Effect of hepatocyte growth factor on inflammatory factors associated with CCL4-induced hepatocyte injury
DUAN Qi, LI Si-yue, LI Qing-lan, WANG Yu, ZHENG Li
Hainan Medical University, Haikou 571199, China
Keywords:Hepatocyte Growth Factor CCL4 Hepatocyte Injury Inflammatory Factor
ABSTRACT
1.Introduction
HGF (Hepatocyte Growth Factor) was discovered in the 20th century during investigations into the regenerative capacity of the liver.Researchers found that after performing a partial liver resection in animal experiments, the remaining liver tissue would regenerate rapidly.Subsequent studies led to the purification and isolation of a protein called HGF from the blood of these experimental animals[1].HGF is a glycosylated protein composed of an α-chain and a β-chain connected by disulfide bonds.It is involved in regulating various life processes, including cell proliferation,migration, and apoptosis[2].The characteristic features of CCl4-induced toxic liver fibrosis include severe hepatocyte necrosis,inflammatory infiltration, panacinar fibrosis, and thick fibrous septa.The development of liver fibrosis is a complex process influenced by multiple factors that interact with each other.Two types of cytokines play a major role in this process: pro-inflammatory and pro-fibrotic factors, as well as anti-inflammatory and anti-fibrotic factors[3].Studies have found that CCl4-induced acute liver injury in mice leads to the secretion of tumor necrosis factor-α (TNF-α),interleukin-8 (IL-8), interleukin-21 (IL-21), and interleukin-4 (IL-4)by the liver[4].The expression levels of TNF-α[5], IL-8, IL-4, and IL-21 in the damaged mice were significantly higher than those in the control group, indicating a close relationship between these cytokines and liver injury[6].
Wu Bin et al.conducted a study on the protective effects of hepatocyte growth factor (HGF) on CCl4-induced acute liver injury.They found that HGF had a certain protective effect on the cytoplasmic membrane of damaged liver cells[7].Further research by Zou Yuan[8] revealed that HGF can enhance the expression levels of liver regeneration-related genes when exerting its protective effects on damaged liver cells[9].Other studies have indicated that HGF can inhibit the release of the inflammatory cytokine TNF-α in acute liver injury in rats[10,11].To investigate the mechanisms underlying the protective effects of HGF on CCl4-induced liver injury in mice,this experiment will establish a mouse model of liver cell injury induced by CCl4.Enzyme-Linked Immunosorbent Assay (ELISA)will be used to measure the expression levels of inflammatory factors TNF-α, IL-8, IL-4, and IL-21 in liver parenchymal cells and white blood cells.The study aims to explore the impact of hepatocyte growth factor on the inflammatory factors associated with CCl4-induced liver cell injury.
2.Materials and Methods
2.1 Materials and Instruments
The mice were obtained from Tianqin Biotechnology Co., Ltd.in Changsha.Percoll cell separation solution (Solarbio), recombinant human HGF protein from Lea BioTech Co., Ltd.in Hainan,RPMI-1640 complete medium from Gibco, mouse IL-8, IL-21,TNF-α ELISA kits from Shanghai Fusheng Industrial Co., Ltd.,mouse IL-4 ELISA kit from Shanghai UniN biological technology Co., Ltd., high-speed tabletop centrifuge from Eppendorf, SPX-250B-Z biochemical incubator from Shanghai Boxun Industrial Co., Ltd.Medical Equipment Factory, electronic balance from Shanghai Shangtian Precision Instrument Co., Ltd., Life Real P-800 multi-wavelength microplate reader from Hangzhou Suizhen Biotechnology Co., Ltd.
2.2 Animal Grouping and Cell Treatment
Six SPF-grade female C57BL/6 mice with a body weight ranging from 18-22 g were randomly divided into a CCl4injury group and a normal control group, with three mice in each group.All mice were acclimated for 7 d with free access to water and food.On the 8th day, the mice in the CCl4injury group were intraperitoneally injected with a dose of 0.2% CCl4castor oil solution at a rate of 10 mL/kg to induce acute liver injury, while the mice in the normal control group were injected with the same dose of castor oil solution at the same site.After 18 h, the mice were anesthetized and blood was collected from the orbital venous plexus.The abdominal cavity of each mouse was exposed, and a circuit was created by connecting the portal vein, peristaltic pump tubing, and inferior vena cava using a threeway tube and indwelling venous needle.A 0.2 mg/mL collagenase solution at 37 ℃ was injected into the portal vein, and the peristaltic pump speed was adjusted to 20 mL/min for perfusion.The liver tissue was then excised, cut into small pieces, and digested with 0.5 mg/mL collagenase solution for 30 min[12].The digested tissue was then crushed and passed through a 200-mesh sieve.The filtered cell suspension was layered on top of 33% Percoll[13], and after gradient centrifugation, the upper layer contained mouse liver parenchymal cells, while the lower layer was a mixture of red blood cells and liver leukocytes.The liver leukocytes were collected after adding red blood cell lysis buffer, washed twice with PBS, and then seeded into a 24-well plate at a cell density of 5×105/mL.
2.3 In vivo Injury Experiment Grouping
The experiment was divided into the injured liver parenchymal cell group, and the experimental groups with 10 ng/mL, 25ng/mL, and 50 ng/mL HGF.A 24-well plate was taken, and 500 μL of liver parenchymal cells from the CCl4injury group were added to each well.For the 10 ng/mL experimental group, 20 μL of HGF was added; for the 25 ng/mL experimental group, 50 μL of HGF was added; and for the 50 ng/mL experimental group, 100 μL of HGF was added.Two replicate wells were set for each group.The stimulation of liver leukocytes was performed as described above.
2.4 In vitro Injury Experiment Grouping
The liver parenchymal cells from the normal control group were counted and seeded into a plate, with 2.5 × 105cells per well in a 24-well plate.After incubation at 37 ℃ for 4 h, 40 μL of 100 mM CCl4was added, and a small amount of dimethyl sulfoxide (DMSO) was used for assistance in dissolution, with a final DMSO concentration of 0.1% (v/v).The cells were incubated with CCl4for 3 h.The treated liver parenchymal cells were divided into the normal liver parenchymal cell group, CCl4injury group, and experimental groups with 10 ng/mL, 25 ng/mL, and 50 ng/mL HGF.For each well, 500 μL of liver parenchymal cells were added, and for the 10 ng/mL experimental group, 20 μL of HGF was added; for the 25ng/mL experimental group, 50 μL of HGF was added; and for the 50ng/ml experimental group, 100 μL of HGF was added.Two replicate wells were set for each group.The stimulation of liver leukocytes was performed as described above.
2.5 ELISA Detection
Supernatants from each group were collected, and the levels of IL-8, TNF-α, IL-4, and IL-21 were measured according to the instructions of the ELISA kits.The absorbance was measured at 450 nm using a microplate reader, and the concentration values were calculated.
1.6 Data Analysis
Data statistical analysis was performed using GraphPad Prism 5.01.The statistical methods used were t-tests or Wilcoxon matched pairs test.When P < 0.05, the null hypothesis was rejected, indicating a significant difference.
3.Results
3.1 Effects of HGF on the secretion of IL-8, TNF-α, IL-4,and IL-21 in liver parenchymal cells in an in vivo CCl4-induced liver injury model
had no significant effect on the production of TNF-α and IL-21 by liver parenchymal cells in the in vivo CCl4-induced liver injury model compared to the injured liver cell group.There were also no significant differences among the HGF groups.(Refer to Figure 1b,1d).For IL-8, the expression level was decreased in the 10ng/ml HGF group compared to the injured liver cell group (t = 2.876, P =0.0452), while there were no significant differences in the 25ng/ml and 50 ng/mL HGF groups.The expression level was increased in the 50 ng/mL HGF group compared to the 10 ng/mL HGF group (t =3.551, P = 0.0238), and there were no significant differences among the other groups.(Refer to Figure 1a).For IL-4, the expression level was decreased in the 25 ng/mL HGF group compared to the injured liver cell group (t = 2.981, P = 0.0452), and the expression level was also decreased in the 50ng/ml HGF group (t = 3.097, P = 0.0238),while there were no significant differences in the 10ng/ml HGF group.(Refer to Figure 1c).
The results showed that HGF (10 ng/mL, 25 ng/mL, and 50 ng/mL)
3.2 Effects of HGF on the secretion of IL-8, TNF-α, IL-4,and IL-21 by liver leukocytes in an in vivo CCl4-induced liver injury model
The results showed that HGF at different concentrations (10 ng/mL,25 ng/mL, and 50 ng/mL) had no significant effect on the production of IL-8, IL-4, and IL-21 by liver leukocytes in the in vivo CCl4-induced liver injury model compared to the CCl4model group.There were also no significant differences among the HGF groups.(Refer to Figure 2a, 2c, 2d).Regarding TNF-α, the expression level was decreased in the 10 ng/mL HGF group compared to the CCl4model group (t = 2.534, P = 0.0322), and the expression level was also decreased in the 25ng/ml HGF group (t = 2.601, P = 0.0300), while there were no differences in the 50 ng/mL HGF group.There were no significant differences among the different concentrations of HGF(10 ng/mL, 25 ng/mL, and 50 ng/mL).(Refer to Figure 2b).
3.3 Effects of HGF on the secretion of IL-8, TNF-α, IL-4,and IL-21 by CCl4-induced liver injury cells in vitro
The results showed that for IL-8 and IL-21, there were no significant differences between the normal control group and the CCl4model group.The HGF groups (10 ng/mL, 25 ng/mL, and 50 ng/mL) had no significant differences compared to the normal control group and the CCl4model group, and there were no differences among the HGF groups.(Refer to Figure 3a, 3d).For TNF-α, there were no significant differences between the normal control group, the HGF groups (10 ng/mL, 25 ng/mL, and 50 ng/mL), and the CCl4model group.Within the normal control group,there were no significant differences compared to the 10 ng/mL HGF group; the 25 ng/mL HGF group showed decreased expression levels(t = 3.236, P = 0.0318), and the 50 ng/mL HGF group also showed decreased expression levels (t = 3.344, P = 0.0287).(Refer to Figure 3b).For IL-4, there were no significant differences between the normal control group, the HGF groups (10 ng/mL, 25 ng/mL, and 50 ng/mL), and the CCl4model group.Within the normal control group, there were no significant differences compared to the 10 ng/mL HGF and 50 ng/mL HGF groups.Compared to the 25 ng/mL HGF group, there was a decrease in expression level (t = 4.946, P =0.0159).(Refer to Figure 3c).
3.4 Effects of HGF on the secretion of IL-8, TNF-α, IL-4,and IL-21 by CCl4-induced liver injury leukocytes in vitro
The results showed that for IL-8 and IL-4, there were no significant differences between the normal control group and the CCl4model group, as well as the HGF (10 ng/mL, 25 ng/mL, and 50 ng/mL)groups.There were no significant differences among the groups.(Refer to Figure 4a, 4c).For TNF-α, there were no significant differences between the normal control group, the HGF (10 ng/mL, 25 ng/mL, and 50 ng/mL) groups, and the CCl4 model group.Comparing the 50 ng/mL HGF group to the 10 ng/mL HGF group,there was an increased expression level (t = 9.168, P = 0.0008),while there were no significant differences between the 25 ng/mLHGF group and the 50 ng/mL HGF group, as well as the 10 ng/mL HGF group.(Refer to Figure 4b).For IL-21, there was a decreased expression level when comparing the normal control group to the CCl4 model group (t = 3.208, P = 0.0094).Compared to the normal control group, the 10ng/ml HGF group, 25 ng/mL HGF group, and 50 ng/mL HGF group showed decreased expression levels (t = 2.889, P = 0.0136; t = 3.020, P = 0.0129; t = 2.789, P =0.0236, respectively).There were no significant differences between the HGF groups (10 ng/mL, 25 ng/mL, and 50 ng/mL) and the CCl4model group, as well as no significant differences among the HGF groups.(Refer to Figure 4d).
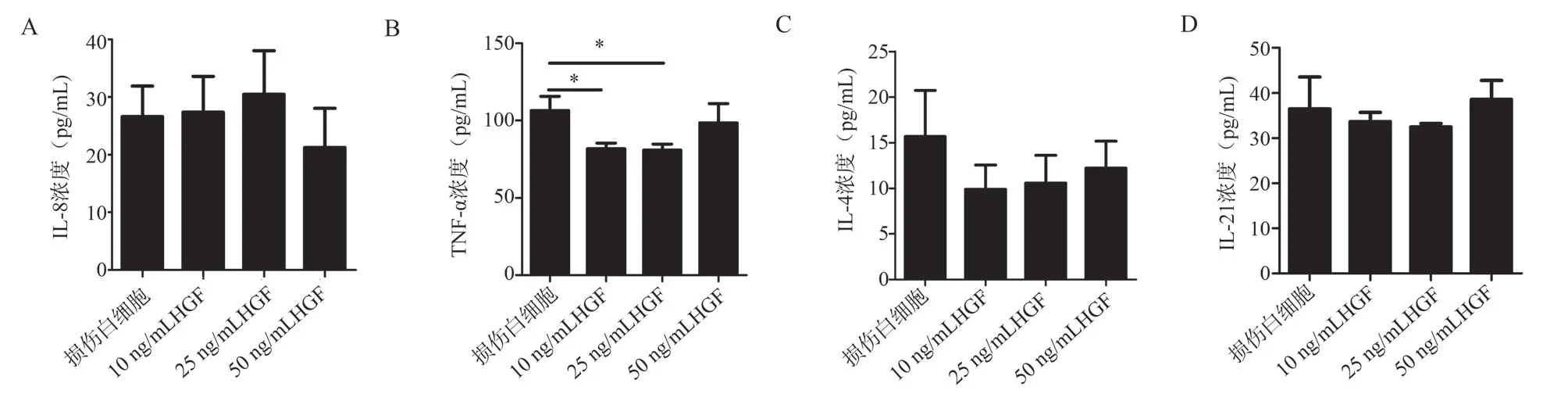
Fig 2 Effects of different concentrations of HGF on the secretion of four cytokines by liver leukocytes in CCl4-induced liver injury mice.a: ELISA measurement of IL-8 levels in the supernatant of liver leukocytes treated with different concentrations of HGF after intraperitoneal injection of CCl4 (10 mL/kg,0.1% CCl4 castor oil solution) for 12 h.b: ELISA measurement of TNF-α levels in the supernatant of liver leukocytes treated with different concentrations of HGF after intraperitoneal injection of CCl4 (10 mL/kg, 0.1% CCl4 castor oil solution) for 12 h.c: ELISA measurement of IL-4 levels in the supernatant of liver leukocytes treated with different concentrations of HGF after intraperitoneal injection of CCl4 (10 mL/kg, 0.1% CCl4 castor oil solution) for 12 h.d: ELISA measurement of IL-21 levels in the supernatant of liver leukocytes treated with different concentrations of HGF after intraperitoneal injection of CCl4 (10 mL/kg, 0.1% CCl4 castor oil solution) for 12 h.*P < 0.05.
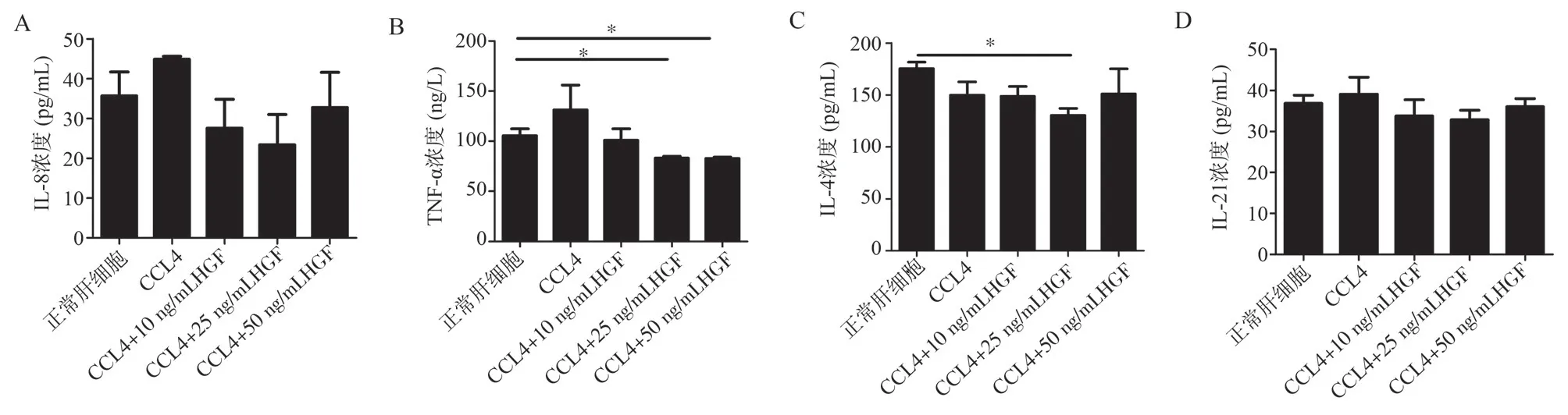
Fig 3 Effects of different concentrations of HGF on the secretion of four cytokines by liver parenchymal cells in CCl4-induced liver injury mice in vitro.a:ELISA measurement of IL-8 levels in the supernatant of liver cells treated with different concentrations of HGF after in vitro CCl4 (100 mmol/mL) injury.b:ELISA measurement of TNF-α levels in the supernatant of liver cells treated with different concentrations of HGF after in vitro CCl4 (100mmol/ml) injury.c: ELISA measurement of IL-4 levels in the supernatant of liver cells treated with different concentrations of HGF after in vitro CCl4 (100mmol/ml) injury.d:ELISA measurement of IL-21 levels in the supernatant of liver cells treated with different concentrations of HGF after in vitro CCl4 (100 mmol/mL) injury.*P< 0.05.
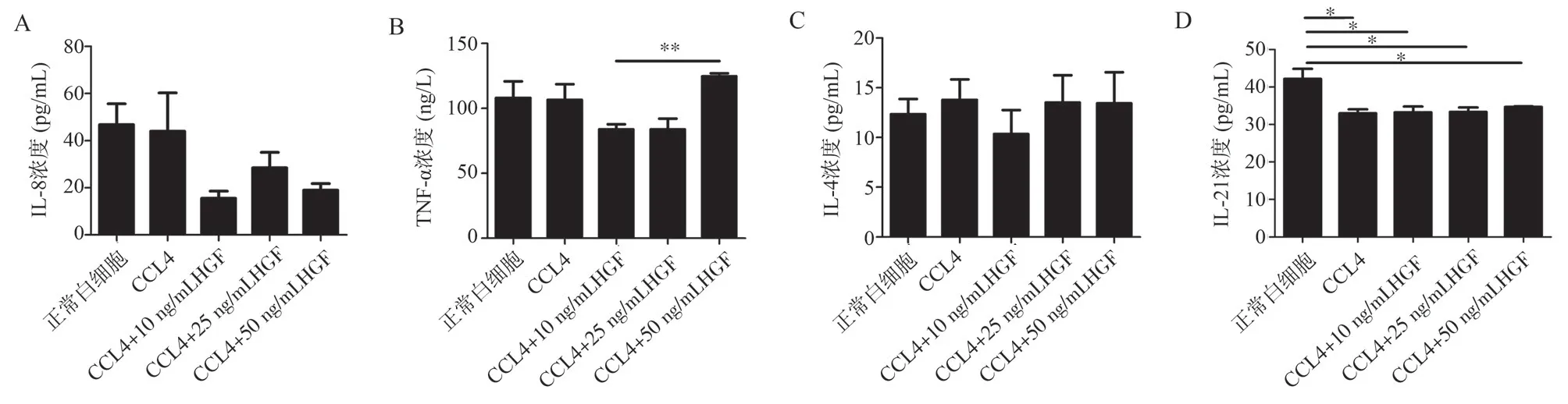
Fig 4 Effects of different concentrations of HGF on the secretion of four cytokines by liver leukocytes in vitro in CCl4-induced liver injury mice.a: ELISA measurement of IL-8 levels in the supernatant of liver leukocytes treated with different concentrations of HGF after in vitro CCl4 (100 mmol/mL) injury.b:ELISA measurement of TNF-α levels in the supernatant of liver leukocytes treated with different concentrations of HGF after in vitro CCl4 (100 mmol/mL)injury.c: ELISA measurement of IL-4 levels in the supernatant of liver leukocytes treated with different concentrations of HGF after in vitro CCl4 (100 mmol/mL) injury.d: ELISA measurement of IL-21 levels in the supernatant of liver leukocytes treated with different concentrations of HGF after in vitro CCl4 (100 mmol/mL) injury.*P < 0.05; ***P < 0.001.
4.Discussion
CCl4is a selective hepatotoxic substance that, after activation by hepatic microsomal cytochrome P450 oxidases in the liver,produces trichloromethyl radicals.This can trigger the production of reactive oxygen species and lipid peroxidation reactions,leading to liver cell damage and the secretion of a large amount of inflammatory factors[14].In order to investigate the role of HGF in the inflammatory response of damaged liver, this study used different concentrations of HGF to stimulate liver parenchymal cells and liver leukocytes in mice with in vivo and in vitro CCl4-induced injury, and detected changes in the pro-inflammatory cytokines IL-8, TNF-α,IL-21, and the anti-inflammatory cytokine IL-4.
The results of the study showed that HGF had an inhibitory effect on the expression of the pro-inflammatory cytokines IL-8 and IL-4 in liver cells of mice with in vivo CCl4-induced injury,and the concentration of 10 ng/mL HGF was the most suitable concentration for inhibiting IL-8 expression levels.IL-8 is an important neutrophil chemotactic factor in the body, which promotes intracellular lysosomal activation and phagocytosis by activating neutrophils, thus causing local inflammatory reactions[15].IL-4 is an anti-inflammatory cytokine, which has the ability to inhibit the inflammatory response and alleviate liver injury[16].In contrast to the findings of Ma Yuzhen et al.[17], this experiment established an acute liver injury model within 18 hours, and the IL-4 level in the mouse liver increased during the early stage of the inflammatory response[18,19].The decrease in IL-4 concentration observed in the experimental results indicates that HGF has an inhibitory effect on the inflammatory response of liver parenchymal cells.
The results of the in vitro stimulation of liver parenchymal cells with CCl4showed that co-stimulation with CCl4and HGF can decrease the secretion of TNF-α and IL-4 by mouse liver parenchymal cells, and the concentration of 10ng/ml HGF is the most suitable concentration for inhibiting TNF-α expression levels in liver parenchymal cells.TNF-α is a monocyte-derived cytokine mainly produced by the mononuclear macrophage system.It has immunoregulatory and pro-inflammatory activities.It can not only activate monocytes and macrophages, enhancing their cytotoxic activity, but also induce the expression of various adhesion factors and the production of chemokines.Abnormal secretion of TNF-α is a major mediator and end-stage mediator of liver injury in response to inflammation, injury, and shock[16,20-23].Research by He Fang et al.[24] showed a positive correlation between TNF-α level and systemic inflammatory response.In this experiment, no significant difference was observed in TNF-α expression levels in mouse liver parenchymal cells after CCl4-induced injury, possibly due to the small number of mice in the experimental groups.However,the experimental results showed a significant decrease in TNF-α expression levels after the addition of HGF, suggesting that HGF may have an inhibitory effect on the secretion of TNF-α by mouse liver parenchymal cells.In conclusion, HGF can inhibit the expression of TNF-α in damaged mouse liver cells, which is consistent with the results of the study by Jia Junqing et al.[25].
In in vivo stimulation, HGF exhibits an inhibitory effect on the secretion of the pro-inflammatory cytokine TNF-α by mouse liver leukocytes, while its effect on liver parenchymal cells is not significant.This is consistent with the results of the study by Li Liyuan et al.[26].The experiment employed density gradient centrifugation to separate liver cells into liver parenchymal cells and liver leukocytes for investigating the inhibitory effect of HGF on the secretion of the pro-inflammatory cytokine TNF-α by these two cell types.The results revealed that HGF exhibited a more pronounced inhibitory effect on the secretion of TNF-α by mouse liver leukocytes in the injured liver.In the in vitro experiment with mouse liver leukocytes, co-stimulation with CCl4 and HGF showed no significant correlation between HGF concentration and IL-21 levels.However, IL-21, as a major pro-inflammatory cytokine that initiates or promotes tissue inflammation and leads to damage, is highly expressed in immune-related diseases such as rheumatoid arthritis and systemic lupus erythematosus.Its expression levels decrease with disease control, indicating a potential role of IL-21 in mediating the generation and exacerbation of inflammation and tissue pathology[27].This finding differs from the study by Ai Guo et al.[28], which reported a positive correlation between IL-21 expression levels and liver inflammation activity.The discrepancy may be attributed to the fact that IL-21 expression in liver leukocytes was not significantly upregulated during the early stages of inflammation.
Studies have shown that HGF improves the symptoms of viral hepatitis and reduces the expression of TNF-β, IFN-γ by infecting mice with adenovirus-induced liver injury models[29].In a liver injury model established by α-naphthylisothiocyanate, HGF was found to reduce the expression of TNF-α and modulate the hepatic inflammatory response[30].HGF can improve liver inflammation in both of these liver injury models by reducing the expression of inflammatory factors.
The results of in vivo liver cell injury induced by CCl4are not entirely consistent with the results of in vitro stimulation.This may be because in vivo liver injury caused by CCl4stimulates systemic immune cell secretion of cytokines, while in vitro experiments only stimulate liver cells.The complex cascade reactions caused by CCl4-induced liver injury in vivo still require further experimental exploration.Inflammatory factors, as precursors of inflammation in the liver, can be used as indicators for researchers to assess the extent of tissue damage and the occurrence of inflammatory reactions.However, the mechanisms by which these factors induce liver inflammation still require further research.
Conflicts of Interest Statement
We declare that we have no financial or personal relationships with any individuals or organizations that could influence our work.There are no professional or other personal interests of any nature or type in any product, service, or company that could be construed as influencing the positions or reviews presented in our content.
Authors’ contribution Qi Duan, Li Zheng is responsible for experimental design.Qi Duan,Siyue Li, Qinglan Li, Yu Wang is mainly involved in experiment implementation.Qi Duan, Li Zheng is responsible for data analysis.Lili is responsible for paper writing and revisions.
 Journal of Hainan Medical College2023年18期
Journal of Hainan Medical College2023年18期
- Journal of Hainan Medical College的其它文章
- Systematic review and meta-analysis of endometrial thickening during endocrine therapy combined with Chinese herbal medicine intervention after breast cancer surgery
- Clinical observation on the efficacy and safety of different doses of alfentanil for painless gastroscopy
- Expression and significance of ADAM12 in bladder cancer
- Effects of intravenous infusion of esketamine on analgesia and postpartum antidepressant after cesarean section
- The expression of TUSC3 in Preeclampsia and the function in trophoblast cell
- Inhibitory effect of water soluble propolis on oxidative damage in rats with ulcerative colitis
