kU·mL-1) into the detection solution.The solution was then incubated in the dark on a shaker for an additional 30 min.The changes in fluorescence were recorded once again using the same excitation wavelength of 565 nm.This experiment was repeated three times in parallel to ensure the reliability and accuracy of the results.
1.6 Antibacterial activities of Se-CDs
To investigate the antibacterial activities of Se-CDs, Se-CD solutions with concentrations of 0.01,0.07, 0.13, and 0.33 mg·mL-1were prepared.Subsequently, 0.1 mL of each Se-CD solution was added to 0.1 mL of anE.colimedium with an OD600(the optical density at 600 nm) value of approximately 0.2 in the wells of a 96-well plate.Control samples were also prepared using theE.colisolution without any Se-CDs.Upon the addition of Se-CD solutions to theE.colisamples, the OD600values were measured every hour using a microplate reader.
2 Results and discussion
2.1 Synthesis and characterization of Se-CDs
Se-CDs were synthesized through a hydrothermal method using BA ando-PDA as precursors.The utilization of BA as a precursor for synthesizing Se-CDs offered several advantages, including accessibility,high selenium content,stability,reactivity,and compatibility with scalable synthesis methods.Specifically,BA is commercially available and relatively affordable compared to other selenium precursors,such as selenocysteine.Moreover, BA has a selenium content of 42.1%,surpassing the selenium precursor 3-selenocyanatopropan-1-amine, which contains 38.7% selenium[10].This higher selenium content enhanced the effectiveness of BA as a dopant for CDs.Additionally, BA demonstrated good stability, making it suitable for handling and storage[11].It was also reactive under appropriate reaction conditions, allowing for efficient conversion into selenium species during the synthesis process.This reactivity facilitated the formation of Se-CDs with high yield and quality.
Following purification, the morphology of the Se-CDs was examined using TEM, as depicted in Fig.1A.The TEM analysis revealed an average diameter of(3.92±0.85) nm for the Se-CDs (Fig.1B).The FTIR(Fig.1C) analysis provided additional molecular confirmation regarding the composition and structure of the Se-CDs.It revealed a broad band around 3 134 cm-1,indicating the presence of hydroxyl (O—H) or amino(N—H)groups on the surface of the CDs.This observation provides important information on the functional groups present in the Se-CDs.These functional groups are derived from nitrogen or oxygen-containing groups in the carbon source.Furthermore, peaks observed at 1 623,1 520,1 400,1 020,and 760 cm-1can be attributed to stretching vibrations of C=O, C=C/C=N,C—N, C—O, and C—H bonds, respectively.To determine the doping state of selenium in Se-CDs, we performed FTIR and XPS analyses on the solid form of dried Se-CDs.XPS analysis further confirmed the composition of Se-CDs, which consists of four elements: C,N, O, and Se.The core levels corresponding to C1s(284.8 eV), N1s(401.4 eV), O1s(532.1 eV), and Se3d(56.8 eV) were identified (Fig.1D).In the high-resolution XPS spectra, specific peak assignments for various functional groups were observed.In the C1sspectrum(Fig.1G), peaks at 284.8, 286.4, and 289.0 eV were identified, corresponding to C—C, C—O, and C=O bonds, respectively.The N1sspectrum (Fig.1H) displayed peaks at 401.8, 400.9, and 399.7 eV, attributed to C=N, N—H, and C—N bonds, respectively.Similarly, in the O1sspectrum (Fig.1E), peaks at 533.3 and 532.0 eV were assigned to C—O and C=O bonds,respectively.The XPS spectrum of Se3ddisplayed peak positions at 56.1 and 54.9 eV,indicating the presence of selenium in different oxidation states (Se4+/Se2+)within the sample (Fig.1F).The peak at 56.1 eV suggested the existence of selenium in a higher oxidation state, potentially corresponding to selenium in the Se4+.Conversely,the peak at 54.9 eV indicated the presence of selenium in a lower oxidation state,potentially corresponding to selenium in the Se2+.This observation aligned with the typical binding energy range for Se in XPS spectra[12].Thus, XPS analysis offered valuable insights into the chemical bonding and composition of the Se-CDs, providing significant information about their molecular structure and elemental composition.Moreover, theζpotential of the Se-CDs was determined to beζ=(8.15±0.68)mV (Fig.1K).The positiveζpotential of the Se-CDs enhances their affinity towards negatively charged oligonucleotides, such as P1, facilitating their further binding and interaction.
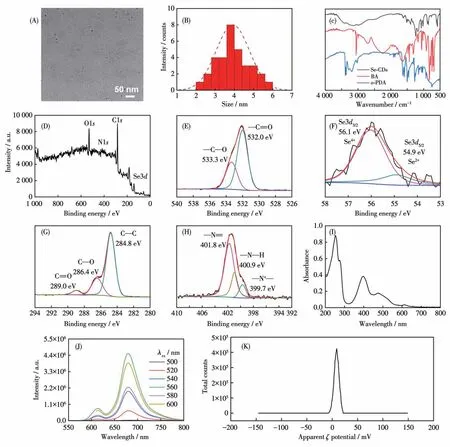
Fig.1 (A)TEM image and(B)hydrodynamic diameter distribution of Se-CDs;(C)FTIR spectra of Se-CDs,BA,and o-PDA;(D)XPS spectra and high-resolution(E)O1s,(F)Se3d,(G)C1s,and(H)N1s XPS spectra of Se-CDs;(I)UV-Vis absorption spectra of Se-CDs;(J)Dependence of fluorescence emission spectra of Se-CDs on excitation wavelength;(K)ζ potential of Se-CDs
Then, spectroscopy methods were employed to characterize the optical properties of Se-CDs, revealing a fluorescence quantum yield (ΦF) of 4.69%.As shown in Fig.1I, Se-CDs demonstrated a broad absorption range from ultraviolet to near-infrared (200-800 nm),with absorption peaks observed at 250, 400, 480, and 615 nm.The two low-wavelength absorptions at 220 and 400 nm can be attributed to theπ→π* andn→π* transitions within the carbon core, respectively.The maximum excitation and emission wavelengths of Se-CDs were determined to be 560 and 675 nm,respectively(Fig.1J).The Se-CDs exhibited a remarkable redshift towards 675 nm,surpassing the red-shift observed in selenium-doped graphene quantum dots (563 nm)[13]and selenium-doped carbon quantum dots synthesized through the hydrothermal treatment of selenocystine(490 nm)[5].This significant red-shift demonstrated the superior fluorescent wavelength property of the Se-CDs synthesized from the precursor BA.Importantly, the fluorescence emission spectrum of Se-CDs remained consistent independent of the excitation wavelength.When the excitation wavelength increased from 500 to 560 nm, the maximum emission wavelength remained unchanged.The stability of the photoluminescence wavelength of CDs is crucial to ensure accurate results in specific detection applications[14].The aforementioned findings contributed to the successful synthesis of Se-CDs and provided valuable insights into their optical properties for potential applications.
2.2 Biosensing of miR-21
miR-21 is known to be dysregulated in various diseases, such as cancer, cardiovascular diseases, and neurodegenerative disorders[15].Its detection can serve as a biomarker for early disease diagnosis and prognosis[16].To enable the detection of miR-21, the Se-CDs need to be assembled with a complementary P1 sequence that recognizes miR-21.The large surface area and positive charge of CDs facilitate the easy modification of negatively charged P1 onto their surface through physical adsorption[17].This assembly process formed the Se - CDs - P1 probe.After removing the unmodified free P1,the obtained Se-CDs-P1 probe was verified by UV-Vis-NIR absorption and circular dichroism spectroscopy analysis(Fig.2A and 2B).The absorption spectra demonstrated that the functionalization of Se-CDs with the P1 sequence resulted in a distinct peak in the absorption intensity at 260 nm, which can be attributed to the characteristic absorption of DNA.In the circular dichroism spectra, the addition of Se-CDs-P1 caused a blue shift in the circular dichroism signal of P1,indicating the interaction between Se-CDs and the DNA bases.Notably, the negative peak around 250 nm exhibited more pronounced changes compared to the positive peak near 280 nm.This observation suggests that the binding between Se-CDs and the nucleic acid primarily impacts the conformation and stacking of the DNA bases, resulting in alterations in the CD spectra.These analyses provide supporting evidence for the interaction between Se-CDs and the P1 sequence.
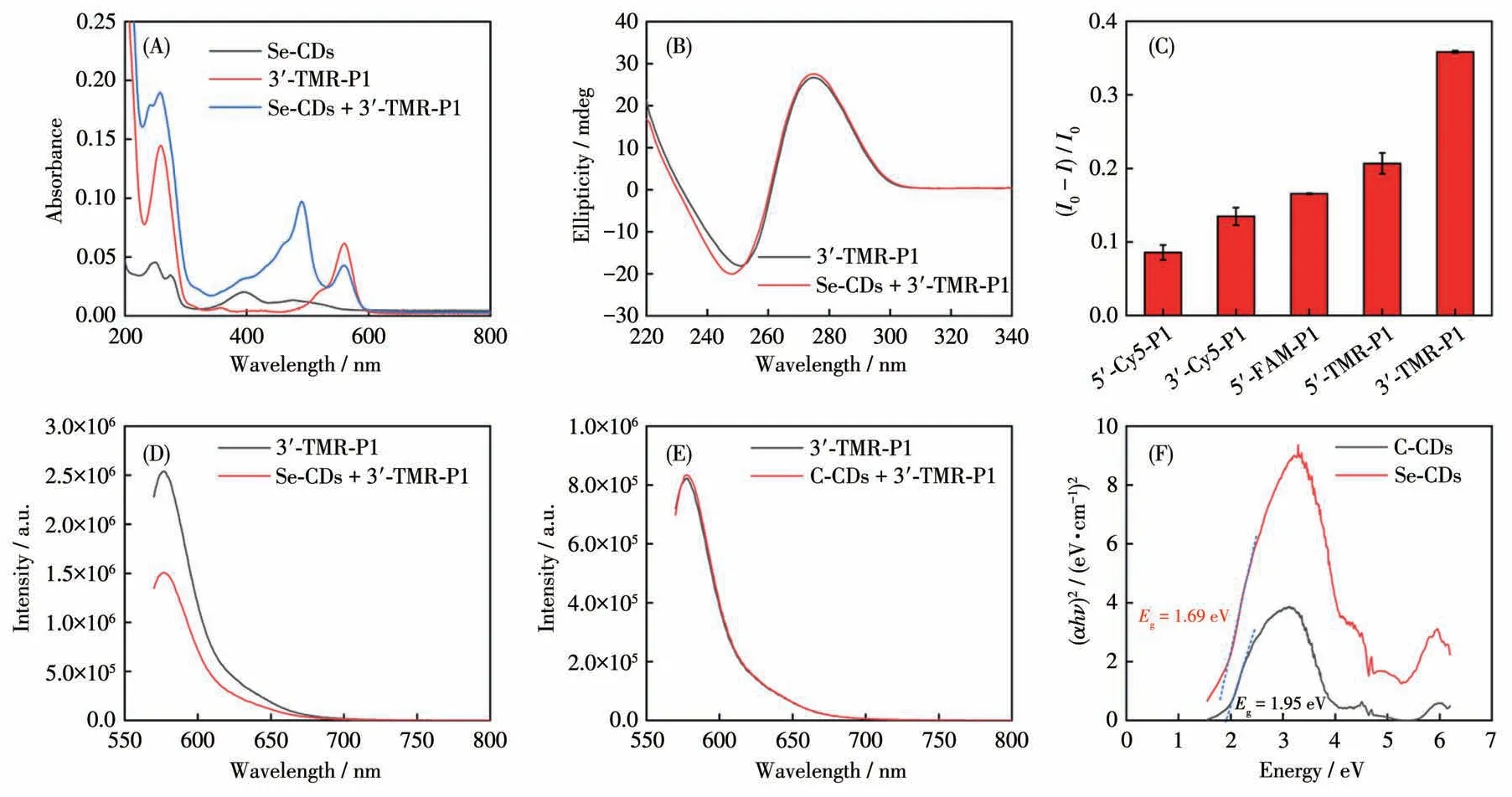
Fig.2 (A)UV-Vis absorption spectra of Se-CDs,3′-TMR-P1,and Se-CDs+3′-TMR-P1;(B)Circular dichroism spectra of 3′-TMR-P1 and Se-CDs+3′-TMR-P1;(C)Detection of quenching efficiency of Se-CDs for different fluorescent dye labeling sequences;Fluorescence response of 3′-TMR-P1 to(D)Se-CDs and(E)C-CDs at the excitation wavelength of 544 nm;(F)Indirect bandgap(Eg)calculation of Se-CDs and C-CDs
To achieve sensitive signal transduction for miR-21 detection, we aimed to incorporate a reporter molecule into the P1 sequence.For this purpose, we conducted several optimization experiments.As shown in Fig.2C, the signal displayed the maximum response when the P1 sequence was modified with the TAMRA(TMR) reporter at the 3′ end of the sequence.Therefore, for subsequent detection, we employed 3′-TMRP1 as the optimal recognition moiety.It is worth highlighting that target-triggered signal transduction occurred because the signal unit of the Se-CDs-P1 probe alone was in the off state due to the quenching effect of selenium, which is similar to that of heavy atoms.However, in the case of CDs in the absence of doped selenium, the reporting unit signal on P1 would remain unaffected (Fig.2D and 2E).To validate this result, indirectEgcalculations were conducted on Se-CDs and non-Se-doped CDs.The indirectEgof both samples was determined using UV diffuse reflectance spectroscopy (Fig.2F).TheEgvalue of Se-CDs was 1.69 eV, whereas that of carbon quantum dots without selenium doping (C-CDs) was 1.95 eV.The lowerEgvalue of Se-CDs signified a stronger light absorption capacity, as photon energy can be utilized to induce electron transition to the conduction band[18].Therefore,Se-CDs are more effective in affecting the signal of TMR, thus accelerating their potential as functional carriers for DNA assembly and constructing sensors.
Since we have demonstrated the signal switch of the Se-CDs-P1 probe, we performed the sensing measurements by using a well-established mimic of miR-21, which is referred to as the target P2 (named as P2)DNA, before introducing the actual miR-21 target.As shown in Fig.3A and 3B, a noticeable recovery in fluorescence intensity was observed after a 3 h incubation of Se-CDs-P1 with P2.As the concentrations of P2 increased, the fluorescence intensity of the probe solution exhibited a linear relationship with the logarithm of the P2 amount (Fig.3C and 3D), thereby confirming the sensing feasibility of Se-CDs.Subsequently, we introduced miR-21 to the Se-CDs+3′-TMR-P1 probe detection system.As depicted in Fig.3E and 3F, we observed a direct positive correlation between the fluorescence intensity of the probe and the concentration of the target miR-21 within the range of 0.2-10 µmol·L-1.The detection limit was 0.078 µmol·L-1at a signal-tonoise ratio of 3 with a regression coefficient of 0.994.To further enhance the sensitivity of the Se-CDs probe,we employed DNase Ⅰ.DNase Ⅰ is an enzyme responsible for degrading DNA molecules into smaller fragments[19].By incorporating DNase Ⅰinto the detection system, we aimed to improve the specificity and reliability of the assay by minimizing the impact of unwanted DNA contaminants[20].Furthermore, the addition of DNase Ⅰresulted in the detachment of 3′-TMR-P1 from the Se-CDs surface, thereby enhancing the chances of recognizing the target miR-21 (Fig.3G).The sensing performance demonstrated an improved detection limit of 6.76 nmol·L-1, and the method achieved a high level of accuracy with a regression coefficient of 0.998(Fig.3H and 3I).Notably,this detection limit was 11.5 times lower compared to the detection limit without DNase Ⅰ,revealing a substantial increase in sensitivity.Therefore, the Se-CDs-based probe showed promise in the sensitive detection of biomolecules when used in conjunction with the DNase Ⅰ-mediated amplification method.
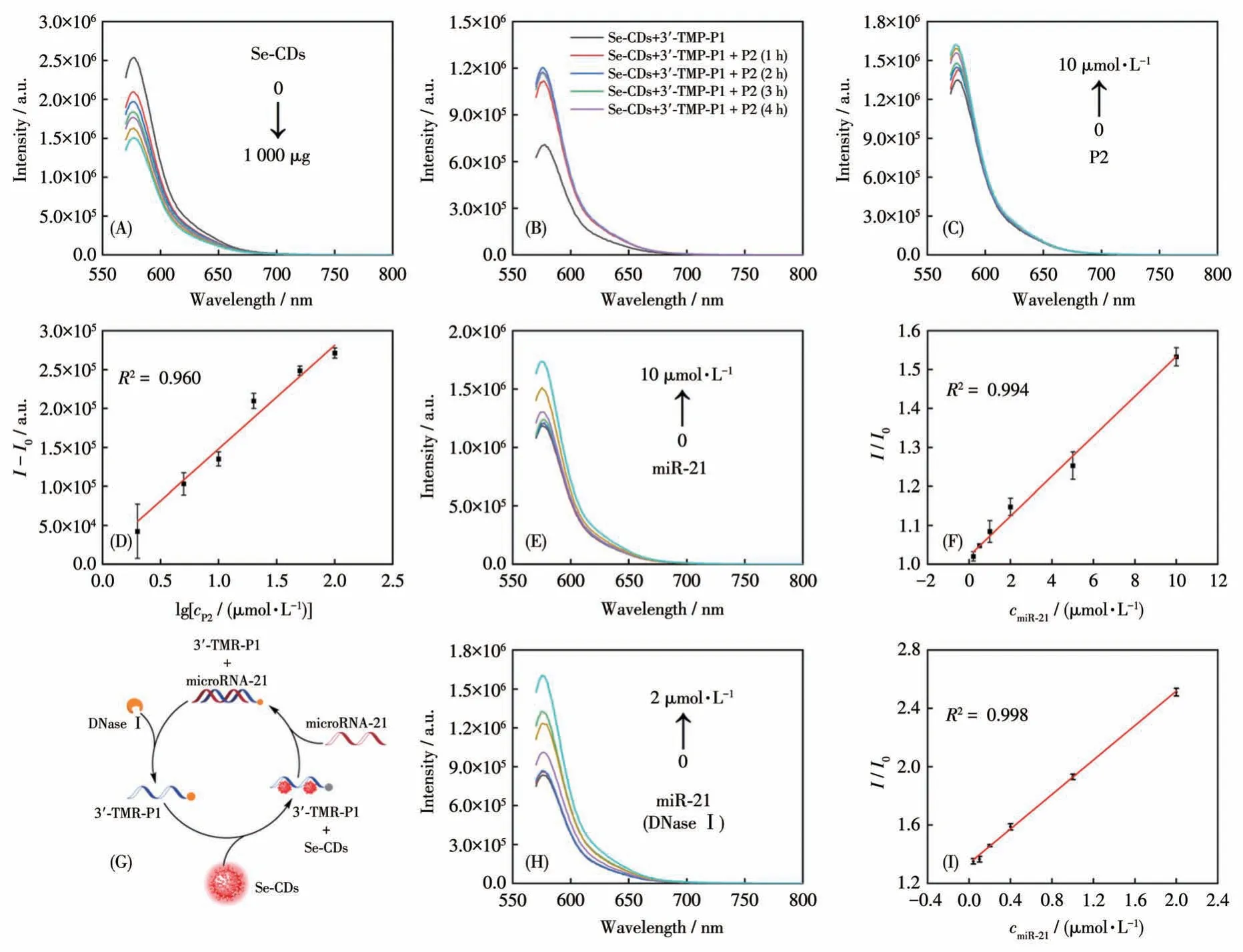
Fig.3 (A)Quenching effect of different concentrations of Se-CDs on 3′-TMR-P1;(B)Fluorescence recovery detection by adding P2 in Se-CDs+3′-TMR-P1;(C)Fluorescence response and(D)linear relationship of Se-CDs+3′-TMR-P1 with different concentrations of P2;(E)Fluorescence response relationship of Se-CDs+3′-TMR-P1 after treatment with different concentrations of miR-21 and(F)linear detection curve of miR-21 sequence(n=3);(G)Detection mechanism of DNase Ⅰ-mediated signal amplification;(H)Fluorescence response and (Ⅰ)linear relationship of Se-CDs-3′-TMR-P1 with different concentrations of miR-21 in the presence of DNase Ⅰ
2.3 Antibacterial activities of Se-CDs
For the demonstration of the antibacterial activities, Se-CD solutions at the concentrations of 0.01,0.07, 0.13, and 0.33 mg·mL-1were utilized as treating agents for the incubation ofE.coli.With the 560 nm light irradiation (among which Se-CDs have the strong absorption),the growth rate ofE.colidecreased with an increase in the weight concentration of Se-CDs, and final OD600values for various weight concentrations were respectively 0.37, 0.35, 0.31, 0.23, and 0.20 after culturing for 180 min (Fig.4A).An obvious inhibition ofE.coliwas observed at weight concentrations of 0.13 and 0.33 mg·mL-1of Se-CDs.As a control,E.colialone in the presence of Se-CDs revealed normal growth and the value of OD600reached 0.38 after the culture for 180 min.Moreover, in the absence of light irradation, with increasing concentrations of Se-CDs,there was a downward trend in the growth rate ofE.coli(Fig.4B).However, even after 180 min, no significant inhibition was observed, suggesting that Se-CDs exhibited a noticeable phototoxic inhibitory effect on bacteria.
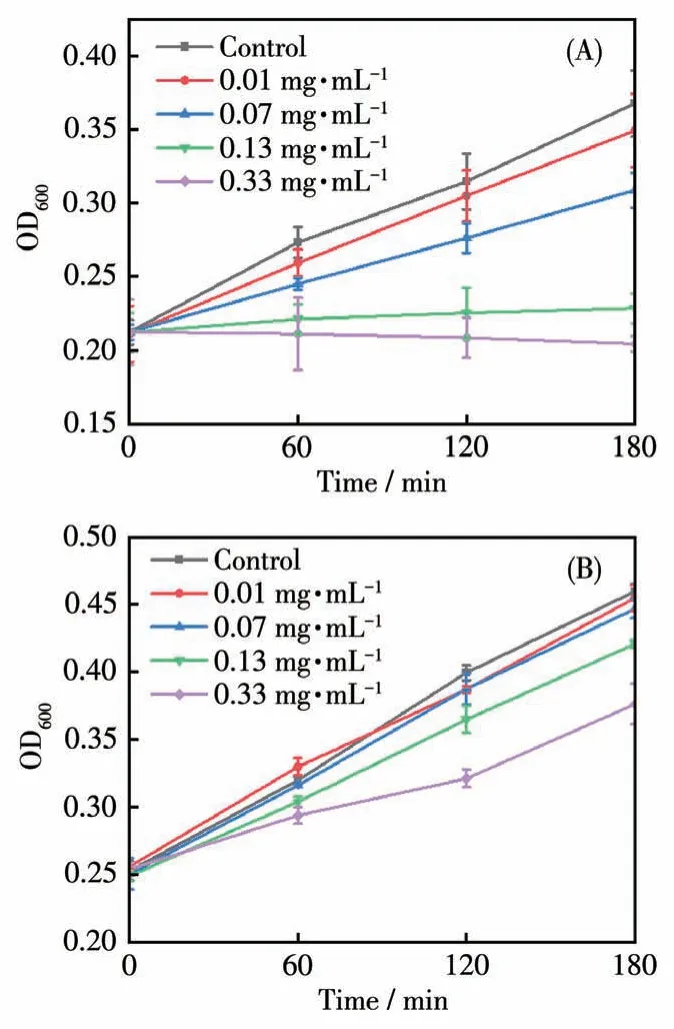
Fig.4 Effects of various concentrations of Se-CDs on E.coli growth in the(A)presence and(B)absence of light radiation
The observed antibacterial activities of Se-CDs againstE.colican be attributed to several factors.Firstly, selenium can undergo redox reactions and generate reactive oxygen species (ROS)[21].These ROS,such as free radicals, can cause oxidative damage to the bacterial cell wall, nucleic acids, proteins, and membrane lipids.This oxidative stress disrupts essential cellular functions and leads to bacterial growth inhibition and cell death.Secondly, the photoactivation of Se-CDs under 560 nm light irradiation played a significant role in enhancing their antibacterial efficacy.The strong absorption of Se-CDs at this specific wavelength enabled efficient energy transfer, triggering the generation of ROS and amplifying their antimicrobial effects[22].This light - induced activation provided a targeted and controlled approach for bacterial inhibition, potentially minimizing any detrimental effects on surrounding healthy cells or tissues[23-24].Overall, the Se-CDs synthesized in this study demonstrated effective photo-antibacterial properties againstE.coli.
3 Conclusions
In summary, Se-CDs were successfully synthesized using a green hydrothermal method and the utilization of simple precursors like BA and amine derivatives.Se-CDs were thoroughly characterized using techniques such as TEM, XPS, and various spectroscopy methods.In terms of their spectral properties, the synthesized Se-CDs displayed a red-shift in fluorescence emission and a reduced indirectEgin comparison to non-Se-doped CDs.Furthermore, the positively charged Se-CDs can be efficiently modified by negatively charged P1.It is worth noting that the presence of selenium can impact the reporter unit of the DNA(P1).The developed Se-CDs-P1 probe demonstrated desirable sensing performance for miR-21 with the assistance of DNase Ⅰ-mediated amplification.Lastly,the redox-active selenium facilitated the Se-CDs to exhibit phototoxicity againstE.coli,making them effective in combating bacterial growth.The results of this work underscore the encouraging properties exhibited by Se-doped carbon nanomaterials, showcasing their significant potential for diverse bioanalytical applications.

