Computational exploration of the significance of COPS6 in cancer:Functional and clinical relevance across tumor types
Shi-Lin Wang,Guang-Zheng Zhuo,Li-Ping Wang,Xiang-Hu Jiang,Guo-Hong Liu,Yun-Bao Pan,Yi-Rong Li
Abstract BACKGROUND The COP9 signalosome subunit 6 (COPS6) has been implicated in cancer progression,while its precise role in most types of cancer remains elusive.AIM To investigate the functional and clinical relevance of COPS6 across various tumor types using publicly available databases.METHODS We used R software and online analysis databases to analyze the differential expression,prognosis,mutation and related functions of COPS6 in pan-cancer.RESULTS Differential expression analysis and survival analysis demonstrated that COPS6 was highly expressed and associated with high-risk profiles in the majority of cancer types.Possible associations between COPS6 expression level and prognostic outcomes were found using data from public databases.Mutational analysis revealed that missense mutations were the predominant type of COPS6 mutation.Additionally,positive correlations were identified between COPS6 expression level and tumor mutational burden and microsatellite instability in most types of cancer.Immune infiltration analysis demonstrated a negative correlation between COPS6 expression level and CD8+T cell infiltration in certain types of cancer.The correlation between COPS6 expression level and cancerassociated fibroblast infiltration exhibited heterogeneity,in which a positive correlation was found in head and neck squamous cell carcinoma and tenosynovial giant cell tumor,and a negative correlation was identified in diffuse large B-cell lymphoma and thymoma.The correlation between COPS6 expression level and macrophage infiltration was closely related to macrophage type.Gene co-expression and enrichment analysis highlighted transcription elongation factor B polypeptide 2 and G protein pathway suppressor 1 were significantly and positively associated with COPS6 expression level.These genes were predominantly involved in processes,such as ubiquitin-mediated proteolysis and human immunodeficiency virus 1 infection.CONCLUSION In conclusion,this study systematically explored the significance of COPS6 across different tumor types,providing a solid foundation for considering COPS6 as a novel biomarker in cancer research.
Key Words: COPS6; Biomarker; Tumor mutational burden; Immune infiltration; Prognostic analysis
INTRODUCTION
According to recent data from the American Cancer Society,it is projected that the United States will witness 1958310 new cancer cases and 609820 cancer-related deaths by 2023[1].While there has been a 1.5% decrease in cancer mortality rates between 2019 and 2020,with an overall decline of 33% since 1991,the incidence of breast cancer,prostate cancer,and uterine cancer is on the rise,imposing potential challenges to the future progress.Furthermore,these types of cancer demonstrate significant disparities in mortality rates among different ethnic groups.Conversely,gastric cancer,esophageal cancer,and cervical cancer have shown downward mortality rates,while lung cancer,colorectal cancer(CRC),and female breast cancer continue to exhibit gradually upward mortality rates[2,3].These trends underscore the persisting challenges caused by cancer.Hence,it is imperative to explore new targets for early diagnosis and personalized treatment,as emphasized by previous studies[4,5].The identification and analysis of novel pan-cancer genes can provide valuable insights into the intricate process of tumorigenesis.Public databases and online analysis tools,such as The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) provide convenient access to comprehensive cancerrelated functional genomics datasets across diverse cancer types,enabling in-depth pan-cancer analysis[6,7].
The COP9 signalosome (COPS) is a multiprotein complex involved in protein degradation,transcriptional activation,signal transduction,and tumor progression[8,9].COPS6,together with its dimerization partnerCOPS5,plays a crucial role in the activation process of deneddylase activity by embedding into the core of the helical bundle[10].While the literature has reported the mechanisms ofCOPS6in human malignancies,such as cervical cancer,papillary thyroid carcinoma (THCA),CRC,breast cancer,lung adenocarcinoma (LUAD),and glioblastoma,the available information is incomplete and the underlying mechanisms remain to be fully elucidated[11].
The present study aimed to comprehensively elucidate the involvement and clinical implications ofCOPS6in many diverse types of cancer.This involved an in-depth analysis of differential expression patterns,prognostic values,gene mutations,immune infiltration,correlation analysis,and functional enrichment assessment,utilizing publicly available databases.
MATERIALS AND METHODS
Differential expression analysis
GEPIA2 (http://gepia2.cancer-pku.cn/#degenes) was utilized for analysis of RNA-seq expression data collected from TCGA and Genotype-Tissue Expression (GTEx) projects,enabling differential analysis,correlation analysis,and survival analysis[12].Genetic difference analysis was conducted using TIMER2 (http://timer.cistrome.org/)[13].COPS6expression level in tumor and normal samples was compared using R (ver.4.0.3),TIMER2,and GEPIA2.Box plots were generated using the ggpubr R (ver.4.0.3) package,and differential expression ofCOPS6in TCGA samples was determined using the Wilcoxon test.For cancers lacking normal controls in the TCGA database,the TIMER2 website was employed for differential analysis of theCOPS6gene.
For protein analysis,UALCAN online portal (http://ualcan.path.uab.edu/analysis-prot.html) was utilized to examine gene,protein,methylation,and phosphorylation differences[14].UALCAN facilitated the comparison of differential expression ofCOPS6protein between tumor and normal tissues.Age-differential expression data was obtained using the limma and ggpubr R packages,while clinical stage differential expression ofCOPS6was obtained from the GEPIA2 website.
Survival analysis
To perform survival analysis forCOPS6,the "Survival Map" feature of GEPIA2 was utilized.This facilitated plotting heatmaps representing overall survival (OS) and disease-free survival (DFS) using data from TCGA database.Forest plots,encompassing OS,progression-free interval (PFI),disease-specific survival (DSS),and disease-free interval (DFI),were generated using the survival and forestplot R packages in association with Cox analysis.
In March 2022,the pan-cancer data were downloaded from the TCGA database,including tumor stage,tumor grade,survival time,and mutation information.The raw data were preprocessed by the R programming language.The survminer R package was utilized to generate Kaplan-Meier survival curves for OS,PFI,DSS,and DFI,with a significance level set atP<0.05.
Genetic alteration analysis
For mutation analysis,cBioPortal (https://www.cbioportal.org/) was utilized[15].In this study,the "Quick Search"feature of cBioPortal was employed to examine the mutation frequency,type,copy number alteration (CNA),and structural variants of TCGA tumors involvingCOPS6.Furthermore,information related to the specific mutation sites and three-dimensional (3D) structure of theCOPS6protein was collected.To assess the impact ofCOPS6alterations on patient survival,"TCGA,PanCancer Atlas" and "Compassion/Survival" modes were utilized to plot OS,DSS,DFS,and progression-free survival curves for TCGA cases with and withoutCOPS6alterations,respectively,using the log-rank test.
Immune infiltration analysis
To investigate immune infiltration,TIMER2 web server was used.In the present study,the presence of CD8+T cells,cancer-associated fibroblasts,natural killer (NK) cells,and macrophages was assessed using the "Immune" module.To explore the relationship between immune inflammatory cells andCOPS6expression,multiple algorithms were utilized,including XCELL,EPIC,TIMER,MCPCOUNTER,CIBERSORT-ABS,TIDE,CIBERSORT,and QUANTISEQ.Pvalues and partial correlation values were obtained using the purity-adjusted Spearman's rank correlation test to quantify the strength and significance of the observed correlations.
Enrichment analysis and correlation analysis
The "Similar Gene Detection" module on the GEPIA2 website was utilized to identify the top 100 genes correlated withCOPS6.Further analysis using the "correlation analysis" module on the same website narrowed down the selection to the top 5 genes with the highest correlation coefficients.Heatmap analysis of these genes was conducted using the"Gene_Corr" module available on the TIMER2 website.The purity-adjusted Spearman's rank correlation test was applied to obtain P-values and partial correlation values.
For protein-protein interaction (PPI) analysis,the STRING database (https://string-db.org/) was employed[16].COPS6was submitted to the database to generate a PPI network withHomo sapiensas the reference organism.The network settings included a full network type,evidence-based network edges,experiments as active interaction sources,a minimum required interaction score of high confidence (0.7),and a maximum number of interactors shown in the 1stand 2ndshells.
To identify overlapping genes between theCOPS6-related genes obtained from the GEPIA2 and STRING,a Venn diagram was generated using GraphPad Prism 9.0.0 software (GraphPad Software Inc.,San Diego,CA,United States).The gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed using the DAVID database (https://david.ncifcrf.gov/home.jsp) with involvement of parameters,such as"OFFICIAL_GENE_SYMBOL","Homo sapiens",and "functional annotation chart".
RESULTS
COPS6 expression level varied in various tumors
The overview of the pan-cancer analysis workflow is shown in Figure 1A.Differential expression analysis ofCOPS6was conducted on TCGA data using R programming language.Significant differential expression (P<0.05) ofCOPS6was found between normal and tumor tissues in several cancer types,including bladder cancer (BLCA),breast invasive carcinoma (BRCA),cholangiocarcinoma (CHOL),colon adenocarcinoma (COAD),esophageal carcinoma (ESCA),glioblastoma multiforme (GBM),head and neck squamous cell carcinoma (HNSC),kidney chromophobe (KICH),kidney renal clear cell carcinoma (KIRC),kidney renal papillary cell carcinoma (KIRP),liver hepatocellular carcinoma (LIHC),LUAD,lung squamous cell carcinoma (LUSC),prostate adenocarcinoma (PRAD),rectum adenocarcinoma (READ),THCA,and uterine corpus endometrial carcinoma (UCEC) (Figure 1B).Differential expression analysis results ofCOPS6in these tumors and normal samples were obtained from the GEPIA2 website.Furthermore,COPS6expression level exhibited significant differences in skin cutaneous melanoma (SKCM),pancreatic adenocarcinoma (PAAD),thymoma(THYM),diffuse large B-cell lymphoma (DLBCL),acute myeloid leukemia (LAML),and CHOL (Figure 1C) compared with TCGA normal samples using GTEx data.
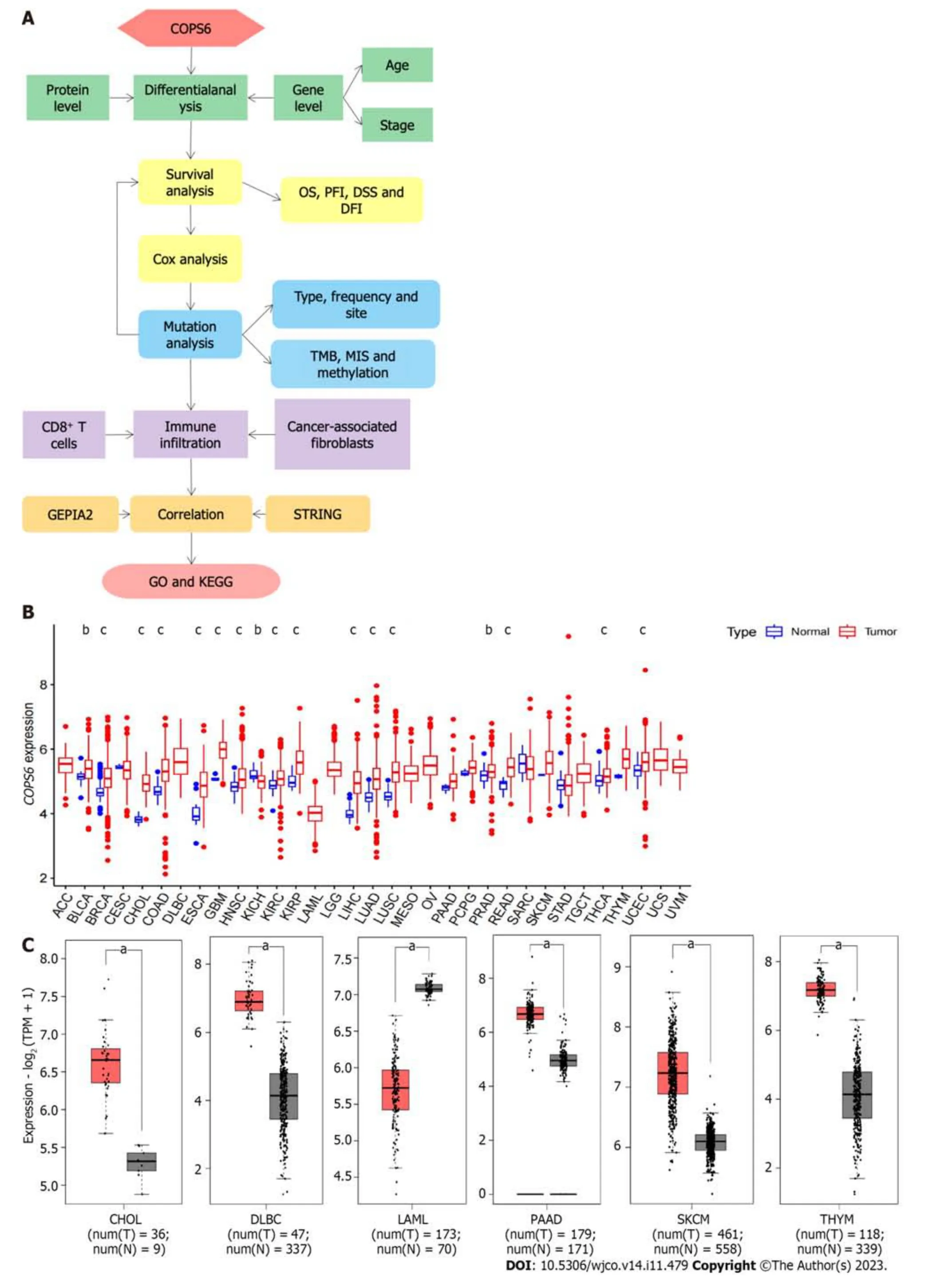
Figure 1 Differential expression analysis of COP9 signalosome subunit 6 in pan-cancer. A: Overview of the pan-cancer analysis workflow;B: Differential expression analysis of COP9 signalosome subunit 6 (COPS6) in the The Cancer Genome Atlas (TCGA) database using the Wilcoxon test in R software;C: Differential expression analysis of COPS6 in matched TCGA normal and Genotype-Tissue Expression data using the GEPIA2 website with specific cutoff criteria.b P : 0.01: cP :0.001.CHOL: Cholangiocarcinoma;DFI: Disease-free interval;DLBC: Diffuse large B-cell;DSS: Disease-specific survival;GO: Gene ontology;KEGG:Kyoto Encyclopedia of Genes and Genomes;LAML: Acute myeloid leukemia;MIS: Microsatellite instability;OS: Overall survival;PAAD: Pancreatic adenocarcinoma;PFI: Progression-free interval;SKCM: Skin cutaneous melanoma;THYM: Thymoma;TMB: Tumor mutational burden.
To compare protein expression level ofCOPS6among multiple types of cancer,protein expression differences between tumor and normal tissues from the CPTAC database on the UALCAN website were compared.The results revealed the elevated expression level ofCOPS6in hepatocellular carcinoma and clear cell renal cell carcinoma tissues (Figure 2A).Additionally,the relationship betweenCOPS6expression level and clinical parameters was investigated using the GEPIA2 website,indicating the presence of association betweenCOPS6expression level and clinical stages of LUAD,KICH,KIRP,and LIHC (P<0.05) (Figure 2B).Furthermore,significantly upregulatedCOPS6expression level in ESCA,LUAD,and LUSC in cases who aged <65-years-old in TCGA database was found,whereasCOPS6expression level was reduced in KIRC (P<0.05) (Figure 2C).
COPS6 expression level was associated with the prognosis of patients with diverse types of cancer
To assess the relationship betweenCOPS6expression level and patient prognosis across various tumors,patients were divided into high and lowCOPS6expression groups based on the medianCOPS6expression level.Utilizing the GEPIA2 website,it was revealed that high expression level ofCOPS6was significantly associated with poor OS in GBM (P=0.017),KICH (P=0.031),mesothelioma (MESO) (P=0.0026),lower grade glioma (LGG) (P=0.007),LIHC (P=0.011),and LUAD (P=0.018) (Figure 3A).Conversely,low expression level ofCOPS6was correlated with poor DFS in KIRP (P=0.042),while high expression level ofCOPS6was associated with poor DFS in LGG (P=0.0013),LIHC (P=0.024),adrenocortical carcinoma (ACC) (P=0.034),KIRC (P=0.009),MESO (P=0.0027),and stomach adenocarcinoma (STAD) (P=0.049) (Figure 3B).Cox regression analysis indicated thatCOPS6was a high-risk gene for OS in HNSC,KICH,KIRC,LGG,LIHC,and MESO (P<0.05),while it was appeared as a low-risk gene for OS in BRCA (P<0.05).Additionally,COPS6was identified as a high-risk gene for DSS in KICH,KIRC,LGG,MESO,and READ (P<0.05),as well as a low-risk gene for DSS in BRCA (P<0.05).Moreover,COPS6was found as a high-risk gene for DFI in ACC,LGG,LIHC,and STAD(P<0.001),as well as a high-risk gene for PFI in KICH,KIRC,LGG,MESO,and STAD (P<0.05),while a low-risk gene for PFI in BRCA (P<0.05).These findings were derived from TCGA database using the survival and forestplot R package(Figure 3C).The association betweenCOPS6expression level and OS (Figure 4A),PFI (Figure 4B),DSS (Figure 4C),and DFI (Figure 4D) was further confirmed through Kaplan-Meier survival analysis in pan-cancer patients from TCGA database.
Correlation between COPS6 mutation and tumor progression
Using the cBioPortal website,comprehensive information was obtained regarding the mutation types,frequency,CNAs,and structural variants ofCOPS6across all TCGA tumors.Missense mutations were identified as the predominant mutation type.Among all TCGA tumors,the highest frequency of variations was found in esophageal adenocarcinoma(9.89%),with amplification being the most frequent alteration (9.34%) (Figure 5A).A 3D representation of theCOPS6protein (Figure 5B) was constructed,revealing a notable mutation site,R197C/H,observed in one case each of adrenocortical carcinoma and endometrioid carcinoma (Figure 5C).Investigation of the relationship betweenCOPS6mutations and prognosis in TCGA cases revealed no significant impact of mutation status on the prognosis of all types of cancer(Figure 5D).
Furthermore,the correlations betweenCOPS6expression level and tumor mutational burden (TMB) and microsatellite instability (MSI) were analyzed.Positive correlations were identified betweenCOPS6expression level and TMB in LUAD,KIRP,LUSC,HNSC,PAAD,KICH,LIHC,KIRC,UCEC,LGG,BRCA,and PRAD (P<0.05),while negative correlations were found in THYM,COAD,ESCA,and LAML (P<0.05) (Figure 5E).Positive associations betweenCOPS6expression level and MSI were observed in BRCA,USC,THCA,SKCM,SARC,PRAD,PAAD,KIRP,KIRC,HNSC,DLBC,and LIHC (P<0.05),with a positive association observed in COAD (P<0.05) (Figure 5F).Additionally,comparison of theCOPS6promoter methylation level between normal and tumor samples revealed a higher methylation level in the tumor group in PRAD,LUSC,HNSC,BRCA,and KIRC,whereas a lower methylation level in BLCA (Figure 5G).
Correlation between COPS6 expression level and immune infiltration
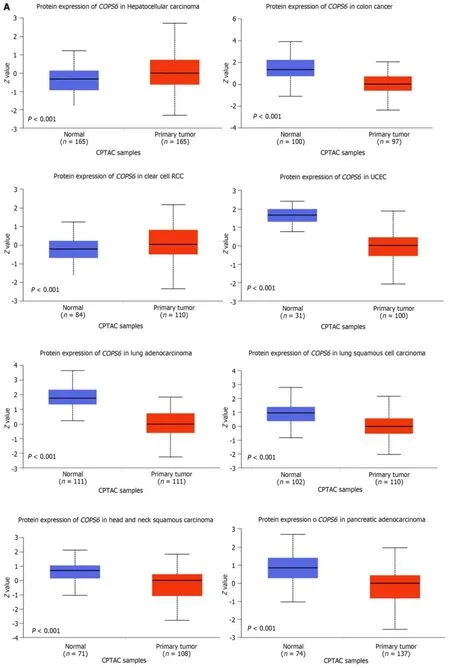
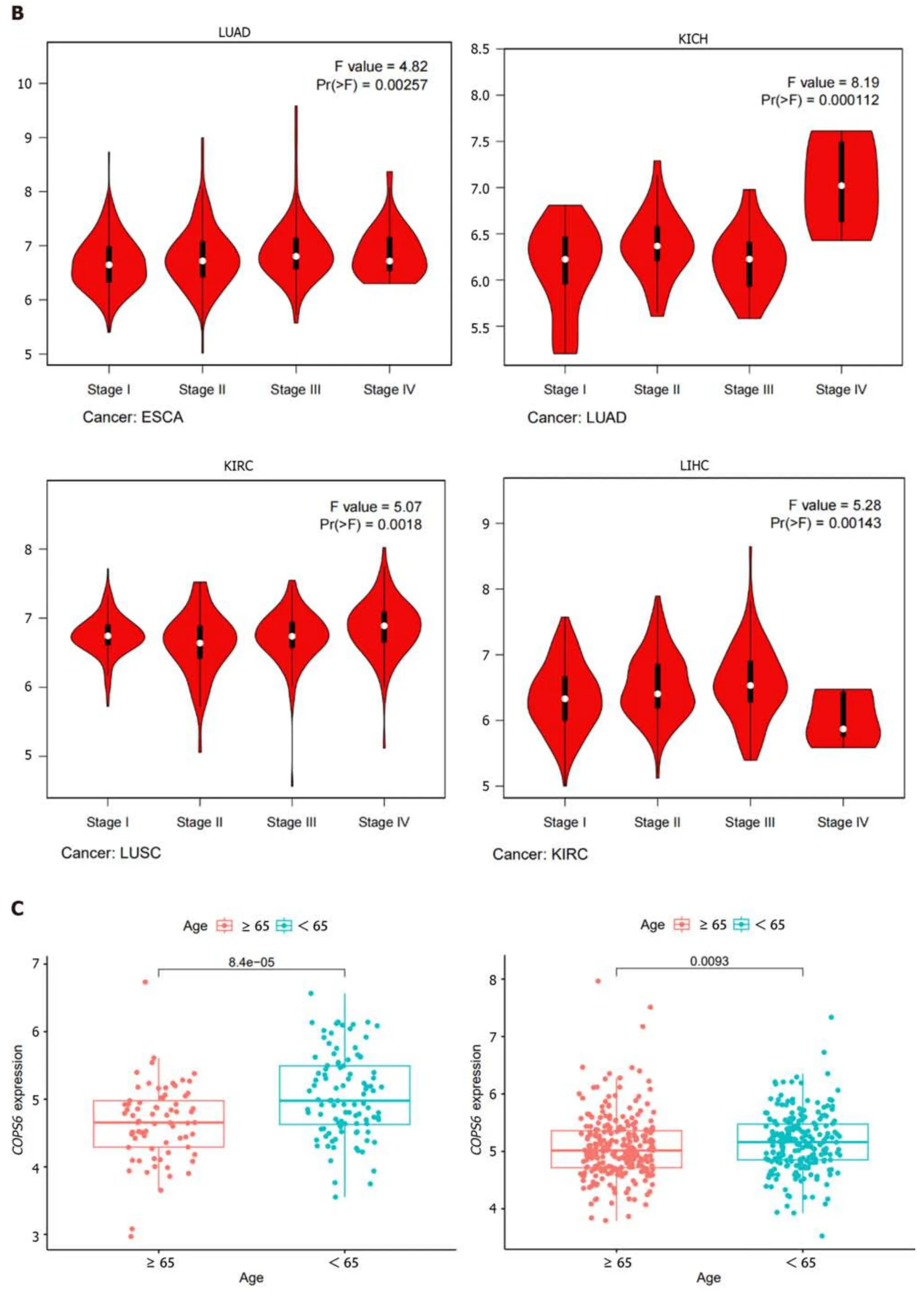
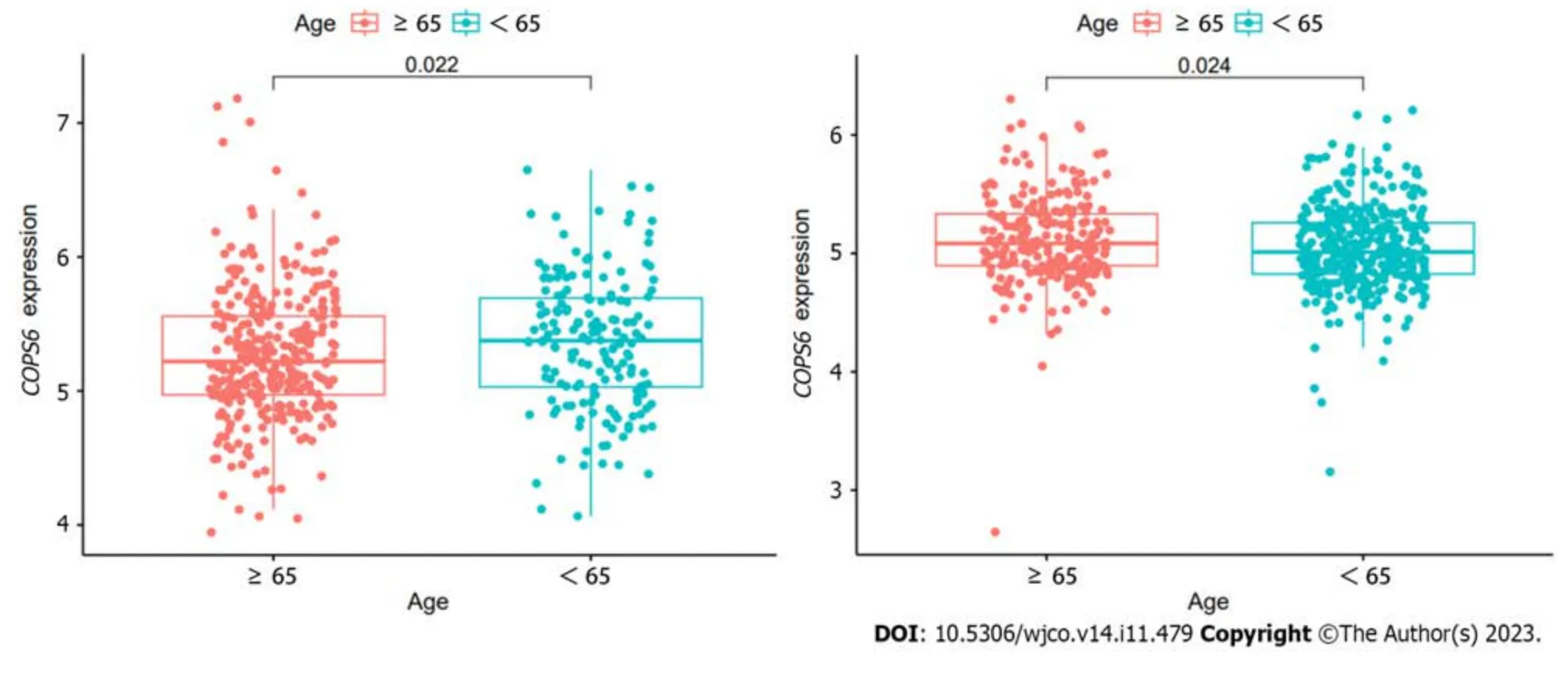
Figure 2 Association between COP9 signalosome subunit 6 and clinical parameters in pan-cancer. A: Differential analysis of COP9 signalosome subunit 6 (COPS6) protein expression in pan-cancers using the CPTAC database accessed through the UALCAN website;B: Relationship between COPS6 expression and clinical stage analyzed with the GEPIA2 website;C: Correlation between COPS6 expression and age using R software.KICH: Kidney chromophobe;KIRC: Kidney renal clear cell carcinoma;LIHC: Liver hepatocellular carcinoma;LUAD: Lung adenocarcinoma.
The relationship betweenCOPS6expression level and immune-infiltrating cells in TCGA tumors was examined using the TIMER2 website.Across multiple algorithms,a negative correlation was found betweenCOPS6expression level and CD8+T cell infiltration in BRCA-LumA,HNSC,HNSC-HPV-,SKCM,SKCM-metastasis,and tenosynovial giant cell tumor (TGCT) (Figure 6A and B).Conversely,the correlation between cancer-associated fibroblast infiltration andCOPS6expression level exhibited heterogeneity.Negative correlations were identified in DLBCL,OV,SARC,THYM,and THCA,while positive correlations were found in HNSC,HNSC-HPV-,and TGCT (Figure 6C and D).The relationship betweenCOPS6expression level and macrophage infiltration varied depending on macrophage subtype.An inverse association was detected betweenCOPS6expression level and M1 macrophages,along with a positive association betweenCOPS6expression level and M2 macrophages in certain tumors (Figure 6E and F).For instance,in DLBCL,four algorithms demonstrated a negative association betweenCOPS6expression level and M1 macrophages,while TIDE algorithm revealed a positive association betweenCOPS6expression level and M2 macrophages.Moreover,in BRCA,KIRC,THCA,and THYM,the TIDE algorithm indicated a positive correlation betweenCOPS6expression level and M2 macrophages.In most of the tumors,NK cell infiltration exhibited a weak correlation withCOPS6expression level,and clear associations were found only in a few tumors (Figure 6G and H).For instance,in THCA and THYM,COPS6expression level showed a negative correlation with NK cell infiltration.Further analysis of NK cell subtypes revealed a negative correlation betweenCOPS6expression level and activated NK cell infiltration,as well as a positive correlation betweenCOPS6expression level and resting NK cell infiltration.
Enrichment analysis and correlation analysis of COPS6-associated genes
To gain deeper insights into the molecular mechanisms involvingCOPS6in growth and progression of tumors,the GEPIA2 website was employed to screen the top 100COPS6-associated genes.Subsequently,the top 5 genes with the highest correlation coefficients were identified and summarized as follows: POLR2J (r=0.69,P<0.001),BUD31 (r=0.65,P<0.001),TAF6 (r=0.66,P<0.001),ALKBH4 (r=0.62,P<0.001),and POP7 (r=0.61,P<0.001) (Figure 7A and B).The PPI network analysis was performed using the STRING website,resulting in the establishment of a network of 35 node genes (Figure 7C).The intersection ofCOPS6-associated genes obtained from the GEPIA2 and STRING led to the identification ofGPS1andTCEB2(Figure 7D).Furthermore,the genes derived from both databases were merged,resulting in the detection of a total of 135COPS6-related genes.Subsequently,GO and KEGG pathway enrichment analyses were conducted (Figure 7E and F).The KEGG pathway analysis revealed thatCOPS6-associated genes were enriched in pathways,such as ubiquitin-mediated proteolysis,nucleotide excision repair,human immunodeficiency virus 1 infection,Parkinson's disease,and circadian rhythm.The GO enrichment analysis indicated enrichment in the proteasomal protein catabolic process,proteasome-mediated ubiquitin-dependent protein catabolic process,protein modification by small protein removal,intrinsic apoptotic signaling pathway,protein deneddylation,COPS,Cullin-RING ubiquitin ligase(CRL) complex,SCF ubiquitin ligase complex,Cul4A-RING E3 ubiquitin ligase complex,Cullin family protein binding,ubiquitin-protein transferase activity,and ubiquitin-like protein transferase activity.
DISCUSSION
The COP9 signalosome (CSN) is a complex protein composed of eight subunits (CSN1-CSN8),participating in various physiological processes.The CSN1,2,3,4,7,and 8 subunits contain a percutaneous coronary intervention domain,which acts as a scaffold in CSN assembly,while theCOPS6andCOPS5subunits possess an Mpr1-Pad 1-N-terminal (MPN)domain[17].COPS5primarily exerts catalytic enzymatic activity,whereasCOPS6,as an essential component of CSN,lacks the metal-binding site and isopeptidase activity associated with theCOPS5MPN domain.The precise function ofCOPS6remains has still remained elusive[18].COPS6is involved in various processes,including the ubiquitin proteasome system,signal transduction,DNA damage response,and tumor progression.It exhibits a high expression level in diverse tumors,and studies have explored its role in cancer[19].
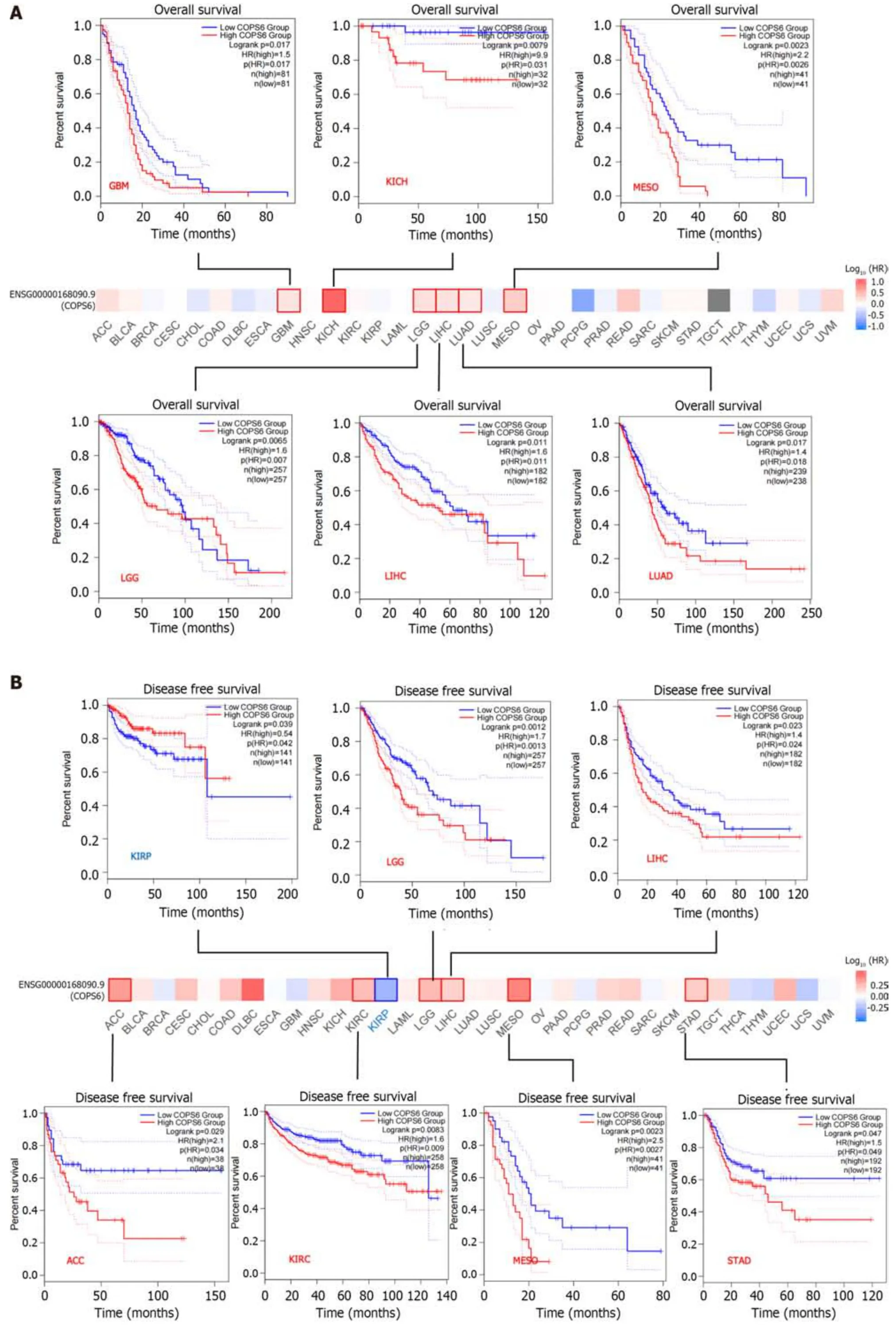
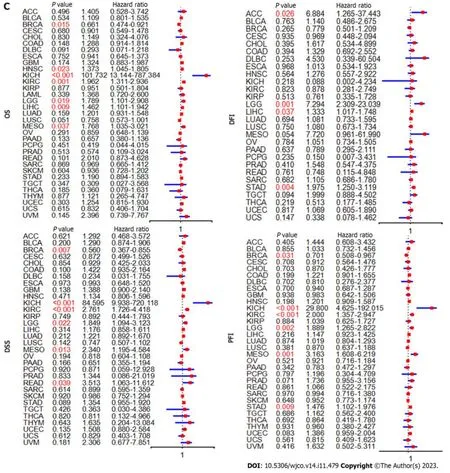
Figure 3 Survival analysis of COP9 signalosome subunit 6. A: Overall survival (OS) and disease-free survival analysis conducted with the GEPIA2 website using the log-rank test (P <0.05);B: Forest plots illustrating disease-specific survival (DSS),OS,progression-free interval (PFI),and disease-free interval(DFI) analyzed with Cox analysis in R software.ACC: Adrenocortical carcinoma;GBM: Glioblastoma multiforme;KICH: Kidney chromophobe;KIRP: Kidney renal papillary cell carcinoma;LGG: Lower grade glioma;LIHC: Liver hepatocellular carcinoma;LUAD: Lung adenocarcinoma;MESO: Mesothelioma;STAD: Stomach adenocarcinoma.
At present,there is a growing interest among researchers in investigating the role ofCOPS6in tumors,as it has been shown to predominantly promote cancer.The CRLs are involved in the ubiquitination of Myc,and Fbxw7,a CRL component,which participates in Myc ubiquitination.In mouse experiments,Chenet al[20] demonstrated thatCOPS6enhances Fbxw7 degradation through binding,thereby maintaining Myc stability and promoting tumor progression.Additionally,in a mouse model,Zhaoet al[21] revealed thatCOPS6attenuates p53-mediated tumor suppression,promotes tumor growth by stabilizing MDM2 protein,and participates in DNA damage-associated apoptosis.In human tumors,COPS6also plays a significant role in tumor progression.Fanget al[22] demonstrated thatCOPS6overexpression in CRC is associated with a worse prognosis.Mechanistic studies suggested that ERK2 directly binds to CSN6 Leu163/Val165 and phosphorylatesCOPS6at Ser148,thereby regulating β-Trcp and stabilizing β-catenin expression,consequently blocking the ubiquitin-proteasome pathway and promoting CRC development.
Programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) checkpoint blockade is an emerging immunotherapy modality in various tumors,while its regulatory mechanism remains uncertain.Suet al[23] demonstrated thatCOPS6expression level could be regulated by the EGFR-ERK pathway,inhibiting PD-L1 degradation and maintaining PD-L1 stability in GBM.Additionally,several studies have reported the involvement ofCOPS6in the epithelial-mesenchymal transition process in various tumors,promoting tumor invasion and metastasis.For instance,Zhanget al[24] revealed that the COPS6-UBR5-CDK9 axis could control melanoma proliferation and metastasis,while Maoet al[25] found thatCOPS6could promote migration and invasion of cervical cancer cells by regulating the expression level of cathepsin L through the autophagy-lysosomal system.Furthermore,COPS6was found to maintain the key transcription factor Snail1,promoting the invasion of breast cancer cells by inhibiting Snail1 ubiquitination[26].
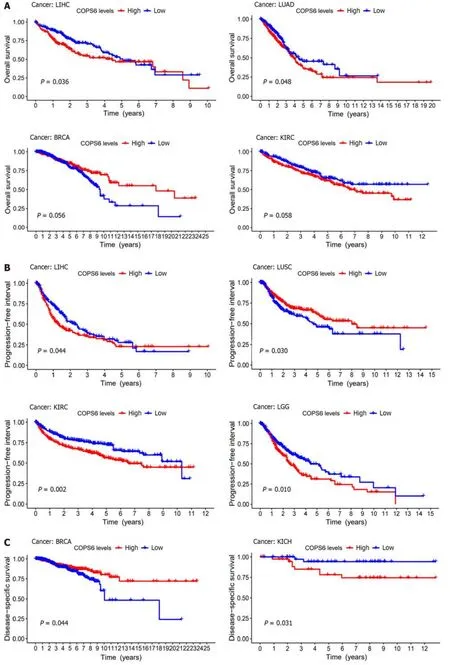
While previous studies have highlighted the significant role ofCOPS6in the progression of specific tumors,the heterogeneity of tumors suggests potential variations in its function across different cancer types.Therefore,a comprehensive analysis and screening are necessary to validate existing findings and provide direction for the futureCOPS6-related studies.In the present study,it was attempted to conduct comprehensive multilevel differential analysis and survival analysis ofCOPS6in pan-cancer data collected from various public databases and online analysis tools,including TCGA,GEO,CPTAC,GEPIA2,TIMER2,and UALCAN.The findings demonstrated that the expression level of theCOPS6gene was significantly upregulated in the most types of cancer compared with normal tissues,except for KICH and LAML.Prognostic analysis revealed that the high expression level ofCOPS6was typically associated with worse prognosis in the majority of tumors,while showing a favorable prognosis in KIRP,BRCA,LUSC,and PCPG.Mutational analysis indicated that missense mutations were the predominant mutation type found inCOPS6.Additionally,TMB and MSI exhibited a positive correlation withCOPS6expression level in most of the tumors,and only few tumors showed a negative correlation.Further exploration of the impact ofCOPS6mutations on patient outcomes revealed that these mutations did not significantly contribute to a worse prognosis in any specific tumor types.However,a comprehensive analysis across all tumors indicated a trend towards a shorter OS associated withCOPS6mutations.Therefore,it can be concluded thatCOPS6mutations have a limited effect on patient prognosis.
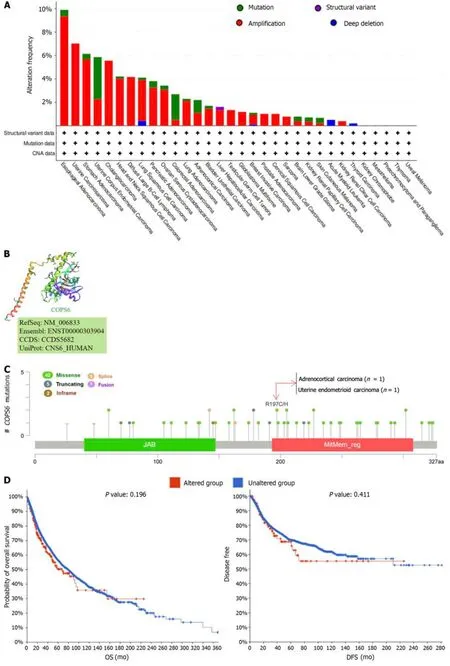

Figure 5 Mutation analysis of COP9 signalosome subunit 6. A: Mutation frequency and types visualized through the cBioPortal website;B: Threedimensional structure highlighting the R197C/H mutation site in COP9 signalosome subunit 6 (COPS6);C: Mutation sites depicted in the cBioPortal website;D:Survival analysis of COPS6 mutations in pan-cancer;E: Correlation of COPS6 with tumor mutational burden (tumor mutational burden) in pan-cancer using R software;F: Correlation of COPS6 with microsatellite instability (microsatellite instability) in pan-cancer using R software;G: Promoter methylation levels of COPS6 in prostate adenocarcinoma (PRAD),lung squamous cell carcinoma (LUSC),head and neck squamous cell carcinoma (HNSC),breast invasive carcinoma (BRCA),bladder cancer (BLCA),and kidney renal clear cell carcinoma (KIRC) accessed through the UALCAN website.OS: Overall survival;DFS: Disease-free survival;PFS:Progression-free survival;DSS: Disease-specific survival.
Subsequently,the association betweenCOPS6expression level and the tumor immune microenvironment (TMIE) was investigated in various types of cancer.The TMIE plays a pivotal role in tumor progression,immune evasion,and therapeutic resistance,involving key components,such as CD8+T cells,cancer-associated fibroblasts,macrophages,and NK cells[27].The findings of the present study demonstrated a negative correlation betweenCOPS6expression level and CD8+T cell infiltration in several tumors,such as BRCA,HNSC,and TGCT.This aligns with Duet al[28]’s results,demonstrating thatCOPS6could inhibit CD8+T cell infiltration within the tumor microenvironment (TME),thereby facilitating tumor immune evasion.Furthermore,a negative correlation was identified betweenCOPS6expression level and the M1 phenotype of tumor-associated macrophages (TAMs),while a positive correlation was found with the M2 phenotype.TAMs,which are macrophages that infiltrate tumor tissue and differentiate from monocytes,predominantly adopt the immunosuppressive M2 phenotype in the TMIE[29].The present study revealed a positive correlation betweenCOPS6expression level and the M2 phenotype in the TIDE algorithm for DLBCL,BRCA,KIRC,THCA,THYM,and other tumors,while other algorithms exhibited a negative correlation with the M1 phenotype.However,it is noteworthy that in some tumors,only the TIDE algorithm yielded consistent results,while other algorithms suggested a negative or no correlation betweenCOPS6expression level and the M2 phenotype.This discrepancy could be attributed to variations in the statistical methods employed by each algorithm,necessitating further experimental validation of these findings.
There is a scarcity of research regarding the interaction betweenCOPS6Level and TME,highlighting the urgent need to explore the role ofCOPS6in the TME.Furthermore,in the present study,correlation and enrichment analyses ofCOPS6were conducted,andGPS1andTCEB2were identified as the two genes,exhibiting the strongest correlation.This investigation sheds light on the potential function and significance ofCOPS6as a novel biomarker in cancer,setting the stage for further research on its molecular mechanisms and the development of targeted therapies.Moreover,the findings emphasize the importance of studying theCOPS6-related TIME.However,it should be noted that the current study ofCOPS6is preliminary,and the specific mechanisms of its action in different types of cancer remain elusive.Therefore,additional resources and efforts are warranted to delve deeper into the role ofCOPS6in cancer.
The present study revealed a potential association ofCOPS6with survival outcomes in various tumors.Notably,GPS1andTCEB2were identified as the two genes exhibiting the strongest correlation withCOPS6at both the gene and protein levels,making them promising targets for future investigations.Additionally,a significant association was found betweenCOPS6expression level and immune infiltration in diverse types of cancer,such as BRCA,HNSC,and TGCT,where research on the TIME remains limited.
CONCLUSION
This study is the first to explore the role ofCOPS6in pan-cancer,taking full use of the existing public database to investigateCOPS6from the aspects of gene expression level,mutation,TIME,and prognosis.However,there are also some deficiencies in this study.For instance,only a multifaceted analysis ofCOPS6was conducted through bioinformatics,while no experiment was carried out to verify the results,hindering the generalization of the findings.
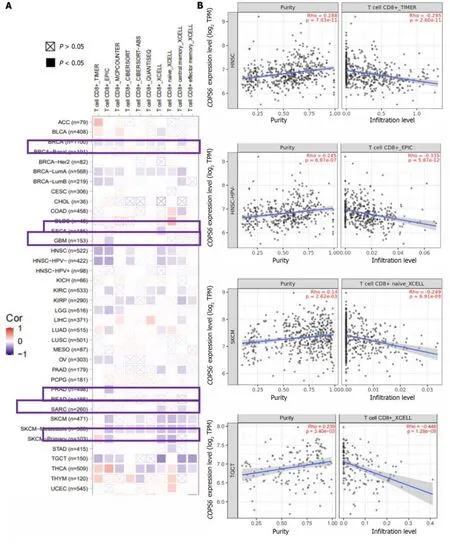
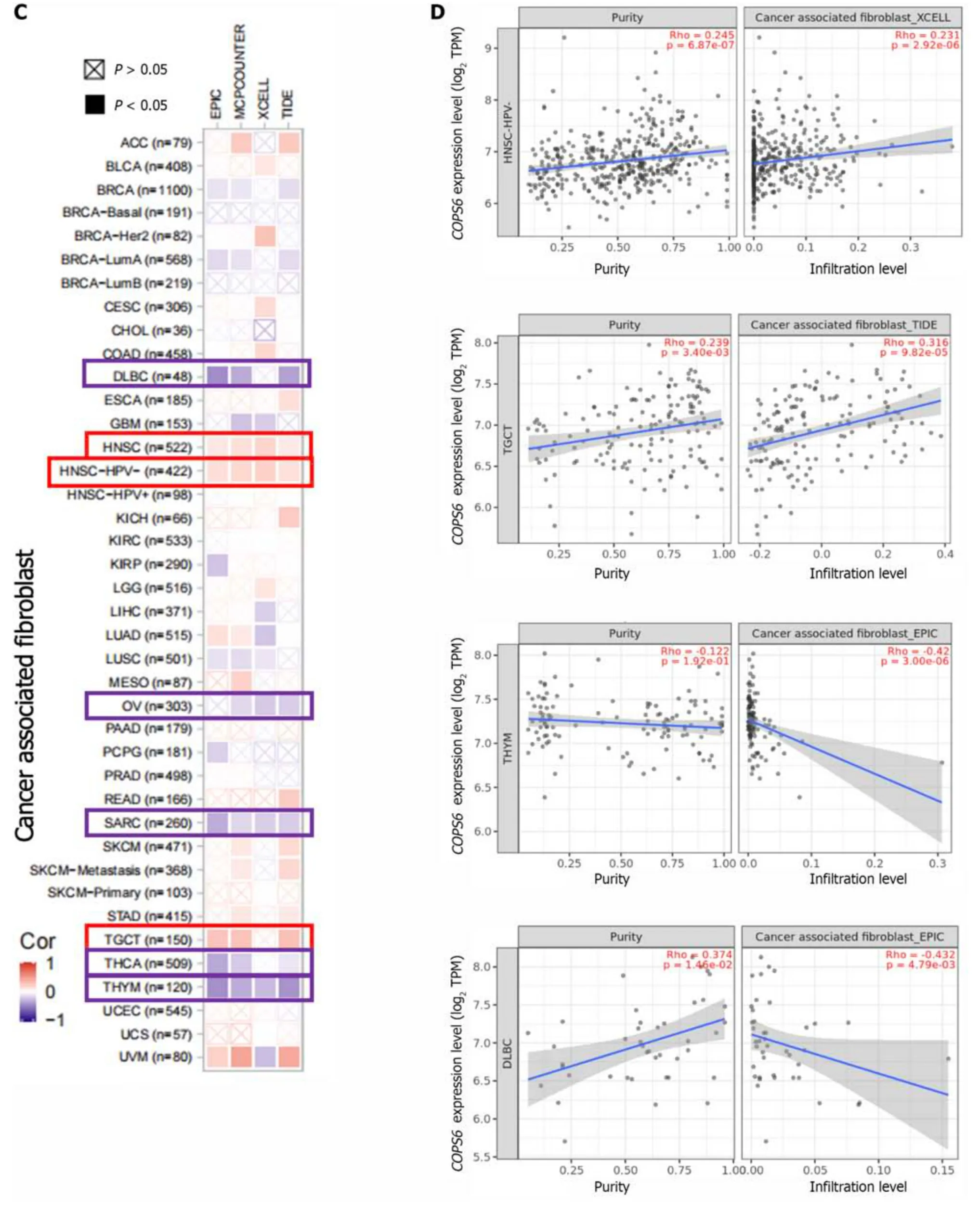
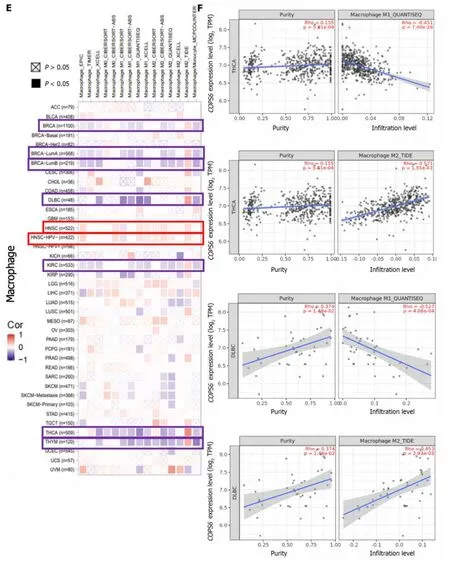
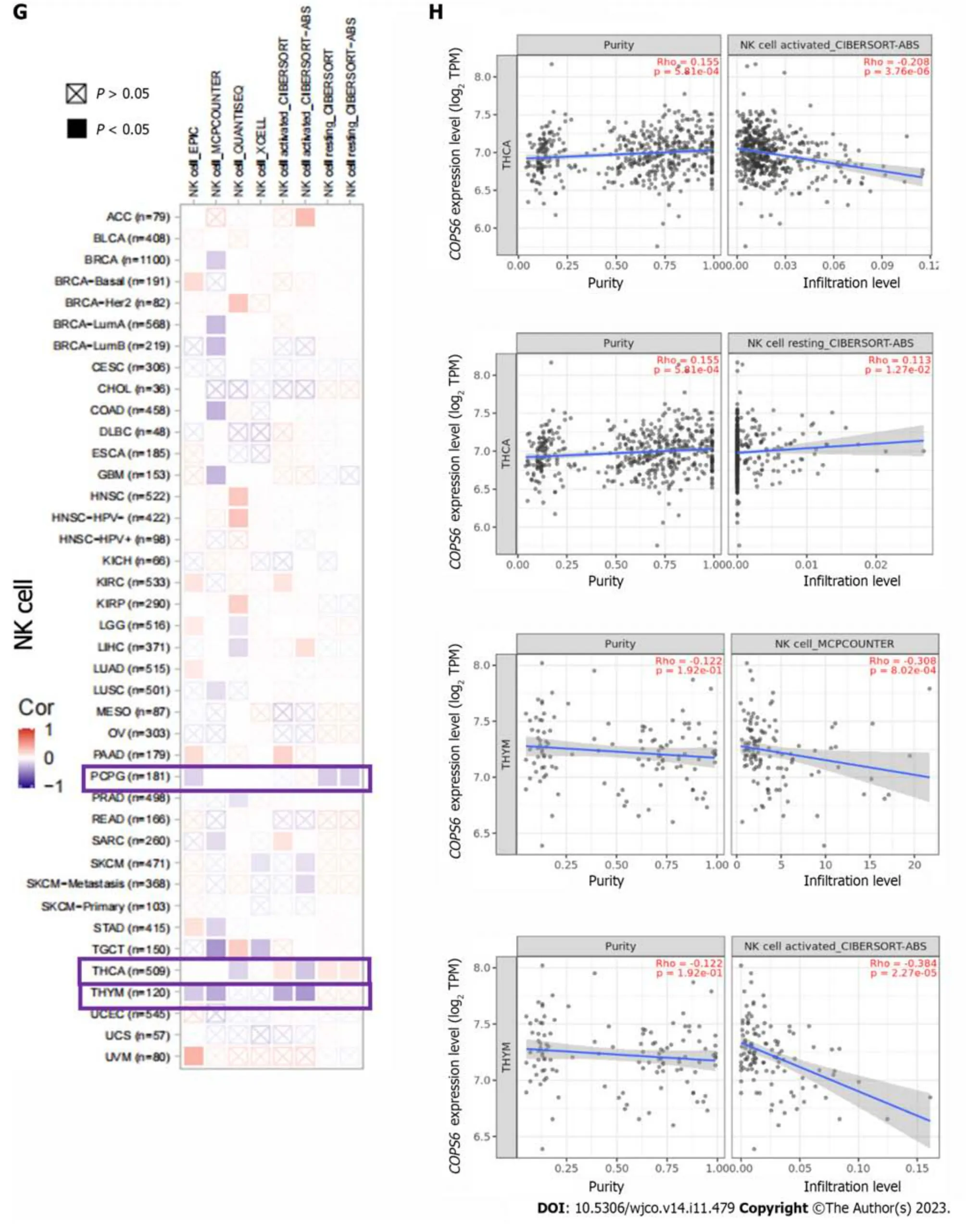
Figure 6 Immune infiltration analysis of COP9 signalosome subunit 6 in the The Cancer Genome Atlas database using the TIMER2 website. A: Heatmap depicting the correlation between COP9 signalosome subunit 6 (COPS6) and CD8+T cells;B: Scatter plot illustrating the relationship between COPS6 and CD8+T cells;C: Heatmap displaying the correlation between COPS6 and cancer-associated fibroblasts;D: Scatter plot demonstrating the relationship between COPS6 and cancer-associated fibroblasts;E: Heatmap indicating the correlation between COPS6 and macrophages;F: Scatter plot showing the relationship between COPS6 and macrophages;G: Heatmap presenting the correlation between COPS6 and natural killer (NK) cells;H: Scatter plot depicting the relationship between COPS6 and NK cells.TPM: Transcripts per million.
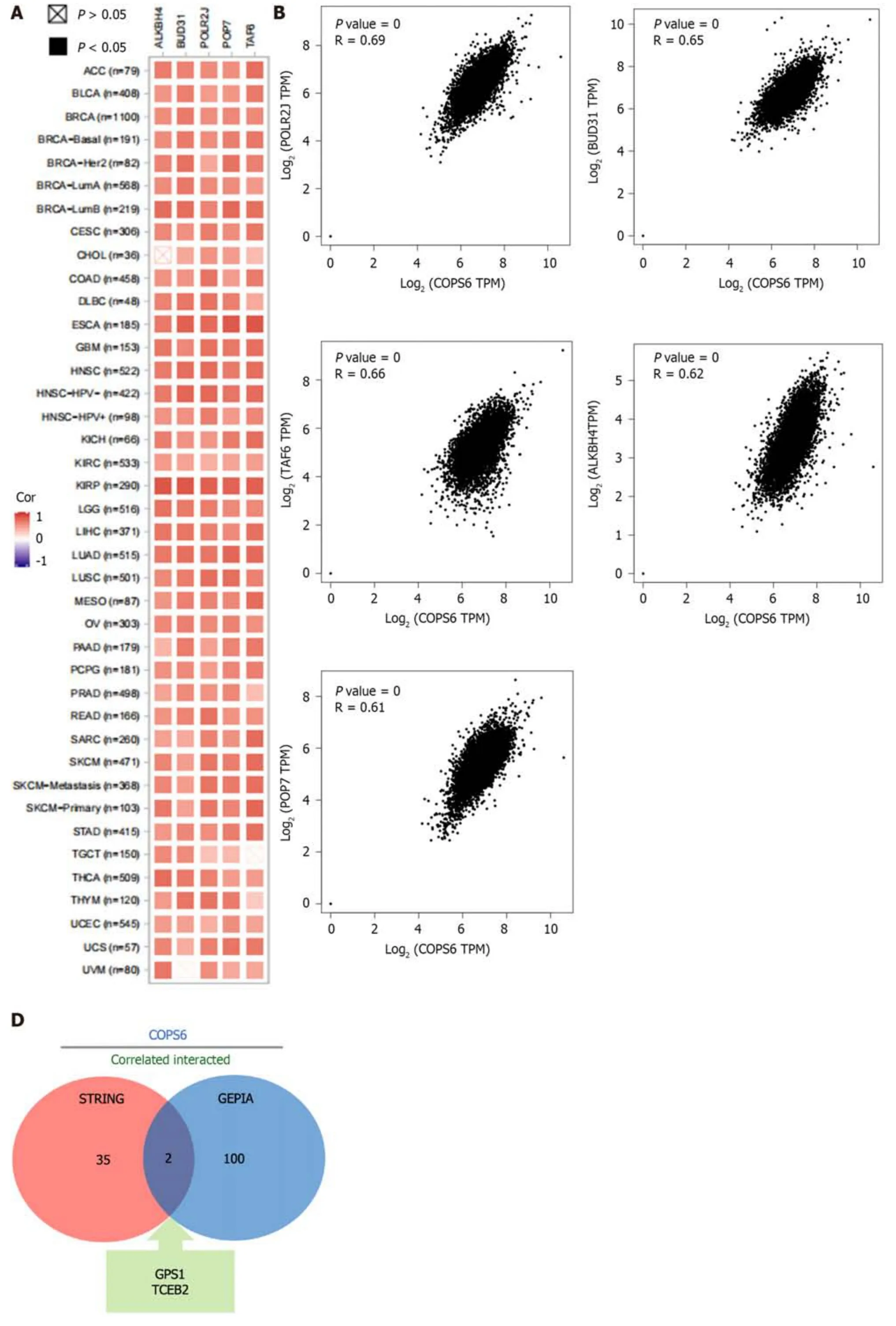
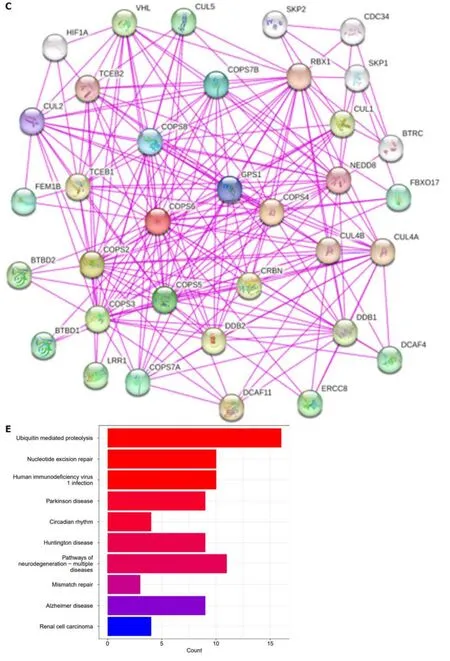
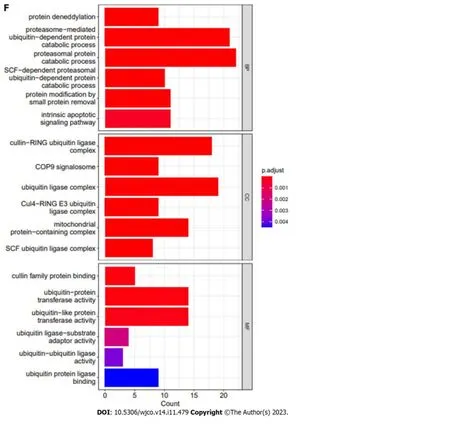
Figure 7 Correlation analysis and enrichment analysis of COPS6. A: Heatmap displaying the top 5 genes correlated with COPS6 in pan-cancer accessed through the TIMER2 website;B: Scatter plot illustrating the correlation between COPS6 and the top 5 genes in pan-cancer using the GEPIA2 website;C:Protein-protein interaction network of COPS6 obtained from the STRING database;D: Intersection of COPS6-related genes screened in GEPIA2 and STRING,resulting in GPS1 and TCEB2;E: Kyoto Encyclopedia of Genes and Genomes enrichment analysis of the combined COPS6-related genes from GEPIA2 and STRING;F: Gene ontology enrichment analysis of the combined COPS6-related genes from GEPIA2 and STRING.TPM: Transcripts per million.
In conclusion,the present study provided early evidence thatCOPS6could be associated with clinicopathological characteristics in various tumors and could play a role in several cancer hallmarks.Additional research is needed to further elucidate the role ofCOPS6in cancer progression.
ARTICLE HIGHLIGHTS
Research background
The COP9 signaling body subunit 6 (COPS6) has been implicated in cancer progression,but its precise role in most types of cancer is unknown.
Research motivation
This study aimed to investigate the functional and clinical relevance ofCOPS6in different tumor types,using publicly available databases.
Research objectives
This study hopes to provide a basis forCOPS6as a novel biomarker for cancer research by exploring the role ofCOPS6in different cancer types.
Research methods
We used R software and online analysis databases to analyze the differential expression,prognosis,mutation and related functions ofCOPS6in pan-cancer.
Research results
Differential expression analysis and survival analysis demonstrated thatCOPS6was highly expressed and associated with high-risk profiles in the majority of cancer types.Missense mutations are the main type ofCOPS6mutations,and in most types of cancer,the levels ofCOPS6expression are positively correlated with tumor mutation burden and microsatellite instability.Immune infiltration analysis foundCOPS6to play different roles in different cancers.Gene coexpression and enrichment analysis highlightedCOPS6-related genes were predominantly involved in processes,such as ubiquitin-mediated proteolysis and human immunodeficiency virus 1 infection.
Research conclusions
This study provides early evidence thatCOPS6may be associated with the clinicopathological features of various tumors and may play a role in several cancer features,providing a basis for subsequent studies related toCOPS6.
Research perspectives
Since this study mainly focused on data analysis,subsequent studies required experimental validation of relevant results.
FOOTNOTES
Co-first authors:Shi-Lin Wang and Guang-Zheng Zhuo.
Co-corresponding authors:Yun-Bao Pan and Yi-Rong Li.
Author contributions:Pan YB designed the research;Wang SL and Zhuo GZ performed the research;Wang SL,Wang LP and Zhuo GZ contributed analytic tools;Wang SL and Zhuo GZ analyzed the data;Wang SL and Pan YB wrote the paper;Pan YB and Li YR were responsible for the supervision.Wang SL and Zhuo GZ contributed equally to this work as co-first authors.The reasons for designating Wang SL and Zhuo GZ as co-first authors are twofold.First,the research was performed as a collaborative effort,and the designation of co-corresponding authorship accurately reflects the distribution of responsibilities and burdens associated with the time and effort required to complete the study and the resultant paper.Second,Wang SL and Zhuo GZ contributed an equally substantial effort throughout the study.They are principal principals of paper writing and data analysis,selecting these researchers as co-first authors,recognizing and respecting this equal contribution.Pan YB and Li YR contributed equally to this work as co-corresponding authors.The reasons for designating Pan YB and Li YR as co-corresponding authors are twofold.First,the research was performed as a collaborative effort.This ensures effective communication and management of post-submission matters,ultimately enhancing the paper's quality and reliability.Second,the overall research team encompassed authors with a variety of expertise and skills from different fields,and the designation of co-corresponding authors best reflects this diversity.Pan YB and Li YR,as heads of both groups,contributed substantially to the experimental design,data analysis and revision,and were therefore listed as co-corresponding authors.
Supported byNational Natural Science Foundation of China,No.31 900558;the Hubei Provincial Youth Talents Program for Public Health,No.WSJKRC2 022013;Wuhan Young and Middle-Aged Medical Backbone Talents Training Project,No.WHQG201904.
Conflict-of-interest statement:The authors have no relevant financial or non-financial interests to disclose.
Data sharing statement:Technical appendix,statistical code,and dataset available from the corresponding author at [panyunbao@
outlook.com].Participants gave informed consent for data sharing.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Yun-Bao Pan 0000-0002-6311-2945; Yi-Rong Li 0000-0002-7493-045X.
S-Editor:Lin C
L-Editor:Filipodia
P-Editor:Zhang XD
 World Journal of Clinical Oncology2023年11期
World Journal of Clinical Oncology2023年11期
- World Journal of Clinical Oncology的其它文章
- Calcitriol induced hypercalcemia -a rare phenomenon in lung cancer: A case report
- Autoimmune diabetes from pembrolizumab: A case report and review of literature
- Bibliometric analysis of the global research status and trends of mechanotransduction in cancer
- Circulating tumor cells as potential prognostic biomarkers for earlystage pancreatic cancer: A systematic review and meta-analysis
- System describing surgical field extension associated with flap reconstruction after resection of a superficial malignant soft tissue tumor
- Clinical study of standard residual liver volume and transient elastography in predicting poor prognosis of patients after hemihepatectomy
