Effects of Groundwater with Various Salinities on Evaporation and Redistribution of Water and Salt in Saline-sodic Soils in Songnen Plain,Northeast China
ZHU Wendong ,ZHAO Dandan ,YANG Fan ,WANG ZhichunDONG Shide,AN Fenghua,MA HongyuanZHANG LuTIBOR Tóth
(1.Northeast Institute of Geography and Agroecology,Chinese Academy of Sciences,Changchun 130012,China;2.Institute of Geographic Sciences and Natural Resources Research,Chinese Academy of Sciences,Beijing 100101,China;3.University of Chinese Academy of Sciences,Beijing 100049,China;4.Binzhou University,Shandong Key Laboratory of Eco-environmental Science for Yellow Rriver Delta,Binzhou 256603,China;5.Research Institute for Soil Science and Agricultural Chemistry of the Hungarian Academy of Sciences (MTA TAKI-RISSAC),Budapest II. Herman O. út 15. 1025,Hungary)
Abstract: Groundwater mineralization is one of the main factors affecting the transport of soil water and salt in saline-sodic areas.To investigate the effects of groundwater with different levels of salinity on evaporation and distributions of soil water and salt in Songnen Plain,Northeast China,five levels of groundwater sodium adsorption ration of water (SARw) and total salt content (TSC mmol/L) were conducted in an oil column lysimeters.The five treated groundwater labeled as ST0∶0,ST0∶10,ST5∶40,ST10∶70 and ST20∶100,were prepared with NaCl and CaCl2 in proportion,respectively.The results showed the groundwater evaporation (GWE)and soil evaporation (SE) increased firstly and then decreased with the increase of groundwater salinity.The values of GWE and SE in ST10∶70 treatment were the highest,which were 2.09 and 1.84 times the values in the ST0∶0 treatment with the lowest GWE and SE.There was a positive linear correlation between GWE and the Ca2+ content in groundwater,with R2=0.998.The soil water content(SWC) of ST0∶0 treatment was significantly (P <0.05) less than those of other treatments during the test.The SWC of the ST0∶0 and ST0∶10 treatments increased with the increase of soil depth,while the other treatments showed the opposite trend.Statistical analysis indicated the SWC in the 0-60 cm soil layer was positively correlated with the groundwater TSC and its ion contents during the test.Salt accumulation occurred in the topsoil and the salt accumulation in the 0-20 cm soil layer was significantly (P <0.05) greater than that in the subsoil.This study revealed the effects of the salinity level of groundwater,especially the Ca2+ content and TSC of groundwater,on the GWE and distributions of soil water and salt,which provided important support for the prevention and reclamation of soil salinization and sodificaton in shallow groundwater regions.
Keywords: groundwater evaporation;sodium adsorption ratio;total salt content;ion composition;soil salinization;water and salt dynamics;Songnen Plain,China
1 Introduction
Soil salinity-sodicity is one of the environmental problems in arid and semi-arid regions worldwide.The mechanism of soil water and salt transport is the core of the environmental changes in saline-sodic soils.It is not only the theoretical basis for understanding the evolution of salinization,but also the main basis for improving the saline-sodic soils (Zhang et al.,2017;Zhu et al.,2022).It is generally believed that the depth and mineralization of groundwater are the important factors leading to groundwater evaporation in saline-sodic areas.Many studies have summarized that the shallower of groundwater depth,the greater the evaporation capacity of groundwater,and some mathematical models have been developed (Lei et al.,1988;Rose et al.,2005;Hernández-López et al.,2014;Xie et al.,2016).However,as an important factor affecting groundwater evaporation,the processes of groundwater mineralization on evaporation need further investigation.Previous studies on groundwater mineralization mainly focuses on the degree of soil salinity (Wang and Tian,2002;Yang et al.,2014).Some studies showed that the salinity in the soil profile increased with the increase of groundwater mineralization,and when the total dissolved substances were more than 1 g/L,the soil often presented severely salinized (Wang and Tian,2002;Li et al.,2012;Jia et al.,2015a;b).Other studies showed that the soil evaporation potential decreased as the groundwater-water salinity increased (Liu and Yang,2002;Zhang et al.,2015).Although the above studies showed that groundwater mineralization had an important influence on evaporation and soil profile salinity,the critical values of groundwater mineralization on groundwater evaporation and soil profile salinity variation still need further investigation.
In addition,as one of the key factors influencing soil evaporation,the ionic composition of groundwater usually varies between different regions.The cation that usually accounts for the largest proportion in saline lands and groundwater is the Na+.In relatively large amounts,Ca2+and Mg2+are also present as common cations in saline soils and groundwater.Different salt ions have different influences on the formation of soil aggregation (Hanay et al.,2004;Wallender and Tanji,2011).Studies showed that the high content of Ca2+,Mg2+,and Al3+in soil was beneficial for the formation of soil aggregates and the increase of soil hydraulic conductivity,while the high content of Na+was usually detrimental to the formation of soil aggregates (Qadir et al.,2001;Mahmoodabadi et al.,2013;Tang et al.,2017).Although the effects of different ions on soil agglomerate structure have been studied,the effects of different ionic composition of groundwater on soil evaporation are less reported.
The Songnen Plain,located in Northeast China with an area of 1.7 × 107ha,has a temperate monsoon climate,and is in a transitional zone of semi-humid and semi-arid region (Deng et al.,2006;Xu et al.,2022).Affected by the local climate and hydrogeological conditions,one of the three major saline-sodic regions in the world gradually formed in the Songnen Plain,China(Liu et al.,2012;2013;Yang et al.,2014).Large area saline-sodic soils in the Songnen Plain not only have high salt-sodium content,but also suffer poor water permeability,poor soil aggregate structure,and poor soil fertility.Only a few extremely salt tolerant plants survive,the proportion of bare surface with few vegetation is large,and the ecological environment is fragile.This has had a significant impact on the lives of local residents and socio-economic development.In addition,salt dust storms occur frequently due to the dry climate and windy in winter and spring,which seriously threaten the ecological environment of the surrounding communities in the plain.In recent years,amendments like desulfurization gypsum have been applied to improve the infiltrability of saline-sodic soils in the Songnen Plain(Zhao et al.,2018).As a result,calcium and magnesium ions from these amendments enter the shallow groundwater and cause changes in its mineralization and ionic composition.However,there is limited research on how these changes affect groundwater evaporation and soil water-salt dynamics.
Considering that both calcium and magnesium ions are divalent cations and their effects on structure of soil colloid are similar,the effects of different ion concentrations on groundwater evaporation and soil salt composition were studied by using calcium ions concentration instead of the sum of divalent cation concentration(Wallender and Tanji,2011).On this basis,the groundwater evaporation tests with different mineralization levels were carried out to reveal the effect of groundwater mineralization degrees on soil evaporation,the law of groundwater evaporation with different mineralization degree on the distribution of soil water and salt,and the effect of different ions on soil evaporation in the process of groundwater evaporation.The purpose of this study is to provide a scientific basis for the dynamics of soil water-salt transport under the conditions of different mineralization groundwater evaporation.
2 Materials and Methods
2.1 Experimental soil
The experimental soil was collected from a wasteland in Baicheng City,Jilin Province,Northeast China,and was classified as a sodic meadow soil (Fig.1).Typical land withLeymuschinensisgrowing was selected in this area,and then a quadrat with an area of 1.5 m × 1.0 m was chosen as the experimental soil.Weeds were cleared and all the soil in 0-30 cm was brought to the laboratory.The candidate soils were sieved through a 2 mm sieve after air drying,triturating,removing impurities,and uniformly mixing.

Fig.1 Location of soil extraction points for the experimental soils in Songnen Plain,China
The soil texture was determined to be sandy loam using a laser particle sizer,with sand,silt and clay accounting for 76.4%,16.51% and 7.45%,respectively.The soil was a mild saline-sodic soil with an initial soil water content of 1.93%.The details of the chemical properties of the experimental soil determined by are shown in Table 1.

Table 1 Chemical properties of experimental soil in Songnen Plain,China
2.2 Experimental design
Soil column experiments were conducted in the laboratory in this study.Five treatments were set up with three replications in the experiment.A set of experimental devices,containing a soil column and a water supplying tank,was arranged in each repetition (Fig.2).A plastic pipe (PVC),with an outer diameter of 20 cm,an inner diameter of 19 cm and a height of 72 cm,was prepared to fabricate the soil column.A hard partition with holes was placed at 60 cm depth to divide the pipe into two parts.A nylon mesh was first placed on the top partition,and then 2 cm thick sand was loaded evenly.Then the prepared soil was filled at 5 cm intervals at a bulk density of 1.5 g/cm3.The 10 cm thick space below the partition was filled with the simulated groundwater to let the soil column bottom was in uniform contact with water.Finally,in-situ sensors were placed on the side walls of the columns at vertical intervals of 10 cm to measure soil EC and water content.The water supply tank was a self-made 18 cm cube on each side of the Markov bottle,made of Perspex,to provide a fixed water head.There was a scale on one side of the tank to record the water level.A balance cup was used to connect the tank to the column.On the one hand,it could prevent water level changes due to tank leakage;on the other hand,it can conveniently record the true groundwater level.
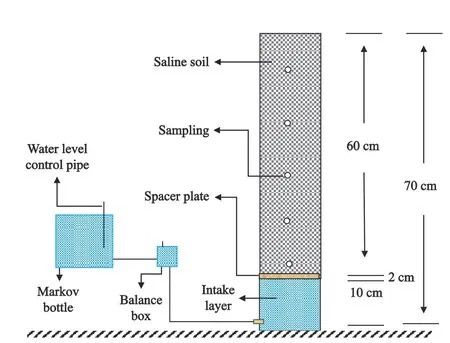
Fig.2 The schematic diagram of experimental device
Five groundwater quality treatments were set up based on the sodium adsorption ratio (SAR) and total electronic cation (TEC) of groundwater.The simulated groundwater of five qualities was prepared with NaCl,CaCl2and pure water according to the equations (1) and(2).The SAR: TEC ratios for the five treatments,marked ST0∶0,ST0∶10,ST5∶40,ST10∶70 and ST20∶100,were 0∶0,0∶10,5∶40,10∶70 and 20∶100,respectively (Table 2).

Table 2 Qualities of groundwater for different treatment in Songnen Plain,China
where [Na+] and [Ca2+] were the concentration of Na+and Ca2+in prepared water,mmol/L;the w inSARw indicates the groundwater.
The relationship between groundwater mineralization and EC could be linearly fitted by groundwater mineralization being 0.63 times of EC.
2.3 Sampling and analysis
The experiment lasted for four months starting on August,2018 to December,2018.The groundwater in all treatments was freely transported upward into the soil column under the action of capillary force.Then the cumulative of groundwater evaporation (GWE) and soil evaporation (SE) were both calculated by stages.The GWE was equal to the reduction of water volume in the groundwater tank,while the SE was calculated based on the water balance principle of the whole soil column at different periods.
One month after the start of the experiment,soil samples were collected at 20 d intervals to determine thewater content,the salt content and ion composition.The soil was sampled at 10 cm intervals in vertical direction by a 2 cm diameter auger.All samples were divided into two parts,one for the determination of soil water content through drying method at 105℃,and the other for the determination of soil salinity and ions.The second part was air-dried,mashed,and sieved to 2 mm sieve firstly,and then an extracted liquid was prepared with a soil-water ratio of 1∶5 (Bao,2000) for further determination.Based on the extract liquid,the pH and EC were determined by pH meter (PHSJ-3F) and conductivity meter (DDS-12DW),the Na+Ca2+,and Mg2+were determined by atomic absorption spectrophotometry,the Cl-was determined by silver nitrate titration.The SAR was calculated by the Equation (3):
where [Na+],[Ca2+] and [Mg2+] respectively indicated the amount of substance concentration of Na+,Ca2+and Mg2+in the extract liquid,and the unit was mmol/L.
2.4 Data analysis
All data were recorded and classified in Microsoft Office Excel 2016.Analyses of variance (ANOVA) were carried out by SPSS 24.0 statistical software (SPSS Inc.,IL,USA).Figures were drawn in Origin 2020 (Origin Lab Inc.,MA,USA).
3 Results and Analysis
3.1 Soil evaporation and groundwater evaporation
The groundwater evaporation and soil evaporation for the five treatments are shown in Fig.3 and Table 3.There were significant differences between treatments for both GWE and SE.The amounts of GWE and SE increased firstly and then decreased with the increase of the groundwater salinity-sodicity.The GWE was the least in ST0∶0 and the largest in ST10∶70,which was 2.09 times the former.A similar phenomenon was found for SE,with the maximum in ST10∶70 being 1.85 times the minimum in ST0∶0 (Fig.3).An increase-decrease type of evaporation response curve with the total salt content (TSC),mineralization and SARw was found,and the TSC,mineralization and SARw influence at the peak position at treatment ST10∶70 (Fig.3).The corresponding TSC,mineralization and SARw in this treatment were 70 mmol/L,5.3 g/L and 13 (mmol/L)0.5,respectively.
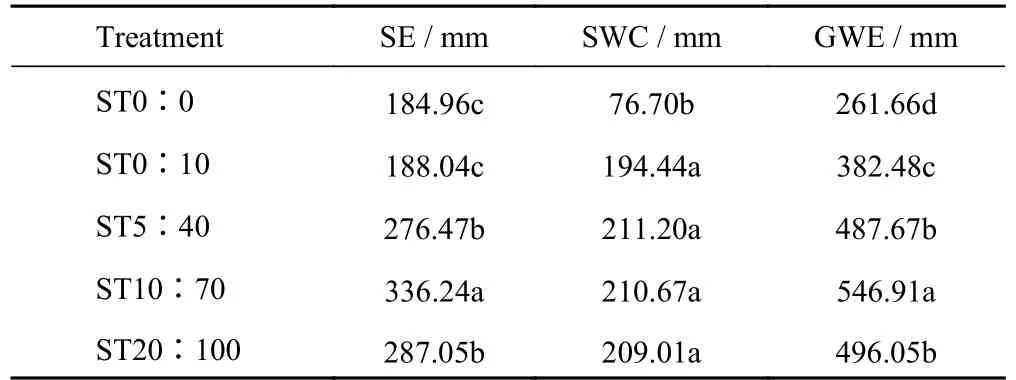
Table 3 The SE (Soil evaporation),SWC (soil water content)and GWE (Groundwater evaporation) under different treatments end the experiment in Songnen Plain,China
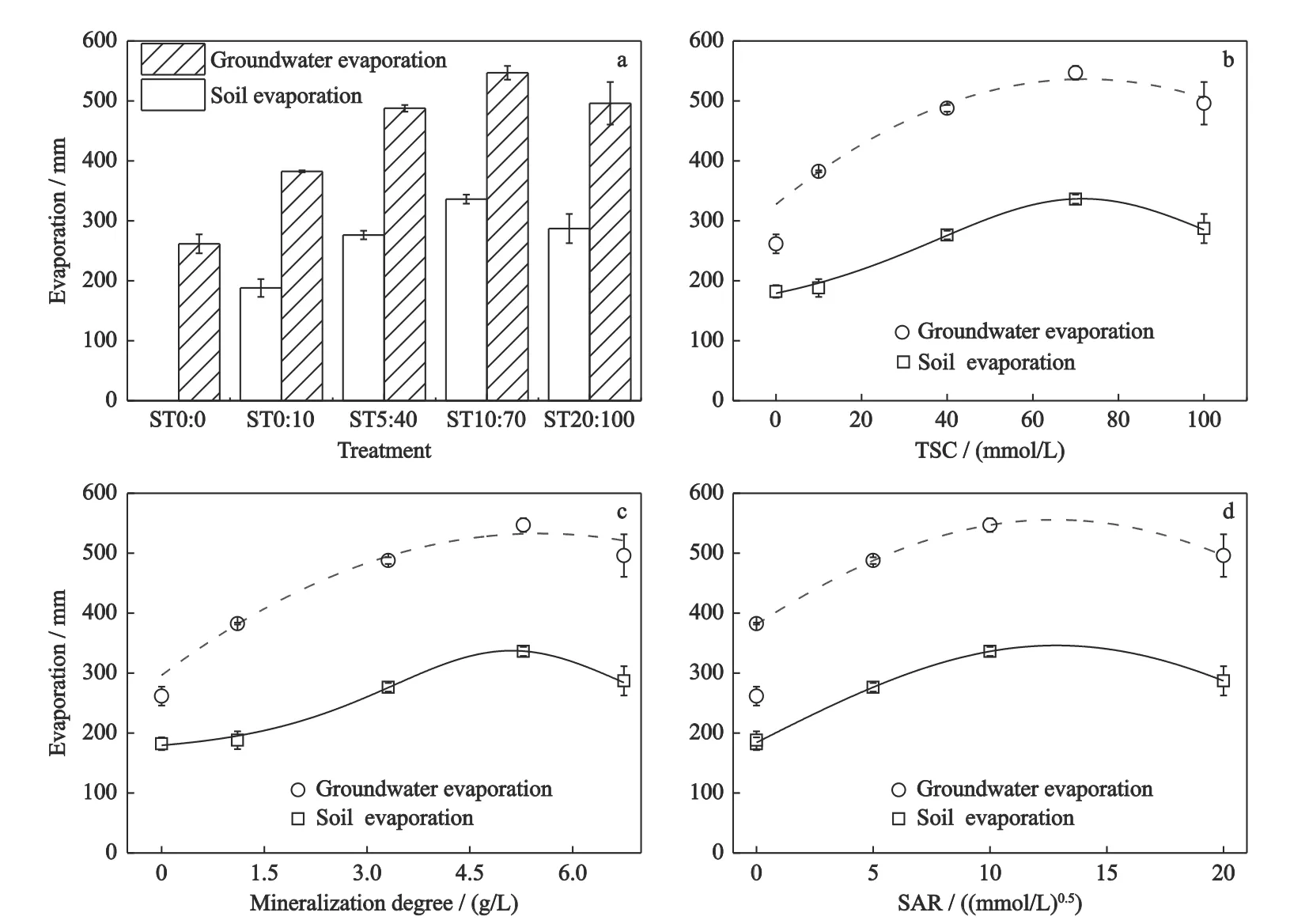
Fig.3 The cumulative groundwater evaporation (GWE) and soil evaporation (SE) in five salinity-sodicity treatments during the experiment in Songnen Plain,China.TSC,total salt content;SAR,sodium adsorption ratio.a,GWE and SE for five treatments;b,GWE and SE for different TSC;c,GWE and SE for groundwater mineralization degree;d,GWE and SE for SAR
The data in Fig.3 and Table 3 indicated that the SE increased proportionally with the increase of GWE.There was no significant difference in SE amounts between ST0∶0 and ST0∶10,while the GWE in ST0∶0 was 31.6% less than that in ST0∶10.
3.2 Water content in the soil profile
SWC in different treatments is shown in Fig.4A.The SWC of ST0∶0 was significantly lower than the other treatments throughout the study period (Table 3).The SWC of ST0∶0 gradually increased with the experimental time and increased by 6.96% in 80 d,while the SWC of the other treatments remained stable along the test time (Fig.4A).In 0-30 cm soil layer,the SWC of ST0∶10 was lower than that of ST5∶40,ST10∶70,and ST20∶100,but the SWC of 30-50 cm soil layer was higher than that of other treatments during the experiment (Fig.4A(b-e)).The SWC increased with the soil depth in ST0∶0 and ST0∶10,however,the SWC in ST5∶40,ST10∶70 and ST20∶100 remained high in the vertical direction,between 25%-35% (Fig.4A)during the experiment.An interesting finding was that the SWC of ST5∶40,ST10∶70,and ST20∶100 was the highest in the topsoil and decreased slightly with the increase of soil depth,while the SWC of ST0∶0 and ST0∶10 was on the contrary.
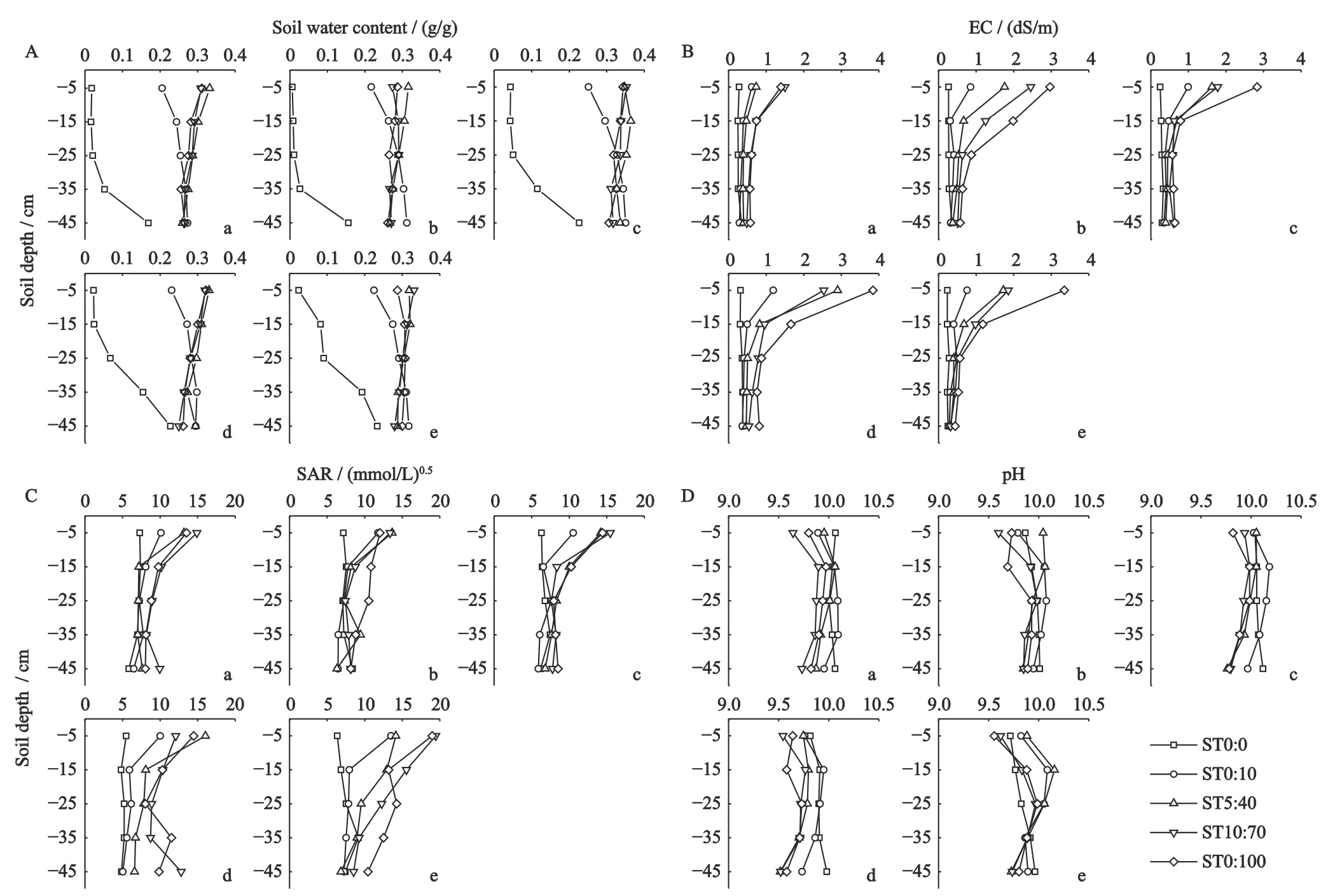
Fig.4 The distribution of soil water content (A),EC (B),SAR (C),pH (D) in soil profile under the different treatments in Songnen Plain,China.a,Sept.27th,2018;b,Oct.7th,2018;c,Oct.27th,2018;d,Nov.17th,2018;e,Dec.16th,2018
3.3 Soil salinity and salt compositions
The soil EC in the profile is shown in Fig.4B.It could be seen that the EC in all treatments except ST0∶0 presented an upward trend compared with the initial EC during the experiment.The accumulation of soil salt mainly occurred in the 0-20 cm soil layer,and the increase of soil salinity in the topsoil was most obvious.The EC in all treatments reached maximum on Nov.7th,2018 (Fig.4B(d)).The order of the maximum values in this stage was ST20∶100 >ST10∶70 >ST5∶40 >ST0∶10 >ST0∶0,which showed the positive relationships among the average EC,TEC and groundwater mineralization.When the experiment terminated (Fig.4B(e)),the average EC values in ST0∶10,ST5∶40,ST10∶70 and ST20∶100 were correspondingly 1.83,2.97,3.23 and 4.92 times that in ST0∶0.
The ion ratios of Na+,Ca2+and Cl-in each treatment soil column and the corresponding ratios of these ions in groundwater are shown in Table 4.As the experiment continued,the proportion of Na+in each soil column was the highest,followed by Cl-,and the proportion of Ca2+was the lowest (Table 4).Except for ST20∶100,the proportion of Ca2+in all treatments gradually increased with time.In addition,the proportion of Ca2+in ST0∶0 increased the most and accounted for the largest proportion.By the end of the experiment (Dec.16th,2018),the proportion of Ca2+in treatment followed the order of ST0∶0 >ST0∶10 >ST10∶70 >ST5∶40 >ST20∶100.In general,the proportion of Ca2+increased first and then decreased in ST0∶0 treatment,and the proportion of Ca2+increased gradually,but the rate of increase gradually slowed down in treatment ST0∶10.For the other treatments,the ion ratios showed a trend being close to the groundwater ions ratios (Table 4).
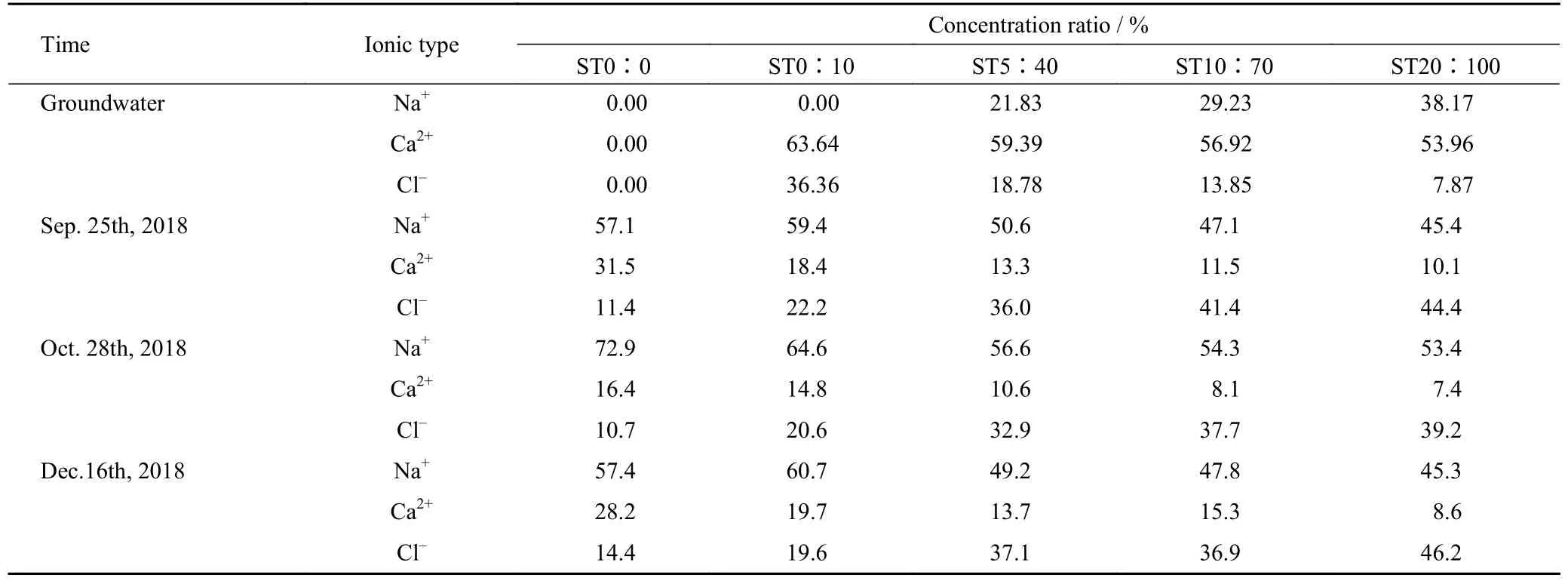
Table 4 Proportion of three major ions in groundwater and soil column profiles by periods under different treatments in Songnen Plain,China
3.4 Soil SAR and pH
The variation of soil SAR in the vertical direction showed that the SAR in all treatment gradually increased from the bottom to the top of the soil column(Fig.4C).Except for ST0∶0,the soil SAR of each treatment had a tendency of increase with the experimental time,and the SAR values in soil profiles reached the maximum at the end of the experiment (Fig.4C(e)).The final average SAR values in soil profiles increased as the groundwater salinity increased,and the values for ST0∶10,ST5∶40,ST10∶70 and ST20∶100 were 7.46,8.82,10.49,13.02 and 13.88 (mmol/L)0.5,which correspondingly increased by 42.11%,67.95%,99.79%,147.93% and 164.32% when compared with the initial SAR.
The soil pH in all treatments at different periods showed an arch distribution in the vertical direction(Fig.4D).The pH values were low at the top and the bottom soil layers and gradually increased to their peaks in 20-40 cm soil layer.The average pH values of the soil profile in ST10∶70 and ST20∶100 treatments were lower than those in ST0∶0,ST0∶10 and ST5∶40 treatments in the whole experimental period.Overall,no significant difference in the soil pH was found over the experimental periods.
4 Discussion
4.1 Influences of mineralization and ion compositions of groundwater on evaporation
According to the results of the study,the salinity of groundwater is a main factor affecting the GWE and SE under the same groundwater depth.The results of the present study are similar to those of previous studies,which all found different rates of water movement through the soil depending on the salinity gradient of the water (Oades,1993;Rose et al.,2005;Liu et al.,2009;Wu and Wang,2010).The difference of GWE and SE may be caused by the different movement of saline water in soil.Infiltration with different salinity gradients can still occur during irrigation (Wu and Wang,2010;Li et al.,2020).It shows that water with different salt concentration will change the turbulence of soil particles,increase soil mass,stabilize the soil structure,and increase the soil porosity and permeability.It facilitates the movement of water in the soil.However,there is a peak value at 5.28 g/L in the process of GWE and SE in this study.The peak value is higher than previous studies 3 g/L (Wu and Wang,2010;Yang et al.,2014).The reason may be related to the different ion composition in groundwater.In particular,the presence of a large number of Ca2+will promote the aggregation of soil particles in ST5∶40,ST10∶70 and ST20∶100.
The relationship between three ion concentrations(Na+,Ca2+and Cl-) in groundwater and GWE and SE are shown in Fig.5.The Ca2+concentration was firmlypositively linearly correlated to the amounts of the both GWE and SE (Fig.5),and the GWE and SE coefficient are 12.63 and 6.86,respectively.Although linear relationships were found between Na+and Cl-concentrations and between GWE and SE,their evaporation coefficients were both lower than that in Ca2+.And the scatter curves of Na+and Cl-were more like arches than straight lines as Ca2+.The main reason is that many Ca2+got into the test soil with the process of GWE and SE.The Ca2+in the soil solution displace Na+in the core of soil colloid to form a soil colloid with Ca2+as the core,which inhibits the dispersion process of soil clay particles,increases the aggregate structure,releases pores,and improves the soil water conductivity (Blume et al.,2002;David and Dimitrios,2002;Wu and Wang,2010;Mahmoodabadi et al.,2013;Li et al.,2020).Therefore,Ca2+as an important ion in saline-sodic soil plays an important role in improving soil water conductivity.It means that in the shallow groundwater area without precipitation,the higher Ca2+concentration in groundwater,the more GWE and SE.

Fig.5 Correlation analysis of ion (a,Na+;b,Ca2+; c,Cl-) concentration in groundwater and groundwater evaporation (GWE) and soil evaporation (SE) in Songnen Plain,China.Dotted line represents the ion concentration relationship between groundwater and GWE and solid line represents the ion concentration relationship between groundwater and SE
4.2 Influences of mineralization and ion compositions of groundwater on SWC
Results showed that the SWC at various period differed among treatments.When the groundwater salinity was low,the average SWC in the profile increased as the groundwater salinity increase,while the influence of the groundwater salinity became weak if the groundwater salinity was higher than a certain value.The change of SWC in groundwater evaporation is similar to that in infiltration (Wu and Wang,2010).This may be caused by a large amount of salt was brought into the soil with the increasing mineralization groundwater evaporation in ST0∶0 to ST20∶100.With the increase of salt concentration in soil column,the diffusion double electron layer compresses to the surface of the clay particles,the repulsive force between soil particles decreases and then enhances the flocculation of soil colloids,which helps to form aggregates and increase soil water carrying capacity (Shao et al.,2006;Meyer and Kassen,2007).Therefore,salt ions can enhance soil water carrying capacity and water holding capacity to some extent.However,with the further increase in salinity,the amount of Na+entering the soil during infiltration also increases,which is the main ion that causes soil degradation.Due to the ionic charge of Na+is small,the radius is relatively large,and the hydration energy is small,the presence of Na+will cause the expansion and dispersion of soil particles.Under the alternating action of dry and wet conditions,the physical properties of soil degradated,and the water permeability and air permeability of soil become worse (Qadir et al.,2003;Panigrahi and Panda,2003;Tejada et al.,2006;Wallender and Tanji,2011;Guo et al.,2014).Therefore,the hydraulic conductivity and water holding capacity of the soil are mainly influenced by the salinity and ionic composition of the water.
The SWC in ST0∶0 and ST0∶10 treatments showed a phenomenon that gradual decrease in soil moisture content from the bottom to the surface layer in soil column,which is similar to the results of previous studies,where the SMC increases the closer groundwater in the absence of rainfall irrigation (Yang et al.,2014).The SWC in ST5∶40,ST10∶70 and ST20∶100 treatments showed a phenomenon that the deeper the soil layer,the lower the groundwater and the closer to the surface,the higher the groundwater,which is inconsistent with the majority of results (Hernández-López et al.,2014;Zhang et al.,2015).Mainly due to the shallow and constant burial depth of the groundwater in the test,the ionic content and composition of the treatments varied greatly,with the evaporation of water,the salts showed a gradual increase from the bottom to the surface in the soil column,the solute potential of the soil column was consistent with the distribution of salt content,and this state of moisture distribution eventually occurred under the action of the water potential gradient (Zhang et al.,2017;Wallender and Tanji,2011).
The relationships between the groundwater ions and SWC in soil profile showed that the correlation between the Ca2+concentration and the SWC was the strongest,while the correlations between the concentrations of Na+and Cl-and SWC were not significant (Fig.6).Previous studies showed that Ca2+entered the soil and replaced the Na+in the soil colloid,forming a stronger colloidal structure,promoting the formation of soil mass structure,changing the physical structure of the soil and increasing hydraulic conductivity (Mahmoodabadi et al.,2013;Zhao et al.,2018).This is consistent with the results of this study.
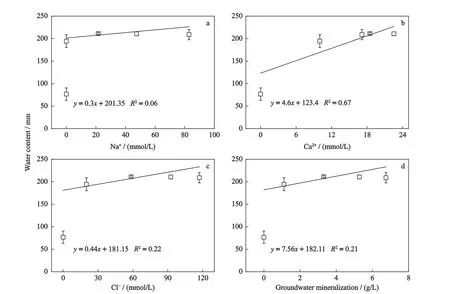
Fig.6 Linear regression analysis of ion concentration (a,Na+;b,Ca2+;c,Cl-;d,groundwater mineralization) and total soil water content in column in the end of the experiment in Songnen Plain,China
4.3 Influences of mineralization and ion compositions of groundwater on soil salinity
This study found that as groundwater evaporates,salt ions accumulated in the soil profile and the greater the groundwater mineralization,the higher the concentration of salt ions in the soil profile.It is similar to that salt was accumulated on the topsoil in the process of groundwater evaporation,resulting in the formation of high salinity zone in the soil surface,which was consistent with the formation process of saline soil in nature(Song and Deng,2000;Hernández-López et al.,2014;Jia et al.,2015b;Rose et al.,2005;Zhang et al.,2015).
Differences in soil salinity among treatments in the soil column are mainly due to differences in GWE determined by groundwater evaporation and groundwater salinity.The average EC in the whole profile and that in topsoil (0-20 cm) were significantly-positively linearly correlated to the total salts brought into the soil columns(Fig.7).The groundwater salinity and GWE together might explain this.With the groundwater salinity increase,the GWE increased first and then decreased.However,the product of groundwater salinity and GWE,that determined the salt amount brought into the soil,increased as the groundwater salinity increased.
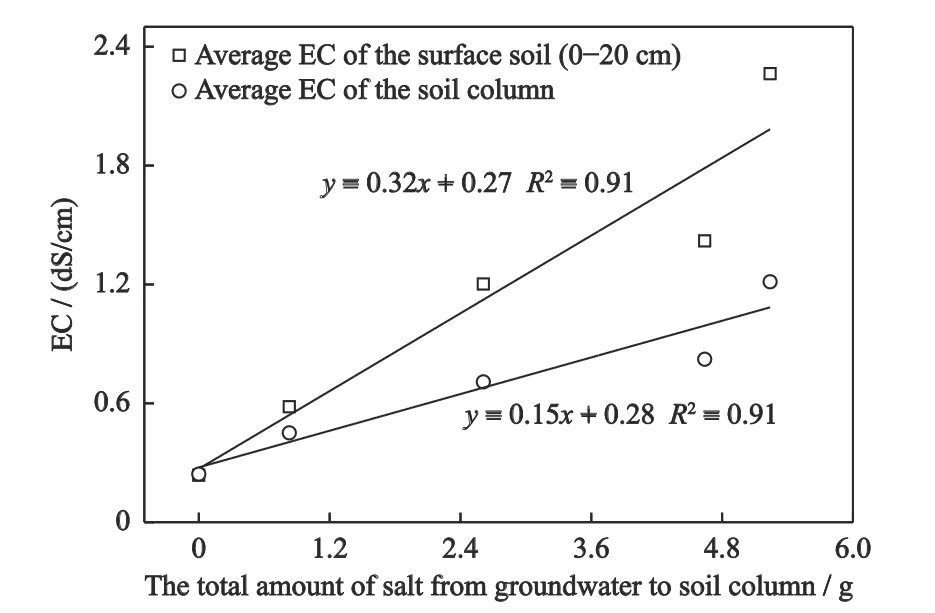
Fig.7 Linear regression analysis between salinity of groundwater and salt content of soil column in Songnen Plain,China.EC,electrical conductivity
The ion concentrations in groundwater had important influences on soil salt ion content and its compositions,especially on soil Ca2+.There was no Na+content in the groundwater in treatment ST0∶10.However,the Na+contents in the whole soil profile and surface layer in this treatment were larger than that in the ST0∶0 as experiment went on,but the Ca2+content decreased instead of increasing.The reason might be related to the adsorption and desorption of ions in colloids (Blume et al.,2002;David and Dimitrios,2002;Li et al.,2020).For the ST0∶0 treatment,the adsorptive ions (Na+and Ca2+,etc.) on the surface of the soil colloid were desorbed into the soil solution under water potential gradient,to improve the osmotic potential of the soil solution and made the solid phase and liquid phase reach the electrolyte concentration balance.In the treatment ST0∶10,the Ca2+was brought into the soil by the capillary rise and exchanged the adsorptive ions (Na+,etc.)to form the new structure-firmer soil colloid,because the charge-mass ratio of Ca2+is larger than that of Na+.This new soil colloid played a role in improving soil physical properties such as the hydraulic conductivity,which was in accordance with results of SE and SWC in this study (David and Dimitrios,2002;Sahin et al.,2020).
The positive linear relationship between the average Na+content in soil profiles and the Na+amount brought by groundwater was extremely significant and similar relationship was also found for Na+content in 0-20 cm(Fig.8A).The linear correlations between the Ca2+contents in soil profile and surface layer and the amount of Ca2+brought by groundwater were not significant,while the linear correlations between the Cl-content and the amount of Cl-was extremely significant (Fig.8B,C).This indicated that the amounts of Na+and Cl-brought by groundwater were closely related to the contents of Na+and Cl-,while the amount of Ca2+was not obviously related to the Ca2+content in the soil.At the beginning,the contents of Na+and Cl-in the soil are relatively high,while Ca2+is relatively low.The Ca2+,entering the soil along with the continuous GWE in different treatments,exchanged the Na+from the colloidal surface due to its superior charge to mass ratio of Na+(Tejada et al.,2006;Cheng et al.,2014).Furthermore,the Ca2+adsorbed in the soil colloid surface gradually entered the colloidal nucleus during the transformation process of the soil colloid and became the solidified Ca2+in the soil solid (Blume et al.,2002;Wallender and Tanji,2011;Li et al.,2020).Therefore,the increasing rate of Ca2+content was lower than those of Na+and Clin soil.
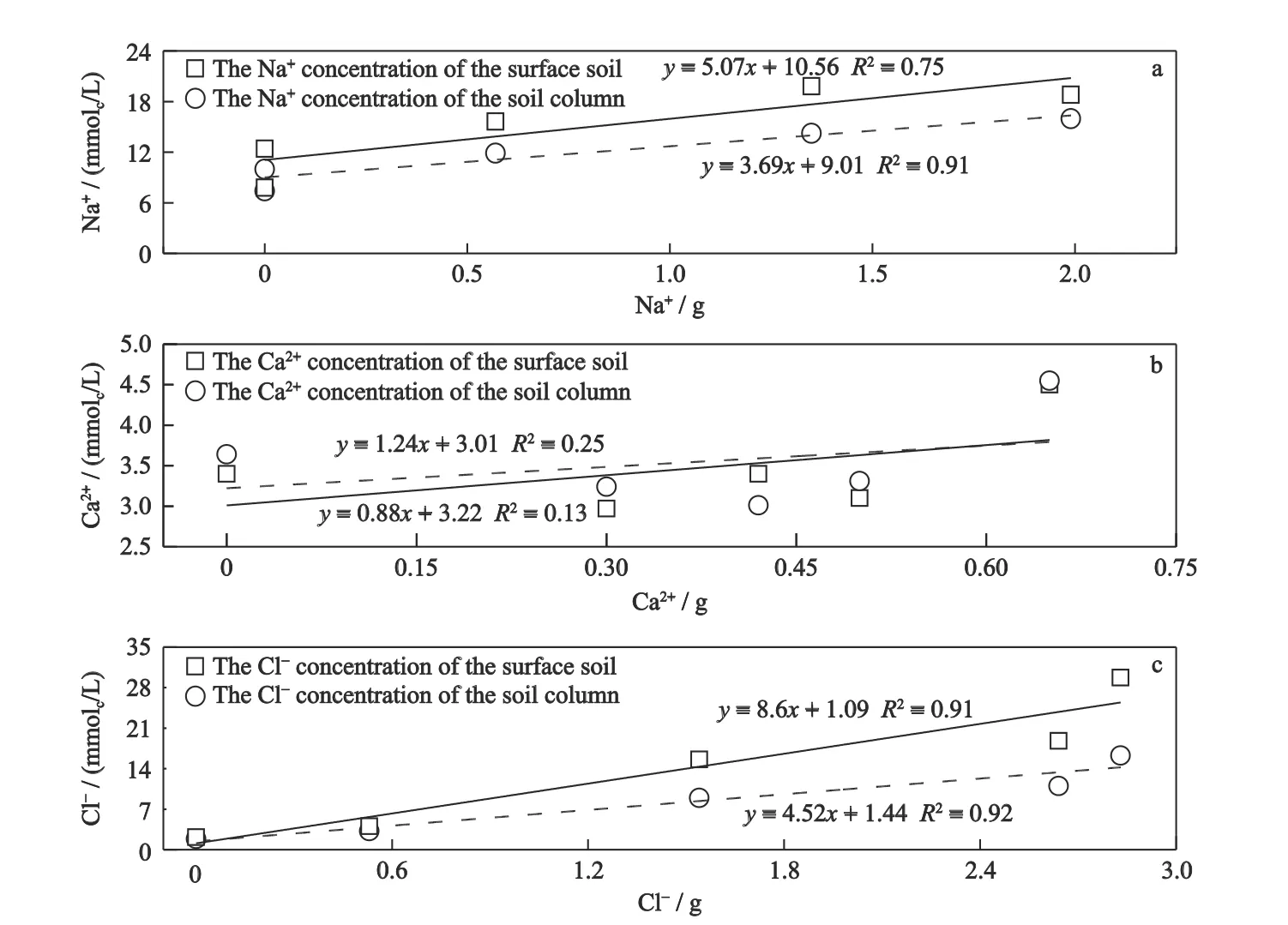
Fig.8 Linear regression analysis between ion mass (a,Na+;b,Ca2+;c,Cl-) entering soil column and ion concentration in soil column in Songnen Plain,China
5 Conclusions
This study provides experimental evidence for the critical role of mineralization and ionic composition in groundwater evaporation and water-salt transport in saline-sodic soils in Songnen Plain,China.With the increase of groundwater salinity,both GWE and SE increased first and then decreased.The GWE values were observed at a groundwater salinity of 5.28 g/L,while the least values were found at 0 g/L.The GWE was positively correlated to the Ca2+content in groundwater with the correlation coefficient >0.99,and the correlations with Na+and Cl-were poor compared with Ca2+.The soil water holding capacity was closely related to groundwater salinity and ion compositions.When the groundwater salinity was larger than 1.1 g/L in a certain range,the SWC was significantly higher than that of the groundwater salinity less than 1.1 g/L.
High groundwater salinity could accelerate the salt accumulation in the soil profile.The soil salt content in column profile showed a gradually increasing trend,compared with the initial soil salt content.The salt increments in the top 30 cm soil layer showed great differences among treatments,and there was a phenomenon of salt accumulation in the soil surface layer of each treatment.The average SAR in the soil profile increased gradually over time.,while pH change was not significant.The proportions of Na+,Cl-and Ca2+in the soil columns in all treatments changed gradually with time,and the ionic ratios in the soil columns in ST5∶40,ST10∶70 and ST20∶100 treatments gradually approached their corresponding groundwater ion ratios.Studies showed there was a close correlation between Ca2+and groundwater evaporation.
Shallow groundwater mineralization and ionic composition have a significant impact on the formation of soil salinization.The findings from this experimental study provide fundamental data necessary for identifying the critical water level in soil salinization.Additionally,these findings enable the establishment of a theoretical basis for assessing the risk of secondary soil salinization and developing a strategy for regulating soil water and salt in this region.
Conflict of Interest
Authors are responsible for correctness of the statements provided in the manuscript.All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript.
Author Contributions
All authors contributed to the study conception and design.Material preparation,data collection and analysis were performed by ZHU Wendong,Yang FAN,ZHAO Dandan and WANG Zhichun.The first draft of the manuscript was written by ZHU Wendong and all authors commented on previous versions of the manuscript.All authors read and approved the final manuscript.
 Chinese Geographical Science2023年6期
Chinese Geographical Science2023年6期
- Chinese Geographical Science的其它文章
- Bibliometrics-based Research Hotspots and Development Trends in Ecohydrology of Dammed Rivers
- Increasing Anthropogenic Mercury Pollution over the Last 200 Years Revealed by Lagoonal Sediments from Hainan Island,South China
- Detection of Multi-dimensional Driving Forces of Public Environmental Concern in China: Based on Spatial Heterogeneity Perspectives
- Projected Regional 1.50°C and 2.00°C Warming Threshold-crossing Time Worldwide Using the CMIP6 Models
- Spatial Distribution Pattern and Influencing Factors of Physical Bookstores of Large Cities: A Case Study of Three National Central Cities in Western China
- Leave or Stay? Antecedents of High-level Talent Migration in the Pearl River Delta Megalopolis of China: From a Perspective of Regional Differentials in Housing Prices
