Quinones from Cordia species from 1972 to 2023: isolation,structural diversity and pharmacological activities
Rostanie Dongmo Zeukang,Jarmo-Charles Kalinski,Babalwa Tembeni,Eleonora D.Goosen,Jacqueline Tembu,Turibio Tabopda Kuiate,Dominique Serge Ngono Bikobo,Maurice Tagatsing Fotsing,Alex de Théodore Atchadé and Xavier Siwe-Noundou*
Abstract Plants of the genus Cordia (Boraginaceae family) are widely distributed in the tropical regions of America,Africa,and Asia.They are extensively used in folk medicine due to their rich medicinal properties.This review presents a comprehensive analysis of the isolation,structure,biogenesis,and biological properties of quinones from Cordia species reported from 1972 to 2023.Meroterpenoids were identified as the major quinones in most Cordia species and are reported as a chemotaxonomic markers of the Cordia.In addition to this property,quinones are reported to display a wider and broader spectrum of activities,are efficient scaffold in biological activity,compared to other classes of compounds reported in Cordia,hence our focus on the study of quinones reported from Cordia species.About 70 types of quinones have been isolated,while others have been identified by phytochemical screening or gas chromatography.Although the biosynthesis of quinones from Cordia species is not yet fully understood,previous reports suggest that they may be derived from geranyl pyrophosphate and an aromatic precursor unit,followed by oxidative cyclization of the allylic methyl group.Studies have demonstrated that quinones from this genus exhibit antifungal,larvicidal,antileishmanial,anti-inflammatory,antibiofilm,antimycobacterial,antioxidant,antimalarial,neuroinhibitory,and hemolytic activities.In addition,they have been shown to exhibit remarkable cytotoxic effects against several cancer cell lines which is likely related to their ability to inhibit electron transport as well as oxidative phosphorylation,and generate reactive oxygen species (ROS).Their biological activities indicate potential utility in the development of new drugs,especially as active components in drug-carrier systems,against a broad spectrum of pathogens and ailments.
Keywords Cordia,Boraginaceae,Quinones,Meroterpenoids,Biogenesis,Pharmacological activities
1 Introduction
Many plants are traditionally used to treat human diseases,including plants from the genusCordia[1].Cordiais among the largest genera in the Boraginaceae family [2-4],with around 300 identified species [2,5,6].Medicines prepared from these plants are commonly used to treat pains,digestive system and blood disorders,urogenital infections,influenza,cardiac and vascular diseases,coughs,asthma,inflammation,worm infestation,ringworm [7-10],syphilis,as well as dermal and mucosal lesions [11].The medical utilization of different parts (leaves,stem,stem bark,roots,flowers,and fruits) ofCordiaspecies is due to the presence of diverse bioactive constituents,such as terpenoids [9,12],cinnamates [13],flavonoids [14],pyrrolizidine alkaloids[15].Cordiaspecies are a source of natural products with an extensive range of pharmacological activities,including antimalarial,antioxidant,antiviral,and wound healing properties [9,16].They are promising sources for discovering and developing new drug formulations.Apart from their pharmacological application in folk medicine,they are grown as ornamental plants [7],and their wood is used for construction work,boat and furniture building [17-19].The genus is known for producing a great diversity of quinone natural products,which are often found to be major phytochemical components,especially in extracts from the heartwood and roots [8].
Quinones have long been considered one of the important natural product classes in developing new drugs due to their valuable biological properties such as antioxidant,anti-inflammatory [20],antimalarial,antibacterial,antifungal,and anticancer activities [21,22].They have the ability to exist in several redox states,can be highly reactive and play a major role in oxidative mechanisms [23].Moreover,they are able to elicit oxidative DNA cleavage [24].Exemplary mitomycin C,a chemotherapy drug used for the treatment of tumors,was isolated from cultures of the bacteriumStreptomyces caespitosusin 1958 [25];daunorubicin,an anthraquinone isolated from the soil bacteriumStreptomyces peucetiusin 1963 is known for its potent antileukemic effect;a close analogue,doxorubicin,was isolated from the same strain in 1969 and is used to treat a variety of malignant tumors [26,27];vitamin K,a naphthoquinone derivative,is indicated to improve blood coagulation [28].Furthermore,oncocalyxone A,a benzoquinone isolated fromCordia oncocalyxand tested in vivo and in vitro models,showed a large spectrum of pharmacological uses such as antiproliferative/cytotoxic activities against mammalian cells,anti-inflammatory,neuroinhibitory and analgesic effects,as well as antimicrobial and antibiofilm activities [26].Previous studies have also reported thatCordiaquinones exhibited pharmacological activities such as antimalarial,antifungal,antimycobacterial and larvicidal activities in addition to cytotoxicity against mammalian cell lines [4,17,29-31].
Quinones occurring inCordiaspecies are primarily classified as meroterpenoid benzoquinones,meroterpenoid hydroquinones,and meroterpenoid naphthoquinones [17,32-34].Moreover,literature reports on their isolation suggested quinones(meroterpenoids and their derivatives) as one of the chemomarkers ofCordiagenus [4,5,32,34].Even though numerous meroterpenoid quinones have been isolated fromCordiaspecies since 1970,no experimentally verified biosynthetic scheme has been reported [34].However,logical deductions have led to the proposal of a potential biosynthetic pathway for some meroterpenoid quinones fromCordiaspecies [17,34-112]
Several studies have investigated the phytochemical and biological studies ofCordiaspecies,and most reports focused on chemical constituents,their biological activities,and the chemical synthesis of meroterpenoid quinones.Some of this work has been reviewed in previous works.For instance,Oza et al.reviewed the pharmacological uses,isolation and biology activities of compounds and extracts from theCordiagenus until 2016 [8].Furthermore,Matias et al.reviewed ethnopharmacological and ethnobotanical uses of the genusCordiauntil March 2014 [7].Most reports discussing quinones ofCordiaspecies focus on South American species used in Brazilian folk medicine.
The relevant information aboutCordiaquinones published between 1972 and 2023,their chemistry,structure,biogenesis and pharmacological activities was obtained through online database search using Scifinder(https:// scifi nder.cas.org),Science Direct (https://www.scien cedir ect.com),PubMed (https:// pubmed.ncbi.nlm.nih.gov),and Google Scholar (https:// schol ar.google.com).The search terms were the following keywords and combinations:Cordiaspecies,quinone compounds,meroterpenoids,biosynthesis,biogenesis,and pharmacological activities.The search results thus obtained were critically reviewed for the descriptions of previously describedCordiaquinones regarding their structure,biogenesis,biological activities,the occurrence of their source organisms,the extraction and purification protocols employed,and the plant parts used.Additional information was obtained by reviewing the cited references in the selected articles.
2 Occurrence of Cordia quinones
Quinones are a diverse natural product class biosynthesized by plants,fungi,algae,and bacteria [38],and numerous protocols for their chemical synthesis were reported [39].They are characterized byorthoorpara-dione substituted cyclic aromatic systems as found in benzoquinones or condensed polycyclic aromatic systems [20] exemplified by naphthoquinones,anthraquinones,and phenanthraquinones [20,21].
Quinones are biosynthesized in plants via different metabolic pathways with diverse precursors.These include acetate-polymalonate,aromatic amino acids,shikimic acid-o-succinoylbenzoic acid,and mevalonic acid pathways [40].They play an essential part in physiological and enzymatic systems due to their principal role as redox agents in many electron-transfer processes in living organisms [21,41].
Up to 2023,approximately 70 quinones were isolated fromCordiaspecies consisting mainly of meroterpenoid quinones,the principal quinone type isolated from this genus.Additionally,meroterpenoid quinones were identified by GC-MS profiling of different extracts ofCordia rothii[42] and by chromatographic fingerprint analysis of bark dichloromethane extract and hexane leaf extract ofCordia dodecandrausing UV-DAD HPLC [10].
Meroterpenoids are a class of natural products derived partially from terpenoid and quinone biosynthetic pathways [43,44],where terpenoid and aromatic quinone moieties are linked by carbon-carbon (C-C) and carbon-oxygen (C-O) bonds [45].Meroterpenoids have been isolated from animals,fungi,marine organisms(algae,microorganisms and invertebrates),and higher plants [46,47].Meroterpenoids exhibit a great diversity of structures.These can be a simple molecular structure comprising a prenyl unit linked to a phenolic derivative moiety such as hydroquinone or more complex structures by ring cyclization and chain rearrangement of various length terpenoid side chains [46,48].
Terpenoids are broadly classified into two major groups depending on their biosynthetic origins:
Firstly,polyketide-terpenoids are grouped according to the number of acyl units that are incorporated to form the polyketide chain (originating from successive condensation of simple carboxylic acids under the control of the polyketide synthases (PKSs)) and the mode of cyclization present.[43,48].Polyketide meroterpenoids can have a tri-,tetra-or polyketide chain connected to the terpenoid moiety [48].
Secondly,non-polyketide-terpenoids in which quinones,protocatechuic acid derivatives,dehydroquinic acid or related subunits originating from shikimate pathways are joined to a terpenoid skeleton by a single carbon-carbon (C-C) bond [43].
Previous chemical studies of meroterpenoids revealed that their purification usually follows maceration and conventional extraction methods using organic solvents or their aqueous mixtures [48].The macerated raw material was extracted with methanol and aqueous methanol (80%) [49-53];ethanol and aqueous ethanol(70-95%) [54-56];ethyl acetate [57-61] and petroleum ether [62].Crude extracts are commonly fractioned by liquid-liquid extraction (hexane;chloroform or dichloromethane,ethyl acetate and butanol) [49,51,54,63];and purify by silica gel column chromatography(CC) (n-Hexane-ethyl acetate;n-hexane-acetone;cyclohexane-dichloromethane-methanol gradient;petroleum ether;ethyl acetate;isooctane-ethyl acetatemethanol;ethyl acetate-methanol [49,56,61-65];Sephadex LH-20 CC (Dichloromethane-methanol (1:1);chloroform-methanol (3:2);methanol) [65-69];MCI gel CHP20P CC (water-methanol (20-100%);methanolwater (60-100%) [55,67,68,70] and RP-HPLC(acetonitrile—0.01% trifluoroacetic acid,88:12 (v/v);acetonitrile-water (80:20-100:0);methanol-water 25%)[55,66,67,71].
The present summarizes quinones from 25Cordiaspecies,among which meroterpenoid quinones were present in 22 species.The summary of various types of isolated meroterpenoid quinones from these 22Cordiaspecies and their biological activities are listed in Table 1.
Quinone constituents ofCordiaspecies are highly diverse,and continuous phytochemical studies of the roots,stem barks,heartwood,wood,leaves,and whole plant extracts ofCordiaspecies led to the isolation and structural identification of various quinone skeletons.The current review reports over 70 quinones (1-70)obtained from twenty-twoCordiaspecies,most of which were isolated from ethanol andn-hexane extracts of the roots.These compounds showed significant pharmacological activities,and their biosynthesis has been hypothesized.Their structural elucidation was achieved by mass spectroscopic (MS),1D and 2D nuclear magnetic resonance (NMR) analysis,chemical derivatization reactions,and X-ray crystallographic analysis.The structures of isolated quinones and their biological activities are summarized in Table 2.
Previous studies reported that the wood ofC.dodecandraused in joinery can cause dermal allergic reactions after prolonged contact [95],and it was explained that the allergy towards woods ofCordiaspecies might be due to the presence of cordiachromes[18,95].Thus,cordiachromes A (1),B (2),E (5) and F(6) fromC.dodecandramixed with 1% of petrolatum elicited high sensitization in experimental animals after 48 h and 98 h of exposure [95].However,another study revealed that cordiachrome F (6) had no noticeable effects on human patients after exposure to these mixtures over the same period.Thus,it was suggested that other cordiachromes that were not tested could be the responsible agents causing allergic reactions [18].
3 Biogenesis and synthesis of quinones from Cordia species
The biosynthesis of meroterpenoid quinones fromCordiaspecies has not been experimentally validated,but their biosynthetic sequences have been proposed based on logical deductions.For instance,Moir et al.[33] proposed that cordiachromes (A-F) can be derived from geranyl pyrophosphate and an aromatic precursor unit followed by oxidation of an allylic methyl group and cyclization totrans,trans-cylodecatriene.Subsequent acid-catalyzed cyclization led to cordiachromes A (1)and B (2).Cis,cis-cylodecatriene afforded cordiachrome C (3) via a Cope rearrangement [33].Cordiachromes D(4),E (5),and F (6) were obtained by methoxylation of the previous cordiachromes,respectively [33].According to Thomson [45],geranylquinol can be another precursor for cordiachromes.He suggested that geranylquinol may be obtained by oxidative cyclization at a terminal allylic methyl group via allylic alcohol pyrophosphate to provide a cyclodecatriene [45].Another cyclization of the latter through boat conformation could then conduct to cordiachromes A (1) and B (2),whereas a cope rearrangement of a cyclodecatriene would lead to cordiachrome C (3) [45].He also suggested that cordiachrome G (61) is more optically active than other cordiachromes because the stereospecific allylic oxygenation occurs before the rearrangement of cyclodecatriene [45].
Dettrakul et al.provide information about the biogenesis of cordiachromes.It was suggested that globiferin(45),isolated fromCordiaspecies,is an intermediate for the biosynthesis of cordiachromes because its structure is similar totrans,trans-cylodecatriene proposed by Moir et al.[17].In addition,the link between the benzoquinone skeleton and the aliphatic chain of globiferin was confirmed by its reduction with Na2S2O4to dihydroxyglobiferin (45a).Cordiachrome C (3) was obtained through Cope rearrangement by refluxing compound45in xylene.Cordiaquinol C (36) was obtained by refluxing compound45in DMSO-d6for two hours.It was also obtained from cordiachrome C (3) under the same conditions.The respective cordiachromes A (1) and B(2) derivatives,diacetylcordiachromes A (71) and B (72),were obtained by cyclization of diacetylglobiferin (45b)under acidic conditions,were obtained respectively [17].The suggestions about biosynthesis and synthesis proposed by Dettrakul et al.are resumed in Scheme 1.
According to Matos et al.and Silva et al.,meroterpenoid quinones fromCordiaspecies are formed viaC-alkylation of thep-hydroxybenzoic acid with prenyl unities which result in the formation of geranyl hydroquinone followed by different chemical reactions such as intramolecular cyclization,oxidation,hydroxylation,o-methylation,epoxidation,and decarboxylation [32,34].Based on this idea,the biogenesis of the cordiachrome derivatives (8to19) isolated fromC.oncocalyxwas established [32].Similarly,the hydroquinones (37,38,42,and43) and naphthoquinones (15and14) isolated fromC.glazovianacould follow the same pathway.
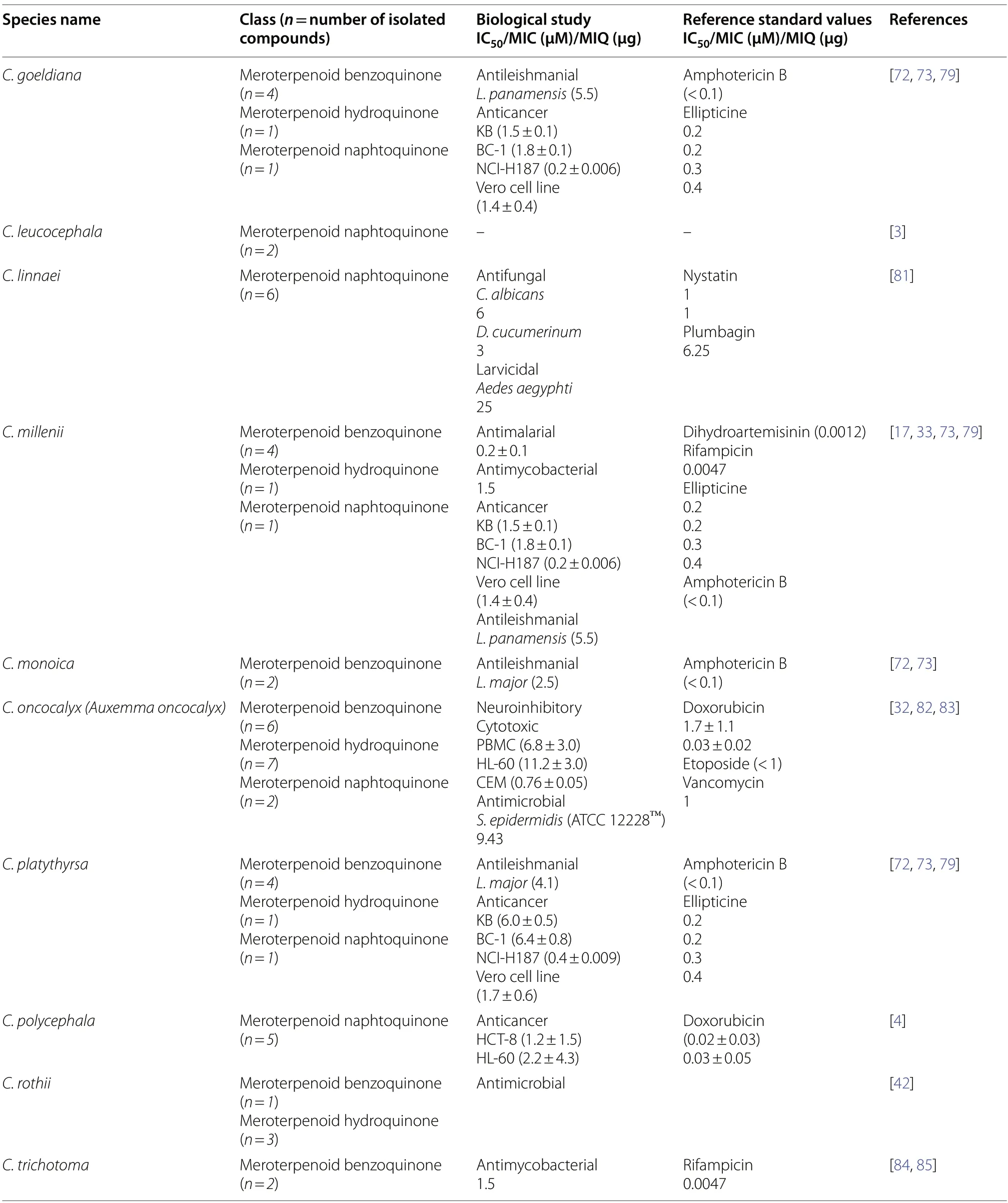
Table 1 (continued)

Table 2 Reported quinones from Cordia species
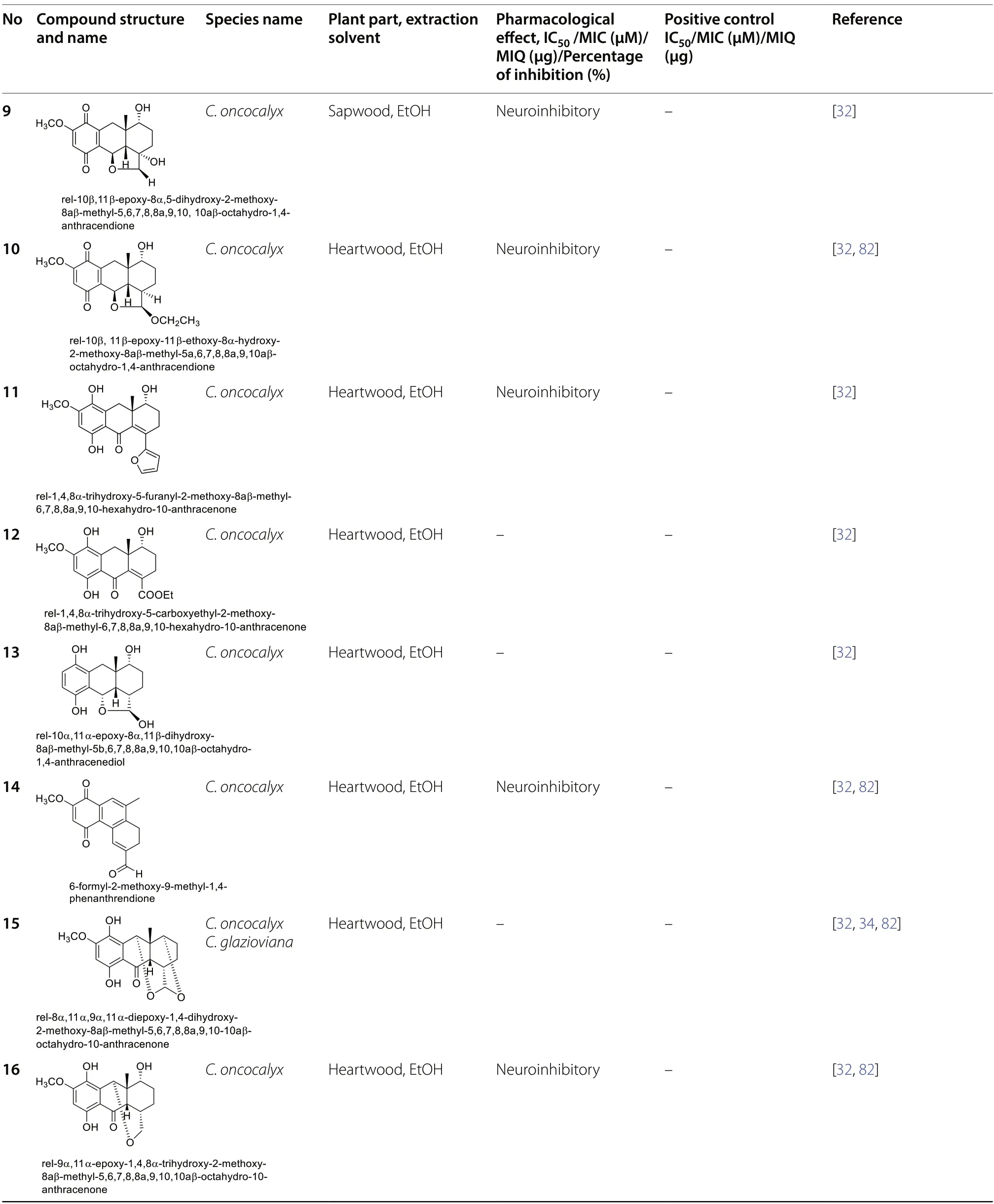
Table 2 (continued)
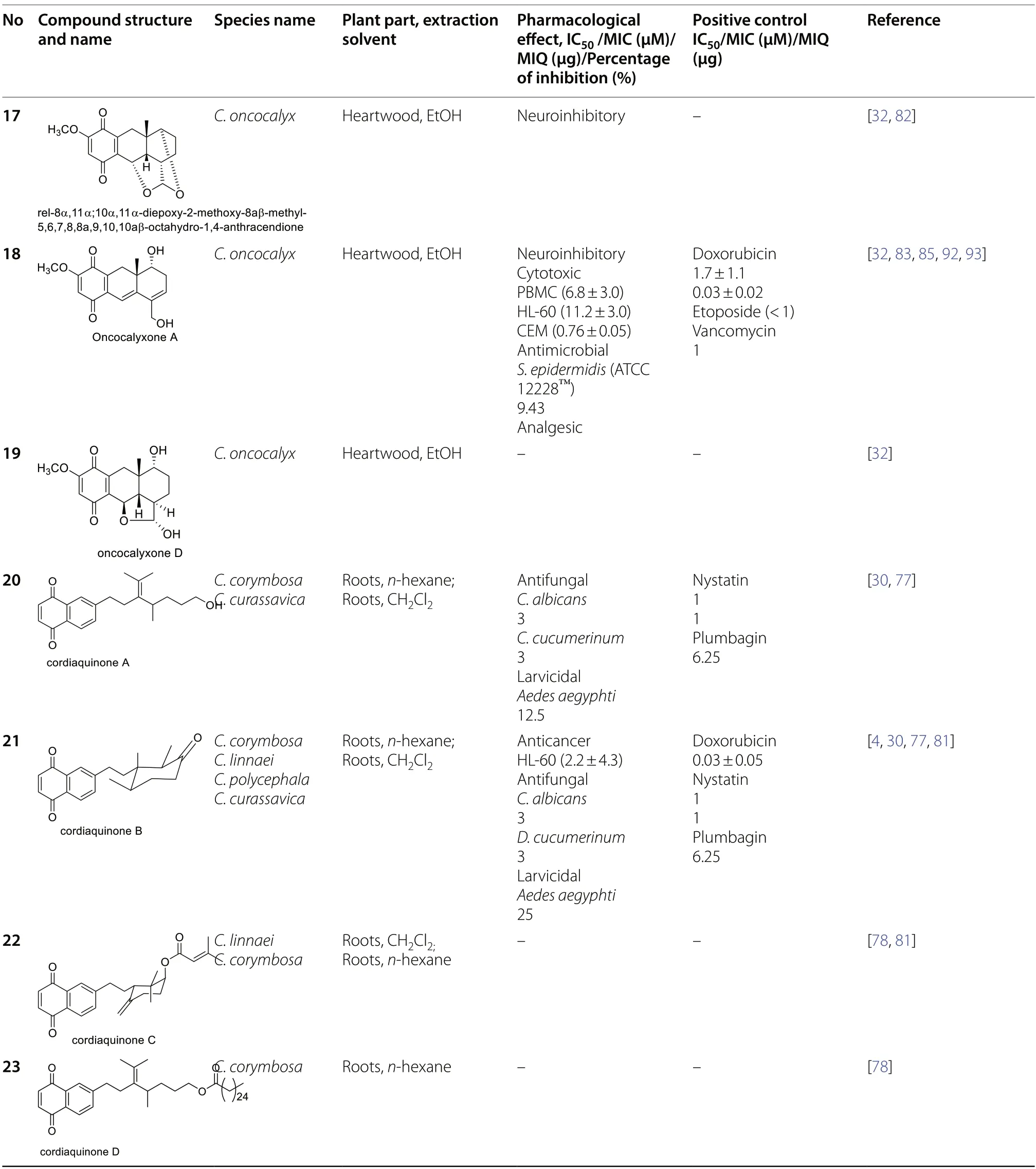
Table 2 (continued)
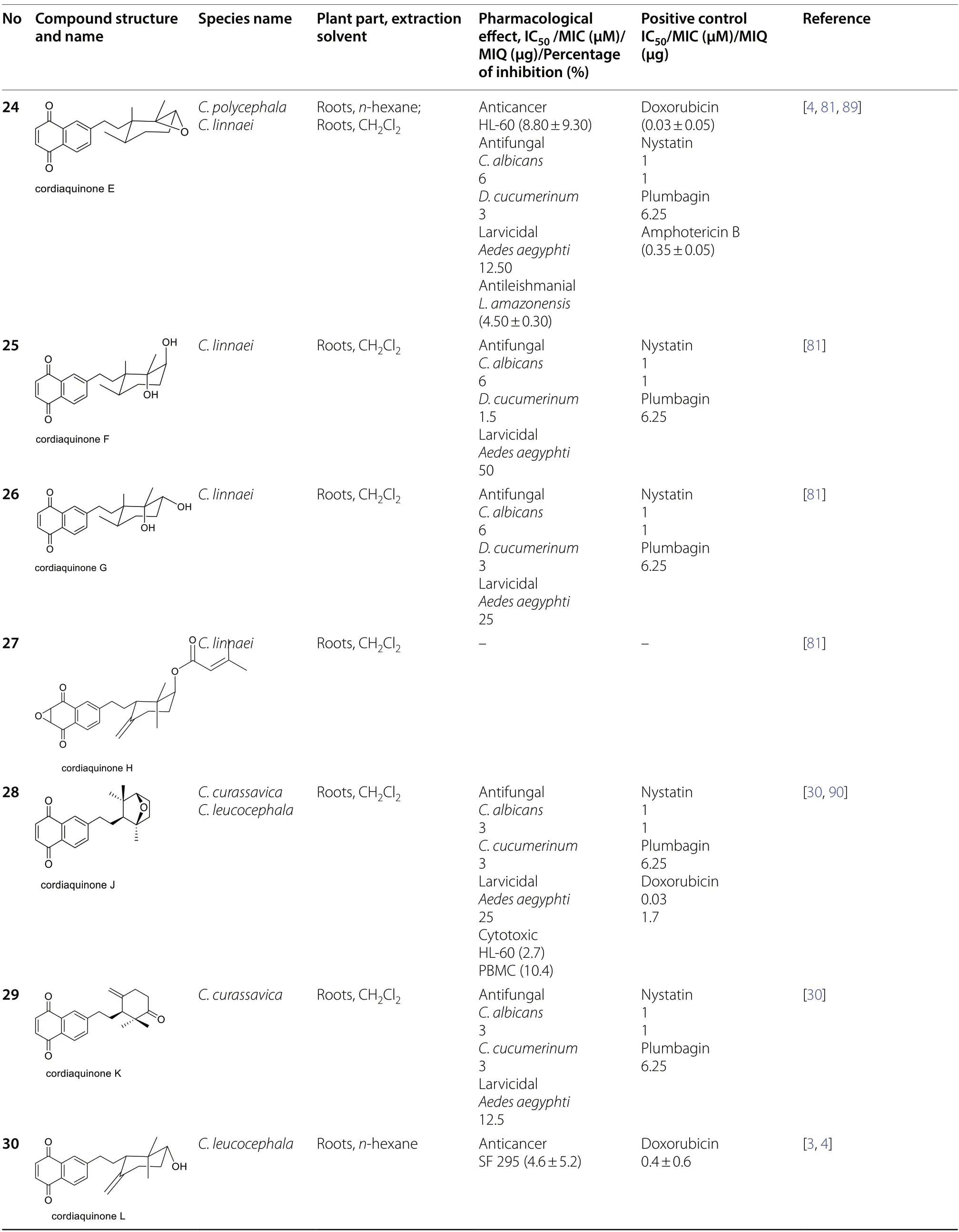
Table 2 (continued)
Table 2 (continued)

Table 2 (continued)
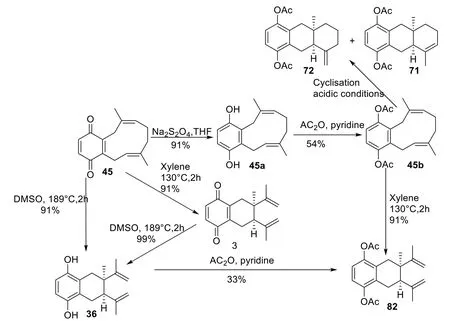
Scheme 1 A proposed synthetic pathway for the cordiachrome skeleton [17]
It has been suggested that alkannin (7),a quinone isolated fromCordia millenii,could be biosynthesized fromp-hydroxybenzoic acid and mevalonate [33].Leistner,this biosynthetic pathway to form alkannin (7)may occur in the Boraginaceae family [40] and,thus,in theCordiagenus.
As for Cordiaquinones biosynthesis,Arkoudis and Stratakis proposed that cordiaquinones are derived from (E)-Naphtoquinone epoxide,their precursor (75)which is obtained from E-trans,trans-Farnesol (73) and benzoquinone (74) through oxidation and Diels-Alder rearrangement,and different cordiaquinones are occurring from precursor through chemical reactions (cyclization,oxidation and esterification) (Scheme 2) [96].
Manners and Jurd suggested the biosynthesis of compounds fromC.alliodora.According to them,the isolation of cordiachromene A (57) fromC.alliodoraconfirms the presence of geranylphenol (76) as a precursor of compounds isolated fromC.alliodora[36].They proposed cyclization of the intermolecular geranyl side chain is due to the acid-catalyzed reaction of phenolic nucleus with geranyl C-3 or C-7 allylic hydroxyl group,which afforded to cordallinol (54) and alliodorol (53),followed by another acid-catalyzed cyclization and intramolecular rearrangement to form cordiol (55),cordiaquinols (36-41),and allioquinol(56),which can also be oxidized to cordiachromes (1-6) and their derivatives (Scheme 3) [36,37].
According to Manners,Cordiacompounds could be provided from a geranylphenol precursor that would then undergo oxidation reactions,intramolecular cyclization and rearrangement to give various geranylhydroquinone and geranylbenzoquinone derivatives occurring fromCordiaspecies woods [76].
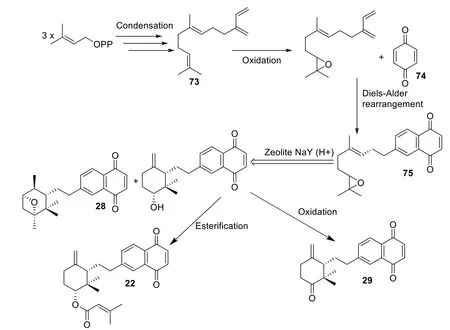
Scheme 2 Proposed biosynthesis and synthesis pathway to obtain cordiaquinone skeletons [35,96]
Many syntheses have been done to elucidate the structures,suggest biosynthetic pathways of isolated quinones from Cordia species,and compare the biological activities of the different compounds.This latter had resulted in other quinone derivatives with biological activities.For instance,the structure of cordiachrome C (3) was confirmed by its hydrogenation in ethyl acetate after reoxidation to obtain dihydrocordiachrome C (77).After reoxidation,its hydrogenation in acetic acid afforded tetrahydrocordiachrome C (78) [72].After the isolation of cordiachromes A-G (1-6,60),cordiachrome H (79)was obtained through oxidation of leucocordiachrome H (61) by silver oxide [75].The absolute configuration of cordiaquinol I (39) was determined by adding (14 mg,0.05 mmol) pyridine (4 mL) andp-bromobenzoylchloride(58 mg,0.26 mmol) and stirring for 24 h at room temperature to afford 1,4-p-dibromobenzoylcordiaquinol I (80)[79].Diacetylcordiaquinol I (81) was obtained through the addition of (8 mg,0.03 nmol),pyridine (0.5 mL),and acetic anhydride (0.5 mL) to cordiaquinol I (39) [79].Cordiaquinol C (36) (83 mg,0.34 mmol),in the presence of pyridine (2 mL) and acetic anhydride (2 mL) afforded diacetylcordiaquinol C (82) [79] (Fig.1).
The abundance of isolated quinones fromCordiaspecies provides a wide range of pharmacological activities that can lead to new drug discovery.
4 Biological studies and therapeutic potential
Prompted by ethnomedicinal uses ofCordiaspecies in preventing and treating various diseases in traditional medicine [7,8],various studies have been undertaken to shed light on the biological activity of extracts and isolated compounds.
4.1 Cytotoxicity
Evaluation of the cytotoxic activities of cordiachromes [B(2),C (3)],cordiaquinol C (36),globiferin (45),alliodorin(46),and elaeagin (66),isolated fromC.globifera,against KB (human epidermoid carcinoma of the mouth),BC-1(human breast cancer cells),NCI-H187 (human small cell lung cancer),and Vero cell lines (African green monkey kidney fibroblast cells),were carried out.Compounds2,3and36exhibited activity against the cell lines mentioned above with IC50values ranging from 0.2 μM to 6.9 μM,while globiferin (45) was active only against NCI-H187 cells with an IC50value of 0.5 ± 0.04 μM [17].
The cytotoxicity of compounds48and49fromC.globosawas evaluated in vitro against human colon adenocarcinoma (HCT-116),ovarian carcinoma(OVCAR-8) and glioblastoma (SF-295) cell lines.None showed antiproliferative effects at maximum concentrations of 20 μM [5].
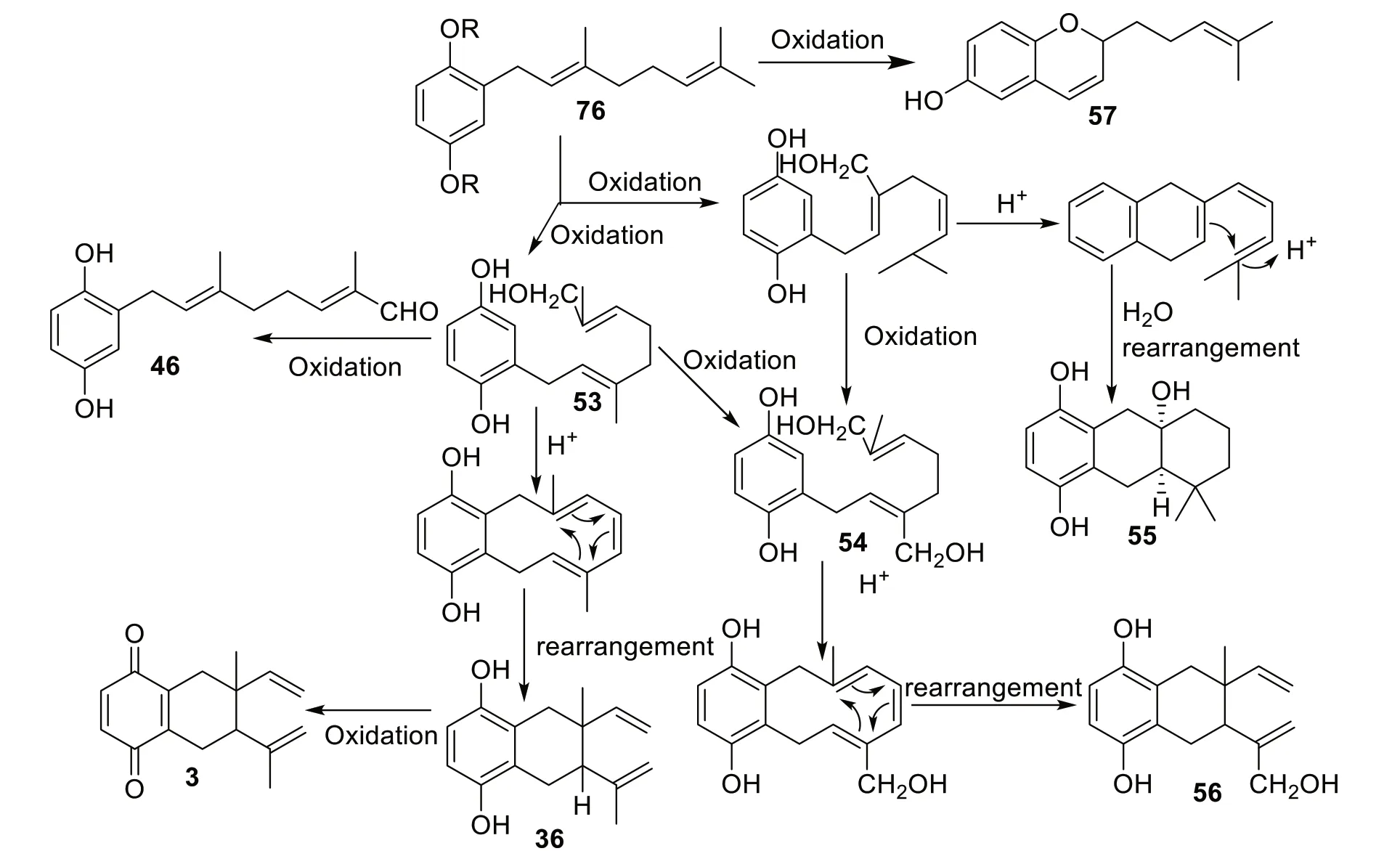
Scheme 3 Proposed biosynthesis scheme of C. alliodora compounds [36,37]
Cordiaquinones B (21),E (24),L (30),N (32),and O (33) fromC.polycephalaroots were tested against HCT-8 (colon),HL-60 (leukemia),MDA-MB-435(melanoma),and SF295 (brain) cancer cell lines [4].All the compounds were active against all these cancer cell lines with IC50values ranging from 1.2 to 11.1 μM,but compounds32and33were most active with IC50values from 1.2 to 3.4 μM.Compound21was most active against HL-60 cells with an IC50value of 2.2 μM (positive reference Doxorubicin with IC50value=0.02-0.8 μM)[4].The authors suggested that the elevated activity of compounds32and33may be related to the presence of the α,β-conjugated carbonyl at the end of the tigloyloxy chain [4].Chemical investigation ofC.globiferaled to the isolation of globiferane (47),which showed weak cytotoxicity against the following cell lines: HepG2(human hepatocellular liver carcinoma),MOLT-3 (acute lymphoblastic leukemia),A549 (human lung carcinoma),and HuCCA-1 (human lung cholangiocarcinoma) with IC50values of 148.6,3.7,148.6,and 66.0 μM,respectively,using an MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazoliumbromide) assay [80]. Its derivative (1aS*,1bS*,7aS*,8aS*)-4,5-dimethoxy-1a,7adimethyl-1,1a,1b,2,7,7a,8,8a-octahydrocyclopropa[3,4]cyclopenta[1,2,b]naphtalene-3,6-dione (50) isolated fromC.globosaroots exhibited significant cytotoxicity activity against colon (HCT-8),leukemia (HL-60,CEM),skin (B-16),and MCF-7 (breast) cancer cell lines,with IC50values ranging between 1.2 and 5.0 μM [31].The observed cytotoxicity exhibited by compound(50)may be due to the electron-donating methoxy groups on the aromatic ring.They are considered essential for anticancer activity [97].According to Liew et al.,compounds with a methoxy group substituted at C-2 of a quinone ring inhibit the growth of cancer cells.In addition,two or more methoxy substituents attached to its side showed more significant cytotoxicity [98].
Pessoa et al.evaluated the cytotoxicity of oncocalyxones A (18) and C (59) isolated fromC.oncocalyxon human cell lines CEM (leukaemia),SW 1573 (lung tumour)and CCD922 (normal skin fibroblasts).Oncocalyxone A revealed toxicity with IC50values of 0.76 ± 0.05,7.0 ± 1.7 and 13.4 ± 0.6 μg/mL on CEM,SW 1573,and CCD922,respectively.Oncocalyxone B (58)also showed cytotoxicity with IC50values of 1.5 ± 0.3,7.5 ± 0.7 and 12.4 ± 0.5 μg/mL on CEM,SW 1573,and CCD922,respectively [93].In addition,the cytotoxicity of oncocalyxone A (18) was evaluated against human normal [PBMC (peripheral blood mononuclear cells)]and tumoral [HL-60 (promyelocytic leukemia),SF-295(glioblastoma),OVCAR-8 (ovarian carcinoma),and HCT-116 (colon carcinoma)] cell lines.It showed high cytotoxic activity on human leukemic cancer cells and normal leukocytes with IC50values of 11.2 and 6.8 μM,respectively while exhibiting IC50values above 16.5 μM against the remaining cell lines [85].
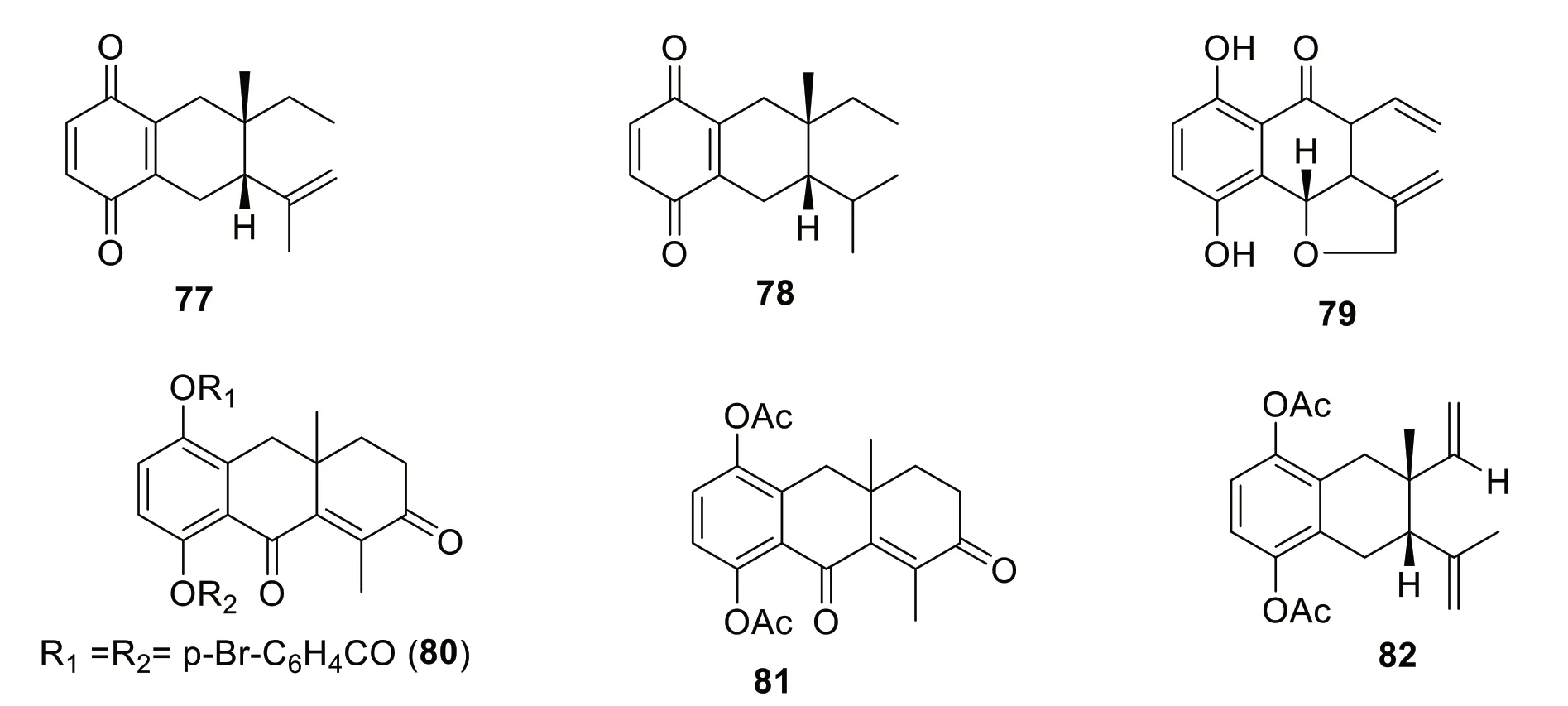
Fig. 1 Synthesized quinone derivatives from the Cordia genus
Moreover,Marinho-Filho et al.examined the cytotoxic effect of (+)-cordiaquinone J (28) isolated from C.leucocephalaon tumor cells.In an MTT assay,(+)-cordiaquinone J (28) demonstrated cytotoxicity activity after 72 h of incubation against HL-60(leukemia),HCT-8 (colon),SF295 (brain),MDA-MB-435(melanoma),and normal PBMC (Lymphocytes) with IC50values of 2.7 μM,4.9 μM,6.6 μM,5.1 μM,and 10.4 μM,respectively compared to doxorubicin as a positive control with IC500.03 μM,0.02 μM,0.4 μM,0.8 μM,and 1.7 μM,respectively [90].
The cytotoxicity of compounds1,2,3,36,39,40,41,and46isolated fromC.fragrantissimaand their synthesized analogues (80,81,and82) against COS-7(African green monkey kidney cells,epithelial-like)and HUH-7 (Human liver cancer cells,epithelial-like)were inactive in an XTT assay compared to MG 132(carbobenzoxy-L-leucyl-L-leucyl-L-leucinal) used as reference [79].
Previous biological studies reported that the cytotoxic activity of quinones is due to their ability to react as dehydrogenating and oxidizing agents [20].The cytotoxicity of quinones can also be explained by their capacity to inhibit electron transporters [99],protein adduct formation [100],oxidative phosphorylation [101],and reactive oxygen species (ROS) production [102]as well as through enzyme SH groups and direct DNA damage [39,90].
4.2 Antifungal and larvicidal activities
Ioset et al.evaluated the antifungal and larvicidal activities of cordiaquinones B (21),E (24),F (25),G(26),and H (27) isolated fromC.linnaeiusing TLC bioautographic and agar-dilution assays [81].The compounds (21,24-26)were active againstCandida albicansandDosporium cucumerinumwith minimum inhibitory concentrations (MIC) ranging from 0.5 to 6 μM compared to nystatin (0.2-1.0 μM) used as a positive reference.However,compound27was inactive on both fungi.Its inability to inhibit the bacterial strains might be due to an epoxide [81].Regarding their larvicidal potential,all the compounds showed activity againstAedes aegyptiwith MIC values between 12.5 and 50 μg/mL compared to reference plumbagin(MIC=6.25 μg/mL),except for compound27,which was not tested [81].
2-(2Z)-(3-Hydroxy-3,7-dimethylocta-2,6-dienyl)-1,4-benzenediol (52),isolated from the roots and bark ofC.alliodora,exhibited weak activity againstCladosporium cucumerinumin bioautography and in agar-dilution assays with an MA (Minimum amount to inhibit growth on the SiO2gel TLC) value of 5 μg and MIC of 15 μM respectively.This compound was inactive againstC.albicanson TLC bioautography,and consequently,it was not tested by agar-dilution assay [27].
Cordiaquinones A (20),J (28),and K (29) showed antifungal activity againstC.cucumerinumandC.albicansin bioautographic and agar-dilution assays with similar values (MA=0.5 μg and MIC=3 μg/mL) as the reference drug nystatin (MA=0.1 μg and MIC=1 μg/mL).These compounds also demonstrated weak larvicidal effects onAedes aegyptiwith MIC values of 12.5—25 μg/mL [28].
The antifungal activity of ehretiquinone (35),isolated fromC.anisophylla,was evaluated onC.albicans(DSY262 and CAF2-1 strains) using bioautography,agardilution assays and mature biofilm [91].The compound was more active against strain DSY262 with a minimum inhibition quantity (MIQ) ≤ 5 μg compared to CAF2-1 with a MIQ of 25 μg.However,the compound (25)was inactive in the agar-dilution assay and mature biofilm[91].
Dettrakul et al.investigated the antifungal activity of cordiachrome B (2) and C (3),isolated fromC.globifera.Both compounds exhibited weak antifungal activity againstC.albicanswith IC50values of 7.7 μM and 4.6 μM,respectively,whereas globiferin (45),cordiaquinol C (38),and alliodorin (46) were inactive with IC50values > 20 μM(positive control amphotericin B,IC50=0.08 μM) [17].The antifungal activity of oncocalyxone A (18) done by Silva et al.showed that it did not inhibit the growth of tested fungi (C.albicansATCC 10234™,C.neoformansATCC 48184™,A.fumigatusATCC 13073™,S.schenckiiATCC 201679™andT.interdigitale73896) with MIC values > 151 μg/mL [103].
4.3 Antileishmanial activity
The chemical investigation ofC.fragrantissimawood extract led to the isolation of several cordiaquinols(36,39,40,and41),cordiachromes (1,2,and3) and alliodorin (46)[73,79].The authors also synthesized related compounds,1,4-p-dibromobenzoylcordiaquinol I (80),acetylcordiaquinol I (81),and acetylcordiaquinol C (82) [79].All the compounds,including their derivatives,were assayed for antileishmanial assay against promastigote forms ofLeishmania major,L.panamensis,andL.guyanensisusing an MTT assay [79].All the compounds were active with IC50values of 1.4-81.4 μM were found more active onL.panamensisandL.guyanensisthanL.major,while compounds1,2,36,40,46,and82exhibited good activity againstL.majorwith IC50values of 4.1,2.5,4.5,2.7,7.0,and 1.4 μM,respectively,compared to Amphotericin B (IC50less than 0.1 μM) used as a positive control [73,79].
In related studies,cordiaquinone E (24),isolated from the roots ofC.polycephala,was evaluated for its activity against promastigote and axenic-amastigote forms ofL.amazonensisin vitro.The compound inhibited the growth of the promastigote form with an IC50value of 4.5 ± 0.3 μM as well as against the axenic-amastigote form with 2.89 ± 0.11 μM,with selectivity indexes (SI) of 54.84 and 85.4,respectively.The evaluation of cordiaquinone E(24) against intracellular amastigotes was carried out to support the notion of antileishmanial activity.It led to a better result with an EC50value of 1.92 ± 0.2 μM and an SI of 128.54 using an MTT assay.The growth inhibition assay of compound24on RAW 264.7 macrophages led to a CC50value of 1246.81 ± 14.5 μM.Antileishmanial activity of compound24onL.amazonensiswas evaluated using Amphotericin B [IC500.35 ± 0.05 μM (promastigote form); IC500.51 ± 0.02 μM (axenic-amastigote form)]and Meglumine antimoniate [IC5021,502 ± 481 μM(promastigote form); IC501730 ± 33.5 μM (axenicamastigote form)],as reference drugs respectively [89].Rodrigues et al.explained the antileishmanial activity of cordiaquinone E.Firstly,by apoptosis,which associates externalization of phosphatidylserine and necrotic cell death,and secondly,by immunomodulation [89].
4.4 Anti-inflammatory activity
Five meroterpenoids (15,38,42,43,and44) isolated fromC.glaziovianawere evaluated for their antiinflammatory activity against RAW 264.7 macrophage murine cells through cellular viability and lipopolysaccharide (LPS) induction.The cytotoxicity of isolated compounds was evaluated by MTT assay [34].Rel-1,4-dihydroxy-8α,11α,9α,11α-diepoxy-2-methoxy-8aβmethyl-5,6,7,8,8a,9,10,10a-octahydro-10-antracenone(15),cordiaquinol E (38),10,11-dihydrofuran-1,4-dihydroxyglobiferin (42),2-[(1'E,6'E)-3',8'-dihydroxy-3',7'-dimethylocta-1',6'-dienyl]-benzene-1,4-diol (43),and 6-[(2'R)-2'-hydroxy-3',6'-dihydro-2H-pyran-5'-yl]-2-methoxy-7-methylnaphthalene-1,4-dione(44) induced inflammation against RAW 264.7 macrophage cells by reducing cells viability with IC50range value 71.66 ± 15.44-609.48 ± 5.05 μM.Lipopolysaccharide production was evaluated by inducing oxide nitric in RAW 264.7 cells.Among these compounds,10,11-dihydrofuran-1,4-dihydroxyglobiferin (42) exhibited the best inhibition of NO (Nitric Oxide) synthesis with IC5050.34 ± 9.88 μM,followed by compounds44(66.73 ± 10.28 μM) and43(105.83 ± 5.09 μM);the rest produced weak inhibition to induced inflammation against RAW 264.7 macrophage compared to dexamethasone (IC501.79 ± 0.04 μM) used as a positive control[34].
Ferreira et al.examined the anti-inflammatory activity of the water-soluble fraction of the heartwood methanolic extract ofC.oncocallyx.The quinone fraction containing mainly oncocalyxone A (18) was very active in inhibiting paw edema induced by a carrageenan injection,with a 57% and 60% reduction three hours after a dose of 10 and 30 mg/kg body weight,respectively [104].
4.5 Antimicrobial,antibiofilm,antimycobacterial and antioxidant activities
Previous biological evaluation ofC.oncocalyxrevealed that oncocalyxone A (18) could inhibit the growth of Gram-positive and Gram-negative pathogenic strains,even clinical specimens.It was more sensitive toStaphylococcusspecies than toEnterococcus,Listeria,Acinetobacter,andStenotrophomonasspecies with an MCI range from 9.43 μg/mL to 151 μg/mL,and it showed high sensitivity againstS.epidermidis(ATCC 12228™)with MIC 9.43 μM compared to vancomycin (MCI 1 μM)used as reference[103].It also inhibited the growth ofS.aureusMED 55 (MIC 18.87 μM),S.aureusCOL andS.epidermidis70D (MIC 37.75 μM);andE.faecalisATCC512999™(MIC 75.5 μM) [103]
It showed inhibition of biofilm production by~70% in methicillin-resistantS.aureusMED 55 strain (resistant clinical specimen) [103]
Khan et al.examined the antimicrobial and antioxidant activities of the GC-MS profile fractions ofC.rothiiroots.Then-hexane fraction,which contained cordiachrome C (3),exhibited weak antibacterial activity against Gram-positive and Gram-negative bacteria.While the MeOH marc extract containing cordiaquinol C (36) and cordiachromene A (57) showed good antibacterial activity againstStaphylococcus epidermidiswith a minimum inhibitory concentration (MIC) 250 μg/disk,EtOAc marc extract containing cordiol A (55) was inactive against all the tested bacteria [42].
Regarding the antioxidant activity of these extracts,MeOH and EtOAc marc left extract ofC.rothiiroots have good activity with EC5093.75 μM thann-hexane extract,which showed weak activity with EC50187.5 μM[42].
Previous biological studies examined the antioxidant activity of the methanol extract of the heartwood ofC.oncocalyx.The quinone fraction (80% oncocalyxone A(18)) was evaluated in a rat model with CCl4-induced hepatotoxicity and the prolongation of pentobarbital sleeping time in mice by measuring plasma GPT and GOT.Only the quinone fraction inhibited the GPT level significantly (29%) with a 30 mg/kg dose.It also caused a significant reduction (45%) of CCl4-induced prolongation of pentobarbital sleeping time with a dose of 10 mg/kg.It confirmed the hepatoprotective effect involving free radical and lipoperoxidation and correlated with the antioxidant properties of quinones [105].The latter is possibly due to the presence of oncocalyxone A,the main constituent [106].Moreover,quinones are renowned for redox cycling ability [107];this is related to their free radical scavenging activity which promotes their antioxidant activity [108].
In addition,cordiachrome C (3) and globiferin (45)showed significant antimycobacterial activity with MIC 1.5 and 6.2 μg/mL,respectively,while cordiachrome B (2) (12.5 μg/mL),cordiaquinol C(36) (25.0 μg/mL),diacetylcordiaquinol C (82) (25.0 μg/mL),alliodorin (46)(12.5 μg/mL),and elaeagin (66) (12.5 μg/mL) displayed weak activity compared to Rifampicin (0.0047 μg/mL),Isoniazid (0.05 μg/mL),and Kanamycin (2.5 μg/mL) used as standard drugs [17].
4.6 Antimalarial and hemolytic activities
Cordiachrome C (3),cordiaquinol C (36),and diacetylcordiaquinol C (82) were evaluated for antimalarial activity againstPlasmodium falciparumusing dihydroartemisinin (IC500.0012 μg/mL),used as reference.They exhibited significant activity with IC500.2 ± 0.1 μg/mL,0.3 ± 0.0 μg/mL,and 0.4 ± 0.1 μg/mL respectively,more than cordiachrome B (2) (IC501.5 ± 0.2 μg/mL),globiferin (45) (IC502.1 ± 0.5 μg/mL),alliodorin (46) (IC503.1 ± 0.5 μg/mL),and elaeagin (66)(3.6 ± 0.1 μg/mL) [17].
Silva et al.evaluated the hemolytic activity of oncacalyxone A (18) through erythrocyte damage due to hemoglobin release.The compound did not show activity at the tested concentrations ≥ 151 μg/mL [103].
Compounds21,24,30,32,and33from C.polycephalaroots were evaluated for hemolytic activity in mice erythrocytes.None was active with EC50> 500 μmol L-1[4].
4.7 Neuroinhibitory effect
Matos et al.(2017) examined the neuroinhibitory effect of different compounds (9-18) isolated fromC.oncocalyxby mice vas deferens bioassay.Compounds10,11and14significantly inhibited the neurogenic contraction by 76%,69%,and 63%,respectively,whereas compounds12and15did not considerably affect neurogenic contraction.Compounds9,10,14,16,17and18showed a completely reversible neuroinhibitory effect upon adding the pharmacological antagonist Promethazine and a partial reversible effect by yohimbine.Neurogenic contraction induced by compound11was irreversible by adding naloxone,famotidine,promethazine or yohimbine antagonists.However,compounds9,10,14,16,17and18did not inhibit neurogenic contractions using the ODQ,famotidine or naloxone antagonists.The authors found that reversible action may be related to presynaptic terminal and pre-synaptic receptor inhibition due to the co-release of histamine and norepinephrine[32].
Although previous reviews reported different isolation methods and biological activities ofCordiaquinones,we noted a lack of information that could help to valorize them.We suggest that future research should focus on the structure-activity relationships and mechanisms of action of the quinones of the genusCordia.More in vivo biological tests and clinical studies should be performed.Up to now,just one clinical study has been done onCordiaquinones (cordiachrome F for allergenic).To improve the number of quinones isolated fromCordiaspecies,pressurized liquid extraction (PLE) could be used.[109].Pressurized hot water extraction to optimize the extraction of volatile components [110] and dry extraction to enrich powder fractions with an extensive range of secondary metabolites could also be done.[111,112].
5 Conclusion
Using Cordia species in traditional medicine to treat various diseases has increased interest in their phytochemistry.This review presents the collective phytopharmacological information onCordiaquinones from 1972 to 2023.The research shows that over 70 (1-70) quinones have been isolated from different parts ofCordiaspecies with different skeletal structures.Meroterpenoid quinones were the major class of compounds isolated,with meroterpenoid benzoquinones being the most predominant in most species.The biosynthesis ofCordiaquinones is not yet well understood,but the biogenesis and some biosynthetic pathways have been proposed to explain the presence of quinones in theCordiagenus.
The extracts and isolated quinones demonstrated antimalarial,antimicrobial,anti-inflammatory,antibiofilm,antioxidant,antimycobacterial,antileishmanial,larvicidal,hemolytic,neuroinhibitory,and cytotoxicity properties.Most studies reported cytotoxicity against particularly cancer cell lines.It may be due to the ethnomedicinal uses of these species and the anticancer properties of the quinones.Although the biological activities of compounds can often be related to their structures,there is currently little information available to explain structure-activity relationships for the quinones occurring inCordiaspecies.This review discussed the potential of the genusCordiaas a promising source of new bioactive compounds that can provide quinones for various pharmaceutical applications.
Abbreviations
AC2O Acetic anhydride
CCl4Tetrachloromethane
CHCl3Chloroform
CH2Cl2Dichloromethane
DMSO Dimethyl sulfoxide
EtOH Ethanol
GOT Glutamate-oxalate-transaminase
GPT Glutamate-pyruvate-transaminase
ODQ Soluble guanylate cyclase inhibitor
RP-HPLC Reverse phase high-performance liquid chromatography
MeOH Methanol
Na2S2O4Sodium dithionite
MTT 3-[4,5-Dimethylthiazole-2-yl]-2,5-diphenyl-tetrazolium bromide
THF Tetrahydrofuran
XTT 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
v/v Volume by volume
1D and 2D One dimension and two dimensions
Acknowledgements
XS-N kindly acknowledges a National Research Foundation (NRF) Competitive Support for Unrated Researchers (CSUR) grant,Reference Number:SRUG2203291031.The authors are grateful to the University of Yaoundé 1,Yaoundé,Cameroon and Sefako Makgatho Health Sciences University,Pretoria,South Africa for a Postdoctoral Fellowship to BT.
Author contributions
Conceptualization,RDZ and XS-N;investigation,RDZ,J-CK,BT and XS-N;validation,ADTA and XS-N;methodology,RDZ,J-CK,BT,EDG,JT,TTK,MTF and XS-N;writing-original draft,RDZ and XS-N;writing-review and editing,RDZ,J-CK,BT,EDG,JT,TTK,DSNB,MTF,ADTA and XS-N;supervision,ADTA and XS-N.All authors have read and agreed to the published version of the manuscript.
Funding
This work did not receive any funding.
Availability of data and materials
All data are included in the manuscript.
Declarations
Competing interests
The authors declare no competing of interests.
Author details
1Department of Organic Chemistry,Faculty of Science,University of Yaounde I,PO Box 812,Yaounde,Cameroon.2Department of Biochemistry and Microbiology,Faculty of Science,Rhodes University,PO Box 94,Makhanda 6140,South Africa.3Department of Pharmaceutical Sciences,School of Pharmacy,Sefako Makgatho Health Sciences University,Medunsa,PO Box 218,Pretoria 0204,South Africa.4Division of Pharmaceutical Chemistry,Faculty of Pharmacy,Rhodes University,PO Box 94,Makhanda 6140,South Africa.5Department of Chemistry,Tshwane University of Technology,Private Bag X680,Pretoria 0001,South Africa.
Received:2 September 2023
Accepted:31 October 2023

 Natural Products and Bioprospecting2023年6期
Natural Products and Bioprospecting2023年6期
- Natural Products and Bioprospecting的其它文章
- Kaemtakols A-D,highly oxidized pimarane diterpenoids with potent anti-inflammatory activity from Kaempferia takensis
- Natural product rhynchophylline prevents stress-induced hair graying by preserving melanocyte stem cells via the β2 adrenergic pathway suppression
- A comprehensive review on the chemical constituents,sesquiterpenoid biosynthesis and biological activities of Sarcandra glabra
- A recent update on development,synthesis methods,properties and application of natural products derived carbon dots
- The alkynyl-containing compounds from mushrooms and their biological activities
- Ginsenoside compound-K attenuates OVX-induced osteoporosis via the suppression of RANKL-induced osteoclastogenesis and oxidative stress
