基于RNA-Seq的甘蔗响应鞭黑粉菌侵染的早花相关基因挖掘
摘""要:甘蔗鞭黑粉菌(Sporisorium"scitamineum)是引起甘蔗鞭黑穗病(sugarcane"smut)的重要病原菌,侵染甘蔗后通常诱导顶端分生组织产生灰黑色“黑鞭”,在部分甘蔗品种(系)中能诱导植株提前开花,但其作用机制仍不明确。为了探究甘蔗响应鞭黑粉菌侵染的开花相关基因及其表达模式,本研究以同期健康和感染鞭黑粉菌的桂糖42甘蔗品种为实验材料进行转录组测序分析,共筛选出了3276个显著差异表达基因,其中1677个基因上调表达(Plt;0.05),1599个基因下调表达(Plt;0.05)。GO富集分析发现差异表达基因主要集中在生物合成过程、核糖体、核糖核蛋白复合体、核糖体结构成分等小类中。KEGG富集结果显示差异表达基因主要集中在氨基酸的生物合成、嘌呤代谢、碳代谢、吞噬作用等代谢通路。筛选到30个与开花途径相关的差异表达基因,并对其中的FT1、PIE1、GID1、GA20ox-1、GA20ox-2等基因进行了qRT-PCR验证,qRT-PCR结果与转录组测序结果基本一致,验证了转录组测序结果的可靠性。本研究结果为解析甘蔗鞭黑粉菌诱导甘蔗提前开花表型的分子机理提供了重要的候选基因,对甘蔗的育种也有重要的参考价值。
关键词:甘蔗;甘蔗鞭黑粉菌;转录组;开花相关基因中图分类号:S566.1""""""文献标志码:A
Identification"of"Early"Flowering"Related"Genes"of"Sugarcane"in"Response"to"Sporisorium"scitamineum"Infection"Based"on"RNA-Seq
WANG"Zhiyuan1,2,"LIANG"Hongcui2,"CAI"Jianhe3,"CHEN"Baoshan1,2,4*,"XU"Xiongbiao1,2,4*
1."Guangxi"Key"Laboratory"of"Sugarcane"Biology,"Nanning,"Guangxi"530004,"China;"2."College"of"Agriculture,"Guangxi"University,"Nanning,"Guangxi"530004,"China;"3."Plant"Protection"Research"Institute,"Guangxi"Academy"of"Agricultural"Sciences,"Nanning,"Guangxi"530007,"China;"4."State"Key"Laboratory"for"Conservation"and"Utilization"of"Subtropical"Agro-bioresources,"Nanning,"Guangxi"530004,"China
Abstract:"Sporisorium"scitamineum,"the"pathogen"of"destructive"disease"smut,"usually"causes"abnormal"growth"of"meristematic"tissue"and"forms"a"“black"whip”"in"meristem"tissues."In"some"cases,"it"induces"formation"of"flowering"structures"in"some"sugarcane"cultivars"(genotypes),"but"the"exact"mechanism"remains"unclear."In"order"to"explore"flowering"related"genes"and"the"expression"patterns"in"sugarcane"in"response"to"infection"by"S."scitamineum,"the"healthy"and"S."scitamineum"infected"‘guitang42’"sugarcane"cultivar"samples"were"analyzed"by"transcriptome"sequencing."A"total"of"3276"differentially"expressed"genes"(DEGs)"were"identified,"of"which"1677"genes"were"up-regulated"(Plt;0.05)"and"1599"genes"down-regulated"(Plt;0.05)."The"DEGs"were"mostly"enriched"in"the"biosynthesis"process,"ribosome,"ribonucleoprotein"complex,"ribosome"structural"components,"etc."according"to"GO"enrichment"analysis."The"KEGG"analysis"showed"that"the"DEGs"were"mostly"enriched"in"amino"acid"biosynthesis,"purine"metabolism,"carbon"metabolism,"phagocytosis,"and"other"metabolic"pathways."Also,"out"of"30"DEGs"were"identified"to"be"associated"with"flowering,"and"the"representative"genes"such"as"FT1,"PIE1,"GID1,"GA20ox-1,"GA20ox-2"were"validated"by"qRT-PCR."The"qRT-PCR"results"were"consistent"with"the"transcriptomic"data,"confirming"the"dependability"of"the"transcriptome"sequencing"results."The"findings"would"present"essential"candidate"genes"for"revealing"the"mechanism"of"early"flowering"of"sugarcane"plants"caused"by"S."scitamineum,"and"lay"the"foundation"for"disease"resistance"breeding.
Keywords:"sugarcane;"Sporisorium"scitamineum;"transcriptome;"flowering-related"genes
DOI:"10.3969/j.issn.1000-2561.2024.06.002
甘蔗(Saccharum"spp.)是世界上最大的商业作物,在全球超120个国家和地区广泛种植。甘蔗是主要的制糖原料,世界上大约80%的糖来源于甘蔗[1],我国90%的食糖也来源于蔗糖[2]。甘蔗黑穗病(sugarcane"smut)是世界范围内为害甘蔗最为严重的病害之一,可造成甘蔗产量和品质的严重损失[3-4]。其病原菌为甘蔗鞭黑粉菌(Spori sorium"scitamineum),通过生长初期的蔗株幼芽顶端实现侵染[5]。蔗株被侵染后表现出分蘖增多、蔗叶淡绿细长、顶叶尖挺、蔗茎细小和节疏等症状[6-7]。随着鞭黑粉菌的不断蔓延,在梢头逐渐形成由植物组织和真菌组成的不分支、方向朝下向内卷曲的鞭状物,俗称“黑鞭”[7]。除上述常见症状外,甘蔗鞭黑粉菌侵染与甘蔗开花密切相关,颜梅新等[8]通过孢子悬浮液接种试验,发现甘蔗鞭黑粉菌能诱导桂柳05-136甘蔗品种提前开花。
开花是被子植物生活史中的重要组成部分,影响植物对不同环境的适应、营养发育、生物量积累和谷物产量等[9-10]。开花是一个由5种基因通路组成的复杂网络协调调控的生物学过程,包括光周期、春化、自主、赤霉素和年龄[9,"11-14]。此外,土壤营养、供水及温度等因素也可以抑制、促进或破坏开花过程[15-17]。开花整合基因FT(FLOWERING"LOCUSnbsp;T)和SOC1(SUPPR ESSOR"OF"OVEREXPRESSION"OF"CONSTANS"1),将上述这些复杂的调节因子整合后进一步传递到下游的花分生组织基因(floral"meristem"identity"gene)中,从而启动开花[12]。甘蔗的开花同样受到许多因素,如昼长、昼夜温度、湿度、甘蔗的生理成熟度,养分有效性,以及纬度等的影响[18]。目前,关于甘蔗开花相关基因的研究相对较少,对于甘蔗鞭黑粉诱导甘蔗提前开花的研究更是未见报道。
甘蔗开花与产量是一个矛盾的过程,一方面生殖生长导致糖分向花序运输,降低了蔗茎中蔗糖的积累;另一方面,甘蔗亲本诱导开花难且花期不遇成为影响甘蔗杂交育种和新亲本创制的重要“瓶颈”。如何精准调控甘蔗的开花过程是平衡甘蔗产量和育种的重点和难点,具有重要的生产实际意义。本研究基于RNA-Seq测序技术,对甘蔗鞭黑粉菌侵染前、后2个时期的甘蔗叶片进行测序分析,挖掘并筛选与甘蔗开花相关的差异表达基因,为甘蔗开花调控和育种提供理论基础。
1""材料与方法
1.1""材料
本研究所用甘蔗品种桂糖42号取自广西亚热带农科新城及广西大学甘蔗试验基地,分别选取被甘蔗鞭黑粉菌侵染但“黑鞭”尚未抽出的甘蔗+1叶为试验材料,以同龄期健康甘蔗植株+1叶为对照,各采集3株叶片样本均匀混合,置于–80"℃超低温冰箱保存。
1.2""方法
1.2.1""RNA提取及质量检测""对照组和试验组甘蔗叶片组织总RNA由北京诺禾致源科技股份有限公司提取,以Nanodrop分光光度计检测RNA纯度,采用Agilent"2100核酸分析仪对提取的总RNA进行质量检测。
1.2.2""文库构建及测序""对质量检测合格的甘蔗叶片总RNA,利用Oligo(dT)磁珠富集含poly(A)尾的mRNA,然后加入NEB"Fragmentation"Buffer将mRNA打断成短片段,以mRNA为模板合成cDNA第一链,然后加入缓冲液、dNTPs和DNA"Polymerase"Ⅰ合成cDNA第二链,随后对双链cDNA进行纯化、末端修复、加尾并连接测序接头,最后进行片段大小选择和PCR富集,获得cDNA文库。文库检测合格后进行Illumina"NovaSeq"6000平台测序。
1.2.3""测序数据处理与分析""测序得到的原始图像数据文件经CASAVA碱基识别(base"calling)分析转化为原始测序序列(original"sequence)。对原始数据(raw"reads)进行过滤,去除带接头和N比率大于10%及低质量的reads,得到高质量序列(clean"reads)。将clean"reads与甘蔗参考基因组数据库(https://sugarcane-genome.cirad.fr/)进行比对和分类注释。
1.2.4""差异表达基因筛选""对测序得到的原始reads进行评估,经TMM标准化处理后,获得对照组和试验组的基因表达量。应用edgeR软件进行样本间差异分析,得到P值后进行多重假设检验校正,通过控制PDR值,并以|log2"(Fold"Change)|≥1和P≤0.05为标准对差异表达基因进行筛选。
1.2.5""差异表达基因本位数据库(GO)和Pathway显著性富集(KEGG)分析""基于Gene"Ontology"(GO),分别从分子功能(molecular"function,"MF)、生物过程(biological"process,"BP)和细胞组分(cellular"component,"CC)3个方面对差异表达基因进行GO注释。基于KEGG数据库,应用超几何检验,找出与整个基因组背景相比,在差异表达基因中显著性富集的Pathway,采用Fisher进行精确检验,通过Bonferroni校正法进行校正,得到差异基因显著富集的GO功能和代谢通路,从中筛选显著差异表达基因进行重点分析。
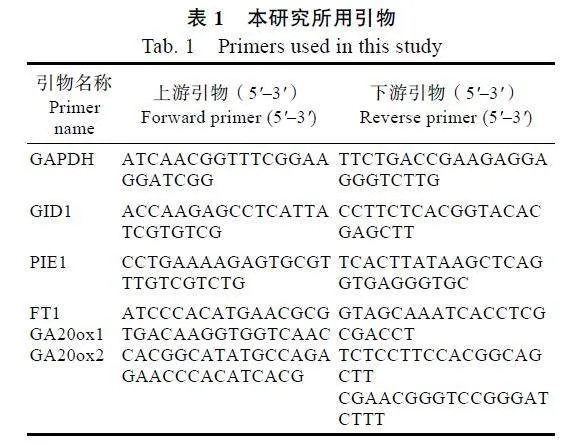
1.2.6""qRT-PCR验证""为验证转录组数据的可靠性,利用qRT-PCR对部分差异表达基因进行验证。利用HiScript"III"RT"SuperMix"for"qPCR(+gDNA"wiper)试剂盒(Vazyme,"Cat."R323-01)合成cDNA第一链,采用ChamQ"Universal"SYBR"qPCR"Master"Mix试剂盒(Vazyme,"Cat."Q711-02)检测基因的表达量,以GAPDH基因作为内参,用SnapGene软件设计qRT-PCR引物(表1)。反应体系为:cDNA模板(cDNA原液稀释10倍)2"μL,10"μmol/L正、反向引物各0.4"μL,2×ChamQ"Universal"SYBR"qPCR"Master"Mix"10"μL,加入Nuclease-free"water至总体积为20"μL,在Light Cycler"96实时荧光定量PCR仪(Roche)上进行qRT-PCR反应。荧光定量PCR反应程序为:95"℃预变性30"s;95"℃变性"10"s,60"℃退火延伸30"s,循环40次。所有试验均设置3次生物学重复和3次实验重复。运用2–ΔΔCT法进行数据处理。
2""结果与分析
2.1""测序数据质控结果分析
试验组(Smut"+1leaf)和对照组(Control"+"1leaf)的原始数据raw"reads数分别为45"626"696条和46"125"906条,经过滤得到clean"reads数分别为42"937"438条和44"276"602条,测序cDNA读取量分别为6.44"Gb和6.64"Gb,Q20分别为98.05%和96.7%,Q30分别为94.17%和91.19"%,GC含量分别为50.48%和52.72"%。说明测序质量较高,满足后续生物信息学分析要求。
2.2""差异表达基因筛选
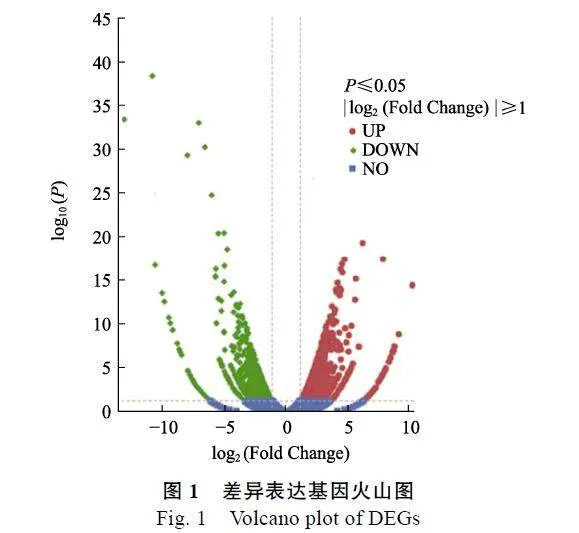
将对照组与试验组的测序数据进行比对分析,结果如图1所示,共有3276个基因差异表达,其中1677个基因显著上调表达(Plt;0.05),1599个基因显著下调表达(Plt;0.05)。
2.3""差异表达基因的GO功能注释和KEGG分析
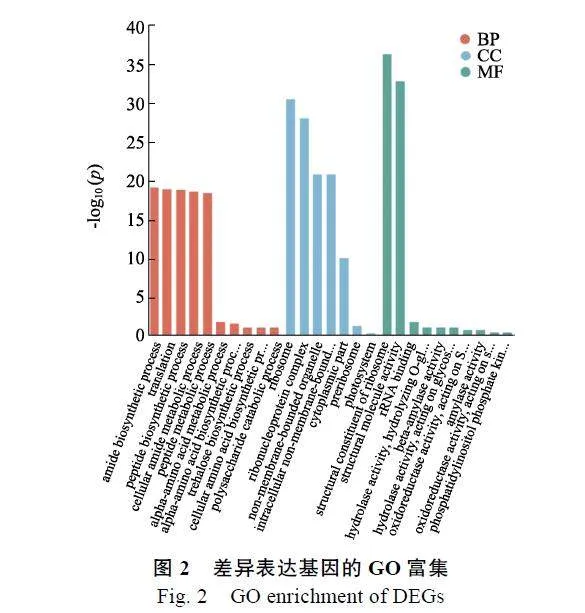
GO功能分析表明,差异表达基因被富集到生物过程、细胞组分和分子功能等3大类和785小类中,其中生物过程390个,细胞组分107个,分子功能288个。生物过程中有28个小类显著富集,细胞组分中有7个小类显著富集,分子功能中有16个小类显著富集。选取3个大类中富集程度高和差异基因占比大的小类作图(图2),其中生物过程主要富集在酰胺生物合成过程(amide"biosynthetic"process)、翻译(translation)、肽生物合成过程(peptide"biosynthetic"process)、细胞酰胺代谢过程(cellular"amidenbsp;metabolic"process)、肽代谢过程(peptide"metabolic"process);细胞组份主要富集在核糖体(ribosome)、核糖核蛋白复合体(ribonucleoprotein"complex)、非膜结合细胞器(non-membrane-bounded"organelle)、胞内非膜结合细胞器(intracellular"non-membrane-bounded"organelle)、细胞质部分(cytoplasmic"part);分子功能主要富集在核糖体的结构成分(structural"constituent"of"ribosome)、结构分子活性(structural"molecule"activity)及rRNA结合(rRNA"binding)等。
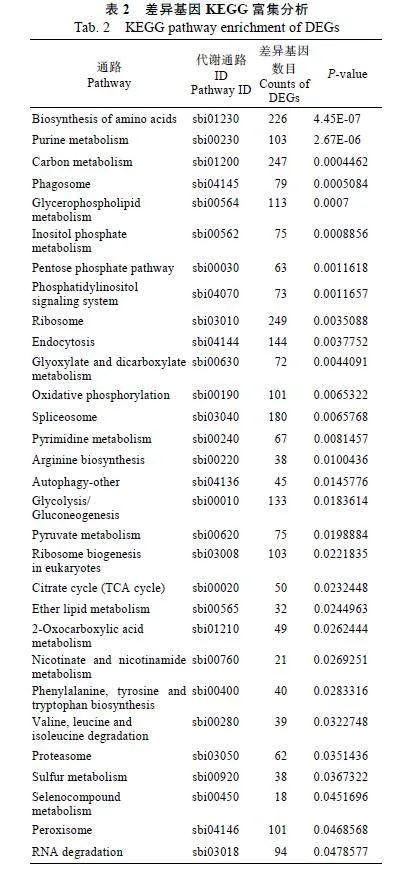
利用KEGG数据库对筛选到的差异表达基因进行功能分类和pathway注释,结果显示(表2):甘蔗鞭黑粉菌侵染前后的差异表达基因定位到了118个代谢通路中,显著富集到了30个代谢通路中,如氨基酸的生物合成(biosynthesis"of"amino"acids)、嘌呤代谢(purine"metabolism)、碳代谢(carbon"metabolism)、吞噬作用(phagosome)、甘油磷脂代谢(glycerophospholipid"metabolism)等。
2.4""开花相关基因的差异表达分析
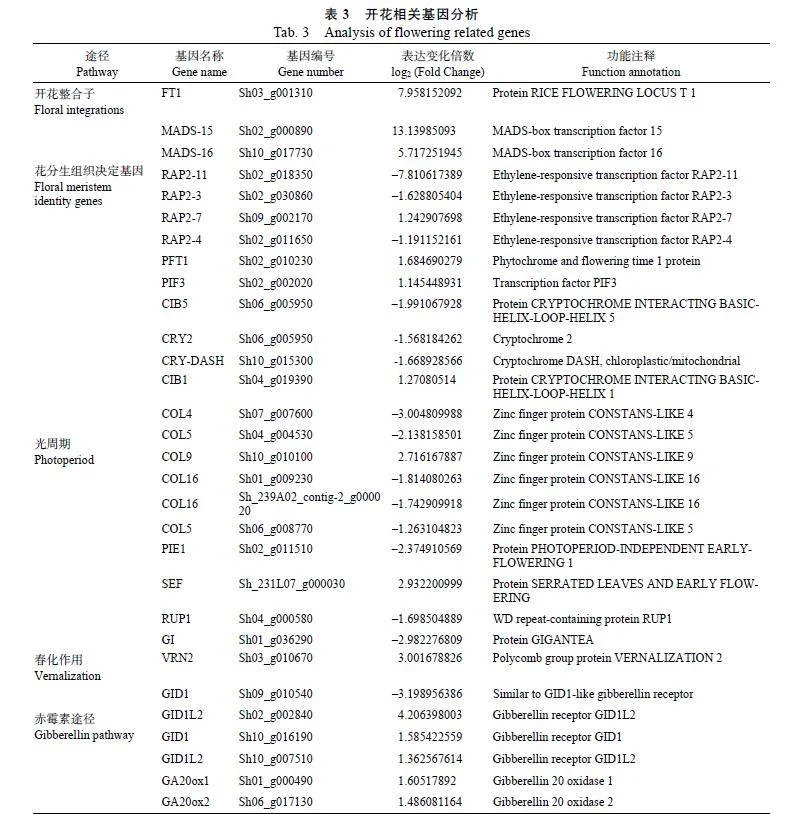
从转录组数据中筛选到30个与开花途径相关的差异表达基因,其中包括开花整合子(floral"integrations)基因1个;花分生组织决定基因(floral"meristem"identity"genes)6个;光周期(photoperiod)途径相关基因16个;春化途径相关基因1个;赤霉素途径相关基因6个(表3)。
2.5""开花相关基因的qRT-PCR验证
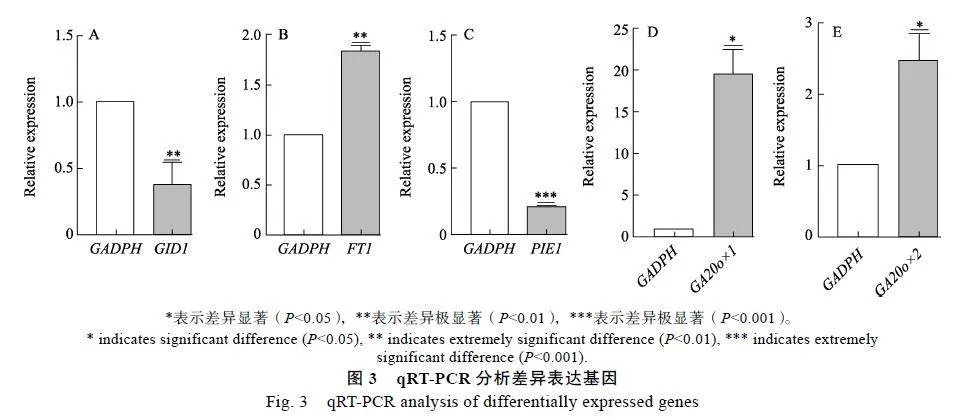
选取GID1、FT1、PIE1、GA20ox-1、GA20ox-2等5个与植物开花途径相关基因进行qRT-PCR验证(图3),结果显示,与健康对照相比,甘蔗鞭黑粉菌侵染的甘蔗+1叶片中FT1、GA20ox-1、GA20ox-2基因显著上调表达,而GID1和PIE1基因显著下调表达。虽然这3个基因的转录组测序结果和qRT-PCR结果在基因差异表达倍数并非完全一致,但是二者之间反映出的差异表达变化趋势是一致的,说明转录组数据的可靠性。
3""讨论
开花是植物由营养生长向生殖生长转变的重要阶段,在植物生活史中扮演着非常重要的作用。甘蔗开花的精准高效调控对于蔗茎产量和遗传育种具有重要意义。本研究通过转录组测序分析,
筛选出3276个差异表达基因,其中1677个基因上调表达,1599个基因下调表达。结合植物开花调控途径对差异表达基因进一步分析,筛选出30个与开花途径相关的差异表达基因。FLOWE RING"LOCUS"T(FT)成花基因作为下游开花整合基因,可以整合多个调控途径中的花发育信号分子,促进开花相关基因的表达,促进植物开花[19]。然而,过表达甘蔗ScFT1基因可抑制拟南芥开花[20]。本研究转录组测序分析发现在甘蔗鞭粉菌
侵染后ScFT1基因呈显著上调表达,同时qRT-"PCR也验证了这一结果。推测甘蔗鞭粉菌侵染后诱导甘蔗提前开花的现象,可能是通过影响上游的开花途径导致的。
赤霉素(gibberellins,"GAs)是一类重要的植物激素,在调节植物生长和抵抗生物和非生物胁迫中具有重要作用[21-22]。在赤霉素信号通路中,GA受体GIBBERELLIN"INSENSITIVE"DWARF1(GID1)感知GA信号后,与下游DELLA蛋白N-端DELLA/TVHYNP结构域互作,促进DELLA经SCFGID2/SLY1泛素/26S蛋白酶体途径降解[23-24],正向调控植株茎伸长、花和果实发育、叶片扩张及种子萌发等生物学过程[25-27]。Gibberellin"20"oxidase(GA20ox)是赤霉素合成过程中的一类重要的限速酶,GA20ox及Gibberellin"3"oxidase"(GA3ox)通过一系列催化反应,将赤霉素前期GA12转化为具有生物活性的GA1和GA4[28-30]。GA20ox-2被认为是一个“绿色革命”基因,其突变导致水稻的矮杆化,从而大大提高了产量[31]。本研究发现与对照相比,甘蔗鞭黑粉菌侵染的甘蔗+1叶中ScGA20ox-1、ScGA20ox-2基因均呈现显著上调表达,而ScGID1基因呈显著下调表达,这与GID1正向调控开花的结果不一致[25-26]。此外,酵母双杂交(yeast"two"hybrid,"Y2H)和双分子荧光互补实验(BiFC)研究发现,ScGID1能与ScGA20ox2在体外和体内发生互作(数据待发表)。推测甘蔗鞭黑粉菌侵染一方面下调ScGID1表达,另一方面促进上游ScGA20ox等赤霉素合成相关基因的表达,干扰了ScGID1与ScGA20ox的相互作用,从而诱导甘蔗早花的表型。
不依赖光周期的早花基因PHOTOPERIOD"INDEPENDENT"EARLY"FLOWERING1(PIE1)能负调控拟南芥开花过程,PIE1突变能抑制FLOWERING"LOCUS"C(FLC)介导的迟花表型,并诱导非FLC依赖的早花表型[32]。本研究发现在甘蔗鞭黑粉菌侵染的甘蔗+1叶中ScPIE1基因转录水平极显著下调表达,推测甘蔗鞭黑粉菌诱导甘蔗早花现象可能与ScPIE1基因的表达下调有关。
在拟南芥中CONSTANS-Like(COL)基因是光周期诱导开花途径中的关键转录因子[33],研究发现AtCOL4是长日照和短日照的开花抑制因子,COL4过表达导致开花延迟[34];而过表达AtCOL5可诱导短日照拟南芥开花[35];COL9通过抑制Ehd1(early"heading"date"1)基因延迟水稻的开花时间[36];COL16基因在水稻中通过上调开花阻碍基因Ghd7(grain"number,"plant"height"and"heading"date"7)来抑制开花[37]。本研究发现ScCOL4和ScCOL16显著下调表达,而COL4和COL16在其他作物中存在抑制开花的现象,推测甘蔗鞭黑粉菌可能通过下调这2个基因的表达从而诱导甘蔗早花。而COL5和COL9在甘蔗中的表达模式和功能可能与其他作物存在一定的差异。
乙烯响应因子(ethylene-responsive"transcription"factor"RAP)RAP2-3、RAP2-4、RAP2-7等属于AP2/ERF类转录因子,参与植物花发育、果实成熟、种子发育和萌发以及响应逆境胁迫的过程[38]。APETALA2(AP2)基因在拟南芥中直接或间接调控花发育过程中其他基因的表达,如FT[39]和SOC1基因[40],进而抑制植物开花。本研究发现RAP2-11、RAP2-3、RAP2-4基因表达下调,推测这3个基因可能响应了甘蔗鞭黑粉菌的侵染,进而诱导甘蔗早花现象。
CRY2(cryptochrome"2)、CRY-DASH(crypto chrome-DASH)都属于隐花色素基因家族,而CIB1(cryptochrome-interacting"basic-helix-loop-"helix"1)、CIB5(cryptochrome-interacting"basic-"helix-loop-helix"5)是一类重要的转录因子,可以在蓝光的作用下与CRY2互作,进而促进拟南芥开花[41]。本研究发现CIB1基因在甘蔗鞭黑粉菌侵染后显著上调表达,推测甘蔗鞭黑粉菌可能通过上调CIB1表达,进而促进甘蔗早花。
关于甘蔗鞭黑粉菌诱导甘蔗早花现象的研究鲜见报道,本研究基于RNA-Seq技术初步筛选了甘蔗响应鞭黑粉菌侵染的与开花途径相关的差异表达基因,并对部分基因的表达模式进行了验证分析,为甘蔗开花调控和育种提供了理论基础。这些基因是如何响应甘蔗鞭黑粉菌侵染,具体互作因子以及诱导提前开花的分子机理,还有待进一步深入研究。
参考文献
[1]"BUDEGUER"F,"ENRIQUE"R,"PERERA"M"F,"RACEDO"J,"CASTAGNARO"A"P,"NOGUERA"A"S,"WELIN"B."Genetic"transformation"of"sugarcane,"current"status"and"future"prospects[J]."Frontiers"in"Plant"Science,"2021,"12:"768609.
[2]"MAGAREY"R"C."Sugarcane-an"old"plantation"crop"that"offers"new"environmentally"friendly"possibilities[J]."IOP"Conference"Series:"Earth"and"Environmental"Science,"2020,"418(1):"012004.
[3]"MANSOOR"S,"KHAN"M"A,"KHAN"N"A,"NASIR"I"R."Effect"of"whip"smut"disease"on"the"quantitative"and"qualitative"parameters"of"sugarcane"varieties/lines[J]."Agricultural"Research"amp;"Technology"Open"Access"Journal,"2016,"2:"67-72.
[4]"RAJPUT"M"A,"RAJPUT"N"A,"SYED"R"N,"LODHI"A"M,"QUE"Y"X."Sugarcane"smut:"current"knowledge"and"the"way"forward"for"management[J]."Journal"of"Fungi"(Basel),"2021,"7(12):"1095.
[5]"BHUIYAN"S"A,"MAGAREY"R"C,"MCNEIL"M"D,"AITKEN"K"S."Sugarcane"smut,"caused"by"Sporisorium"scitamineum,"a"major"disease"of"sugarcane:"a"contemporary"review[J]."Phytopathology,"2021,"111(11):"1905-1917.
[6]"ZHANG"H"Y,"YANG"Y"F,"GUO"F,"SHEN"X"R,"LU"S,"CHEN"B"S."SsRSS1"mediates"salicylic"acid"tolerance"and"contributes"to"virulence"in"sugarcane"smut"fungus[J]."Journal"of"Integrative"Agriculture,"2023,"22(7):"2126-2137.
[7]"QUE"Y"X,"LIN"J"W,"SONG"X"X,"XU"L"P,"CHEN"R"K."Differential"gene"expression"in"sugarcane"in"response"to"challenge"by"fungal"pathogen"Ustilago"scitaminea"revealed"by"cDNA-AFLP[J]."Journal"of"Biomedicine"Biotechnology,"2011,"2011:"160934.
- 颜梅新,"宋修鹏,"王泽平,"张小秋,"雷敬超,"黄海荣,"黄冬梅,"李秋芳."一种诱导甘蔗品种05-136开花的甘蔗鞭黑粉菌菌剂的制备方法:"CN202110605812.9[P]."2021-09-10.YAN"M"X,"SONG"X"P,"WANG"Z"P,"ZHANG"X"Q,"LEI"J"C,"HUANG"H"R,"HUANG"D"M,"LI"Q"F."A"preparation"method"of"Sporisorium"scitamineum"agent"for"inducing"flowering"of"sugarcane"variety"05-136:"CN202110605812.9[P]."2021-09-"10."(in"Chinese)
- HILL"C"B,"LI"C."Genetic"architecture"of"flowering"phenology"in"cereals"and"opportunities"for"crop"improvement[J]."Frontiers"in"Plant"Science,"2016,"7:"1906.
- MANECHINI"J"R"V,"SANTOS"P"H"D"S,"ROMANEL"E,"BRITO"M"D"S,"SCARPARI"M"S,"JACKSON"S,"PINTO"L"R,"VICENTINI"R."Transcriptomic"analysis"of"changes"in"gene"expression"during"flowering"induction"in"sugarcane"under"controlled"photoperiodic"conditions[J]."Frontiers"in"Plant"Science,"2021,"12:"635784.
- WELLMER"F,"RIECHMANN"J"L."Gene"networks"controlling"the"initiation"of"flower"development[J]."Trends"in"Genetics,"2010,"26(12):"519-527.
- SRIKANTH"A,"SCHMID"M."Regulation"of"flowering"time:"all"roads"lead"to"Rome[J]."Cellular"and"Molecular"Life"Sciences,"2011,"68(12):"2013-2037.
- YAMAGUCHI"A,"ABE"M."Regulation"of"reproductive"development"by"non-coding"RNA"in"Arabidopsis:"to"flower"or"not"to"flower[J]."Journal"of"Plant"Research,"2012,"125(6):"693-704.
- SONG"Y"H,"SHIM"J"S,"KINMONTH-SCHULTZ"H"A,"IMAIZUMI"T."Photoperiodic"flowering:"time"measurement"mechanisms"in"leaves[J]."Annual"Review"of"Plant"Biology,"2015,"66:"441-464.
- JACKSON"S"D."Plant"responses"to"photoperiod[J]."New"Phytologist,"2009,"181(3):"517-531.
- HONG"Y,"JACKSON"S."Floral"induction"and"flower"formation--the"role"and"potential"applications"of"miRNAs[J]."Plant"Biotechnology"Journal,"2015,"13(3):"282-292.
- BRAMBILLA"V,"GOMEZ-ARIZA"J,"CERISE"M,"FORNARA"F."The"importance"of"being"on"time:"regulatory"networks"controlling"photoperiodic"flowering"in"cereals[J]."Frontiers"in"Plant"Science,"2017,"8:"665.
[18]"PAVANI"G,"MALHOTRA"P"K,"VERMA"S"K."Flowering"in"sugarcane-insights"from"the"grasses[J]."3"Biotech,"2023,"13(5):"154.
[19]"WICKLAND"D"P,"HANZAWA"Y."The"FLOWERING"LOCUS"T/TERMINAL"FLOWER"1"gene"family:"functional"evolution"and"molecular"mechanisms[J]."Molecular"Plant,"2015,"8(7):"983-997.
[20]"COELHO"C"P,"MINOW"M"A,"CHALFAN-JUNIOR"A,"COLASANTI"J."Putative"sugarcane"FT/TFL1"genes"delay"flowering"time"and"alter"reproductive"architecture"in"Arabidopsis[J]."Frontiers"in"Plant"Science,"2014,"5:"221.
[21]"COLEBROOK"E"H,"THOMAS"S"G,"PHILLIPS"A"L,"HEDDEN"P."The"role"of"gibberellin"signalling"in"plant"responses"to"abiotic"stress[J]."Journal"of"Experimental"Biology,"2014,"217:"67-75.
[22]"HUANG"S,"ZHANG"X,"FERNANDO"W"G"D."Directing"trophic"divergence"in"plant-pathogen"interactions:"antagonistic"phytohormones"with"no"doubt?[J]."Frontiers"in"Plant"Science,"2020,"11:"600063.
[23]"FENG"S,"MARTINEZ"C,"GUSMAROLI"G,"WANG"Y,"ZHOU"J,"WANG"F,"CHEN"L,"YU"L,"IGLESIAS-PEDRAZ"J"M,"KIRCHER"S,"SCHAFER"E,"FU"X,"FAN"L"M,"DENG"X"W."Coordinated"regulation"of"Arabidopsis"thaliana"development"by"light"and"gibberellins[J]."Nature,"2008,"451(7177):"475-479.
[24]"MURASE"K,"HIRANO"Y,"SUN"T"P,"HAKOSHIMA"T."Gibberellin-induced"DELLA"recognition"by"the"gibberellin"receptor"GID1[J]."Nature,"2008,"456(7221):"459-463.
[25]"BAO"S,"HUA"C,"SHEN"L,"YU"H."New"insights"into"gibberellin"signaling"in"regulating"flowering"in"Arabidopsis[J]."Journal"of"Integrative"Plant"Biology,"2020,"62(1):"118-131.
[26]"CHENG"H,"QIN"L,"LEE"S,"FU"X,"RICHARDS"D"E,"CAO"D,"LUO"D,"HARBERD"N"P,"PENG"J."Gibberellin"regulates"Arabidopsis"floral"development"via"suppression"of"DELLA"protein"function[J]."Development,"2004,"131(5):"1055-1064.
[27]"LANTZOUNI"O,"ALKOFER"A,"FALTER-BRAUN"P,"SCHWECHHEIMER"C."GROWTH-REGULATING"FACT ORS"interact"with"DELLAs"and"regulate"growth"in"cold"stress[J]."Plant"Cell,"2020,"32(4):"1018-1034.
[28]"YAMAGUCHI"S."Gibberellin"metabolism"and"its"regulation[J]."Annual"Review"of"Plant"Biology,"2008,"59:"225-251.
[29]"GIACOMELLI"L,"ROTA-STABELLI"O,"MASUERO"D,"ACHEAMPONG"A"K,"MORETTO"M,"CAPUTI"L,"VRHOVSEK"U,"MOSER"C."Gibberellin"metabolism"in"Vitis"vinifera"L."during"bloom"and"fruit-set:"functional"characterization"and"evolution"of"grapevine"gibberellin"oxidases[J]."Journal"of"Experimental"Botany,"2013,"64(14):"4403-4419.
[30]"HEDDEN"P,"THOMAS"S"G."Gibberellin"biosynthesis"and"its"regulation[J]."Biochemistry"Journal,"2012,"444(1):"11-25.
[31]"LOPEZ"-CRISTOFFANINI"C,"SERRAT"X,"JAUREGUI"O,"NOGUES"S,"LOPEZ-CARBONELL"M."Phytohormone"profiling"method"for"rice:"effects"of"GA20ox"mutation"on"the"gibberellin"content"of"Japonica"rice"varieties[J]."Frontiers"in"Plant"Science,"2019,"10:"733.
[32]"NOH"Y"S,"AMASINO"R"M."PIE1,"an"ISWI"family"gene,"is"required"for"FLC"activation"and"floral"repression"in"Arabidopsis[J]."Plant"Cell,"2003,"15(7):"1671-1682.
[33]"PUTTERILL"J,"ROBSON"F,"LEE"K,"SIMON"R,"COUPL AND"G."The"CONSTANS"gene"of"Arabidopsis"promotes"flowering"and"encodes"a"protein"showing"similarities"to"zinc"finger"transcription"factors[J]."Cell,"1995,"80(6):"847-857.
[34]"STEINBACH"Y."The"Arabidopsis"thaliana"CONSTANS-"LIKE"4"(COL4)–a"modulator"of"flowering"time[J]."Frontiers"in"Plant"Science,"2019,"10:"651.
[35]"HASSIDIM"M,"HARIR"Y,"YAKIR"E,"KRON"I,"GREEN"R"M."Over-expression"of"CONSTANS-LIKE"5"can"induce"flowering"in"short-day"grown"Arabidopsis[J]."Planta,"2009,"230:"481-491.
[36]"LIU"H,"GU"F,"DONG"S,"LIU"W,"WANG"H,"CHEN"Z,"WANG"J."CONSTANS-like"9"(COL9)"delays"the"flowering"time"in"Oryza"sativa"by"repressing"the"Ehd1"pathway[J]."Biochemical"and"Biophysical"Research"Communications,"2016,"479(2):"173-178.
[37]"WU"W,"ZHENG"X"M,"CHEN"D"B,"ZHANG"Y"X,"MA"W"W,"ZHANG"H,"SUN"L"P,"YANG"Z"F,"ZHAO"C"D,"ZHAN"X"D,"SHEN"X"H,"YU"P,"FU"Y"P,"ZHU"S"S,"CAO"L"Y,"CHENG"S"H."OsCOL16,"encoding"a"CONSTANS-like"protein,"represses"flowering"by"up-regulating"Ghd7"expression"in"rice[J]."Plant"Science,"2017,"260:"60-69.
[38]"FENG"K,"HOU"X"L,"XING"G"M,"LIU"J"X,"DUAN"A"Q,"XU"Z"S,"LI"M"Y,"ZHUANG"J,"XIONG"A"S."Advances"in"AP2/ERF"super-family"transcription"factors"innbsp;plant[J]."Critical"Reviews"in"Biotechnology,"2020,"40(6):"750-776.
[39]"CASTILLEJO"C,"PELAZ"S."The"balance"between"CONSTANS"and"TEMPRANILLO"activities"determines"FT"expression"to"trigger"flowering[J]."Current"Biology,"2008,"18(17):"1338-1343.
[40]"YANT"L,"MATHIEU"J,"DINH"T"T,"OTT"F,"LANZ"C,"WOLLMANN"H,"CHEN"X,"SCHMID"M."Orchestration"of"the"floral"transition"and"floral"development"in"Arabidopsis"by"the"bifunctional"transcription"factor"APETALA2[J]."Plant"Cell,"2010,"22(7):"2156-2170.
[41]"LIU"Y,"LI"X,"MA"D,"CHEN"Z,"WANG"J"W,"LIU"H."CIB1"and"CO"interact"to"mediate"CRY2-dependent"regulation"of"flowering[J]."EMBO"Reports,"2018,"19(10):"e45762.

