Studies on ammonium dinitramide and 3,4-diaminofurazan cocrystal for tuning the hygroscopicity
Dongdong Hu,Yinglei Wang,Chuan Xiao,Yifei Hu,Zhiyong Zhou,,Zhongqi Ren,
1 State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China
2 Xi’an Modern Chemistry Research Institute, Xi’an 710065, China
3 China Research and Development Academy of Machinery Equipment, Beijing 10089, China
Keywords:Ammonium dinitramide 3,4-Diaminofurazan Hygroscopicity Cocrystallization Adsorption Kinetics
ABSTRACT Ammonium dinitramide (ADN) is a promising oxidizer with high energy characteristic,which is a relatively new environmentally friendly oxidizer without halogens and carbon elements.However,ADN has high hygroscopicity when exposed to high humidity air,restricting its applications on the solid propellants.In this paper,a novel energetic cocrystal composed of ammonium dinitramide and 3,4-diaminofurazan(DAF)was proposed and successfully synthesized by antisolvent crystallization method,and the properties of the cocrystal were systematically investigated by analytical characterization and theoretical simulation calculations.The formation of the cocrystal was confirmed by powder X-ray diffraction,differential scanning calorimetry,scanning electron microscopy,infrared spectroscopy and Raman spectroscopy,indicating that the synthesized product was a cocrystal.Through theoretical studies,the ADN/DAF cocrystal structure was predicted,and the powder X-ray diffraction,morphology,water sorption capacity of ADN/DAF cocrystal were calculated,which was consistent with experimental phenomena.The results showed that newly prepared cocrystal of ADN/DAF had lower hygroscopicity compared to pure ADN,and the water sorption capacity was reduced from 15.35%to 7.90%.This may be due to the formation of N-H···O medium-strength hydrogen bonds between the ammonium ion of ADN and the O atom of DAF in the cocrystal,which prevents the binding of water molecules in the air and ammonium ions and reduces the probability of ADN binding to water molecules,leading to the reduction of cocrystal hygroscopicity.The newly prepared energetic cocrystal can provide theoretical and technical guidance for the study of the anti-hygroscopicity of ADN and advance the practical application of ADN.
1.Introduction
Ammonium dinitramide (ADN,NH4NO2NNO2) is an ionic compound containing an ammonium cation (NH4+) and a dinitramide anion [(NO2NNO2)-].Its crystal structure was determined by Gilardiet al.[1] and can be found in the Cambridge Crystallographic Database (CCDC 854801).ADN has been wildly applied in composite solid propellants,and the ADN-based propellants have more high impulse than other traditional propellants.For example,Flonet al.[2,3] theoretically calculated the energy change after replacing ammonium perchlorate(AP)with ADN in the rocket propellant using hydroxyl terminated polybutadiene propellant(HTPB) as the binder and aluminum as the fuel system,and it was found that the theoretical specific impulse of the propellant was increased by 3%,and the combustion temperature was reduced by 4%,and it was found the maximum specific impulse of the propellant in the ADN/ glycidyl azide polymer (GAP)/Al(59% ADN,20% GAP,21% Al) system was 296 s,which was higher than AP/HTPB/Al(68%AP,12%HTPB,20%Al)system(284 s).These results indicated that the replacement of AP by ADN as a propellant oxidant formula can increase the energy performance of the propellant.
Although ADN has obvious energy advantage,ADN is strongly hygroscopic at high temperature and high humidity condition,which limits its application in solid composite propellants.The critical relative humidity of ammonium dinitramide was 55.2% at 25 °C [4].Up to now,the anti-hygroscopicity technology of ADN contains prilling,surface coating,and co-crystallization three technologies [5-7].Co-crystallization is a supramolecular assembly process by which a variety of solid substances are precipitated from vapor,solution or melt in a co-crystalline state.Cocrystal is an effective method to solve the hygroscopicity of ADN.Cocrystal technology is the combination of host component and cocrystal guest in a certain molar ratio to form a crystal.Different from the formation of salt crystals,the formation of cocrystal is through intermolecular hydrogen bonds,π-π bonds,van der Waals forces and other non-covalent bonds,especially hydrogen bonds are the main driving force for the formation of cocrystal [8].Xieet al.[9]prepared a cocrystal of ADN/1,4,7,10,13,16-hexaoxacyclooctade cane(18-crown-6)with a molar ratio of 1:1 by solvent evaporation method.At 30 °C and 80% relative humidity,the water sorption rate of ADN was from 18% to 1.2%,the anti-hygroscopic performance of ADN has been greatly improved.Bellas and Matzger[10] prepared a cocrystal of ADN/pyrazine-1,4-dioxide (PDO) with a stoichiometric ratio of 2:1,which proved that the critical relative humidity of ADN was 53.5% and the critical relative humidity of ADN/PDO cocrystal was 79.5%,and it was found that the calculated specific impulse of ADN,ADN/PDO cocrystal was 202 s and 259 s by calculations,respectively.These results showed that the ADN/PDO cocrystal not only reduced hygroscopicity but also improved the energy performance.In order to improve the hygroscopic properties of ADN crystals,our group proposed a theoretical design method for ADN anti-hygroscopic cocrystal ligands [11].The crystal structure prediction and hygroscopicity calculation of ADN cocrystal were carried out for nine energetic materials using molecular simulation software of Materials Studio.Four energetic materials,hexanitrohexaazaisowurtaitane (CL-20),octogen(HMX),cyclotrimethylenetrinitramine (RDX)and 1,3,3-trinitroazetidine (TNAZ),were selected as the cocrystal ligands of ADN.The results showed that the cage molecular structure can prevent water molecules from approaching the crystal surface,and had a good anti-water absorption effect under high humidity conditions.Our previous work provided a theoretical basis and technical support for the cocrystal method to solve the hygroscopicity problem of ADN.
3,4-Diaminofuran (DAF) is an important precursor for some high energy density materials such as 3,4-di(nitramino)furazan(DNAF).From the thermal studies,DAF does not have adverse effect on vulnerability and chemical stability of the propellant formulation,and it shows promising application prospect on propellant [12].The molecular structure of ADN and DAF are shown in Fig.1.
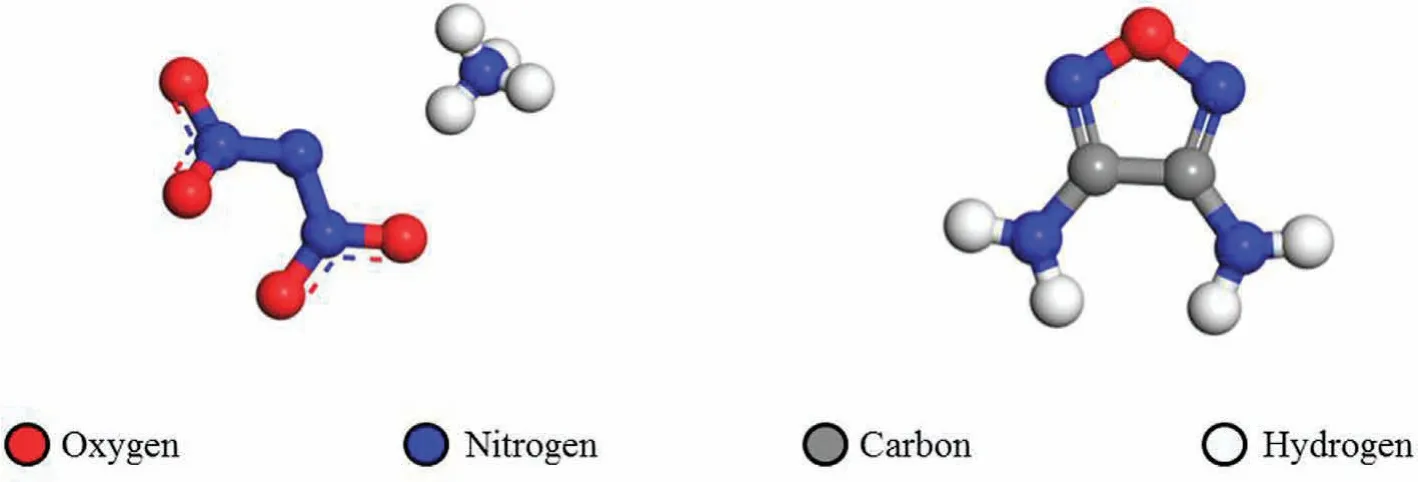
Fig.1.The molecular structures of ADN (left) and DAF (right).
In this study,a new cocrystal with ADN as the host component and DAF as the cocrystal guest was systematically investigated using simulated calculations and experimental studies to improve the hygroscopicity of ADN.The powder X-ray diffraction(PXRD),differential scanning calorimetry (DSC),scanning electron microscopy(SEM),infrared spectroscopy (IR) and Raman were used to characterize the formation of cocrystal,which indicated that the synthesized products were cocrystal.Using the material simulation software of Materials Studio,the structure,morphology,PXRD and hygroscopicity of the cocrystal were determined by theoretical calculation method.The calculations can provide guidelines for the preparation of ADN/DAF cocrystal.This work can provide substantial insight into co-crystallization for tuning the hygroscopicity of ADN.
2.Computational Methodology
2.1.Cocrystal structure prediction
With the development of crystal structure prediction,lattice energy and density scatterplot are fast and efficient method to predict cocrystal structures[13-15].Generally,for crystals,the lowest lattice energy means the most stable thermodynamics structure;for explosives,density is an important parameter that determines the detonation performance,high density means good detonation performance.Therefore,low lattice energy and high-density crystal form are the goals pursued by cocrystal research.
The cocrystal structure of ADN/DAF was predicted by Polymorph tools.The prediction calculations of cocrystal structures were based on Monte Carlo method.From the results of experimental preparation process and PXRD comparison between experimental and computational results,the molar ratio of cocrystal formation should be 1:1.Since the Dreiding force field,as a general force field,was especially suitable for describing the system containing carbon,hydrogen,oxygen,and nitrogen atoms,and can well describe the interaction between ADN and other energetic materials,the Dreiding force field was used for Optimization process for molecular and crystal structures [16].
First,the topology of the molecules needed to be optimized before the cocrystal structure prediction calculation.Based on the calculation of the electrostatic potential,the general position of the intermolecular interaction was analyzed and placed.During the optimization process,the relative positions of the molecules were changed for many times.A tentative optimization would yield the cocrystal dimer combination conformation with the lowest total energy as the cocrystal prediction file.Then,using the Polymorph module to predict the unit cell structure of the ADN cocrystal,a few predicted crystal structures were obtained during Monte Carlo simulations.By successively placing the relative positions of the molecules in the unit cell,the space group structure,lattice energy,density and hydrogen bond energy of the crystal can be obtained.The Dreiding force field was used for the calculation force field,the Gasteiger calculation method was used for the electric charge,the electrostatic force was set to Ewald,the van der Waals force was set to Atom based,and ten space groups including P-21/C,P-1,P212121,C2/C,P21,PBCA,PNA21,CC,PBCN and C2 were selected.
2.2.PXRD spectra simulation
The Reflex module is used to simulate X-ray,neutron and electron powder diffraction patterns of crystalline materials,which can determine the crystal structure,analyze the diffraction data.Also,it can verify the calculated and experimental results and be widely used in the acquisition of crystal information at the atomic level[17].Among them,powder diffraction simulation is used to calculate the diffraction pattern,establish a model for the material to be analyzed,and select the experimental conditions to be simulated.
After obtaining the cocrystal structure,we use the Reflex module.First,we select powder diffraction simulation and set the simulation parameters according to the experimental data,2-theta was set to 5°-90°,the step size was set to 0.02°,the diffraction line type was selected as X-ray,the wavelengths λ1=1.5406 Å(1 Å=0.1 nm),and the anode material was copper.Then the XRD pattern of ADN/DAF cocrystal simulation was obtained.Finally,the experimental data and theoretical data were compared to verify the accuracy of the simulation calculation.
2.3.Cocrystal morphology prediction
Morphology can both study the shape of particles and consider the effect of changing the growth rate of a particular surface on crystal morphology.Bravais-Friedel-Donnay-Harker(BFDH)theory predicted crystal morphology from the arrangement of molecules and atoms.The BFDH method was a geometric calculation method that generated a set of possible growth faces and their relative growth rates,from which the morphology of the crystal can be inferred.This method was an approximation and was mainly used to identify those faces that are important in the growth process[18-22].
First,the important crystal planes of the predicted cocrystal in a vacuum environment were determined.Using the Morphology module,the BFDH method was selected to predict the crystal morphology of the cocrystal.The minimumdhklparameters were adjusted to 1.30 Å,the maximumh,k,andlwere 3/3/3,and the maximum number was 200.Then,the important crystal planes of ADN/DAF cocrystal prediction were obtained.
2.4.Water sorption capacity prediction
The moisture absorption rate is the water sorption capacity calculated by examining the number of water molecules adsorbed by the cocrystal under different relative humidity conditions.When the ADN/DAF cocrystal reaches adsorption saturation under the specified temperature and relative humidity for an infinite period of time,the calculated water sorption capacity is converted into the mass of adsorbed water per gram of substance,which is the definition of saturated moisture absorption rate [23].The crystal planes with high water sorption capacity indicate that the crystals are arranged according to these crystal planes and have strong hygroscopicity,and the crystal planes with low water sorption capacity indicate that the crystals arranged according to these crystal planes have weak hygroscopicity.Therefore,it is necessary to accelerate the growth of crystal planes with low water sorption capacity in order to prevent moisture absorption.
The saturated adsorption capacity is calculated by Eq.(1) [24]and gas phase density is calculated by Eq.(2) [25].
wherenexis the saturated adsorption capacity,mol·g-1;Nis the total adsorption capacity,mol·g-1;Va,VgandVpare the adsorption phase volume,gas phase volume,and free volume,cm3;ρgis the gas phase density,g·cm-3;Pis the atmospheric pressure andPSis the saturated vapor pressure,Pa;φ is the humidity,%.
The hygroscopicity of the basic crystal planes,important crystal planes of the ADN crystal,ADN/DAF cocrystal were investigated.Firstly,2×2×2 supercells of ADN crystal and ADN/DAF cocrystal were established,and cut to a thickness of 0.10 nm to obtain(1 0 0),(0 2 0),(1 1 0),(0 1 1) and (1 1) crystal surfaces of ADN [23];(0 2 0),(1 1 0),(0 1 1) and (0 1) crystal surfaces of ADN/DAF,respectively.To eliminate boundary layer effects,a 1.0 nm vacuum layer was added,the excess vacuum layer was removed by adjusting the height of the model,and the middle of the model represented the moist air region.The Sorption module was used for calculation,the task was selected as fixed pressure,the method was selected as Metropoil,the Quality was selected as fine,the temperature was 20 °C,the humidity was 40%;the van der Waals force was calculated by Atom based;properties selected energy distribution,density field and Energy field.Finally,the water sorption capacity and total crystal water sorption capacity of different crystal planes of ADN crystal and ADN/DAF cocrystal were calculated.
3.Experimental Methods
3.1.Reagents and materials
Ammonium dinitramide (ADN) stored in the dark desiccator at room temperature was synthesized in Xi’an Modern Chemistry Research Institute (China).3,4-Diaminofurazan (DAF) was synthesized in Xi’an Modern Chemistry Research Institute.Acetone was purchased from Tianjin Kemiou Chemical Reagent Co.,Ltd(China).Dichloromethane was purchased from Chengdu Kelong Chemical Reagent Co.,Ltd (China).
3.2.Experimental methods
3.2.1.Preparation method
ADN is freely soluble in the acetone (at 20 °C,the solubility is 84.97 g/100 g solvent),and poorly soluble in the dichloromethane(at 20°C,the solubility is 0.003 g/100 g solvent)[26].DAF is freely soluble in the acetone,and poorly soluble in the dichloromethane[27].ADN/DAF was prepared by antisolvent crystallization method.DAF(0.2 g,0.002 mol)and ADN(0.248 g,0.002 mol)were dissolved in 10 ml acetone.Then,the solution was placed in the KQ-250DB CNC ultrasonic cleaner under the ultrasonic oscillation condition,and the solutes were completely dissolved in the solution after 1 h.Then,the solution was slowly added to 150 mL dichloromethane under the condition of stirring,and there was continuous solid precipitation.The time of dropping should be more than 0.5 h for controlling dropping process stability.After dropping completion,the suspension liquid was stirred for 0.5 h.Then,the cocrystals were filtered,and dried in vacuum drier to obtain ADN/DAF cocrystal,showing pale yellow color.
3.2.2.Analytical methods
(1) FT-IR.The raw material ADN,DAF and prepared ADN/DAF were operated by tableting method.Then,the samples were tested by MPA infrared spectrometer (Germany Bruker Corporation).
(2)PXRD.The PXRD data of the raw ADN,raw DAF and prepared ADN/DAF cocrystal were recorded under ambient conditions on D/MAX-2400 XRD diffractometer (Japan Rigaku Industrial Corporation).
(3)SEM.The images of samples were tested by Quanta 600 FEG scanning electron microscope (UK FEI Corporation).
(4) DSC.The DSC of samples was test by DSC204HP/2 equipment (Germany NETZSC Corporation),and the heating rate was 10 °C·min-1,and the temperature range was 10-450 °C,and the samples mass was 0.5-1.2 mg and the test was in the condition of N2gas.
(5) Raman.The Raman spectra of ADN and ADN/DAF cocrystal were tested by InVia Reflex laser Raman spectrometer (UK Renishaw Corporation).
4.Results and Discussion
4.1.Cocrystal structure prediction
Electrostatic potential is the most important factor in the formation of non-covalent bonds between molecules.As shown in Fig.2,the red electron-poor region in the ADN molecule,that was the H atom of the ammonium ion,and the blue part of DAF with dense electrons,that was the O atom,attract and complement each other,thereby forming a stable non-covalent bond force.The cocrystals were mainly constructed based on the interaction between the ammonium radicals in ADN and the oxygen atoms in DAF.The crystal structure was predicted and an ADN/DAF cocrystal with a molar ratio of 1:1 was obtained.Fig.3 was the lattice energy-density prediction diagram of the cocrystal,in which the red point was the point of the selected cocrystal,and Fig.4 was the three-dimensional crystal structure of ADN/DAF cocrystal.
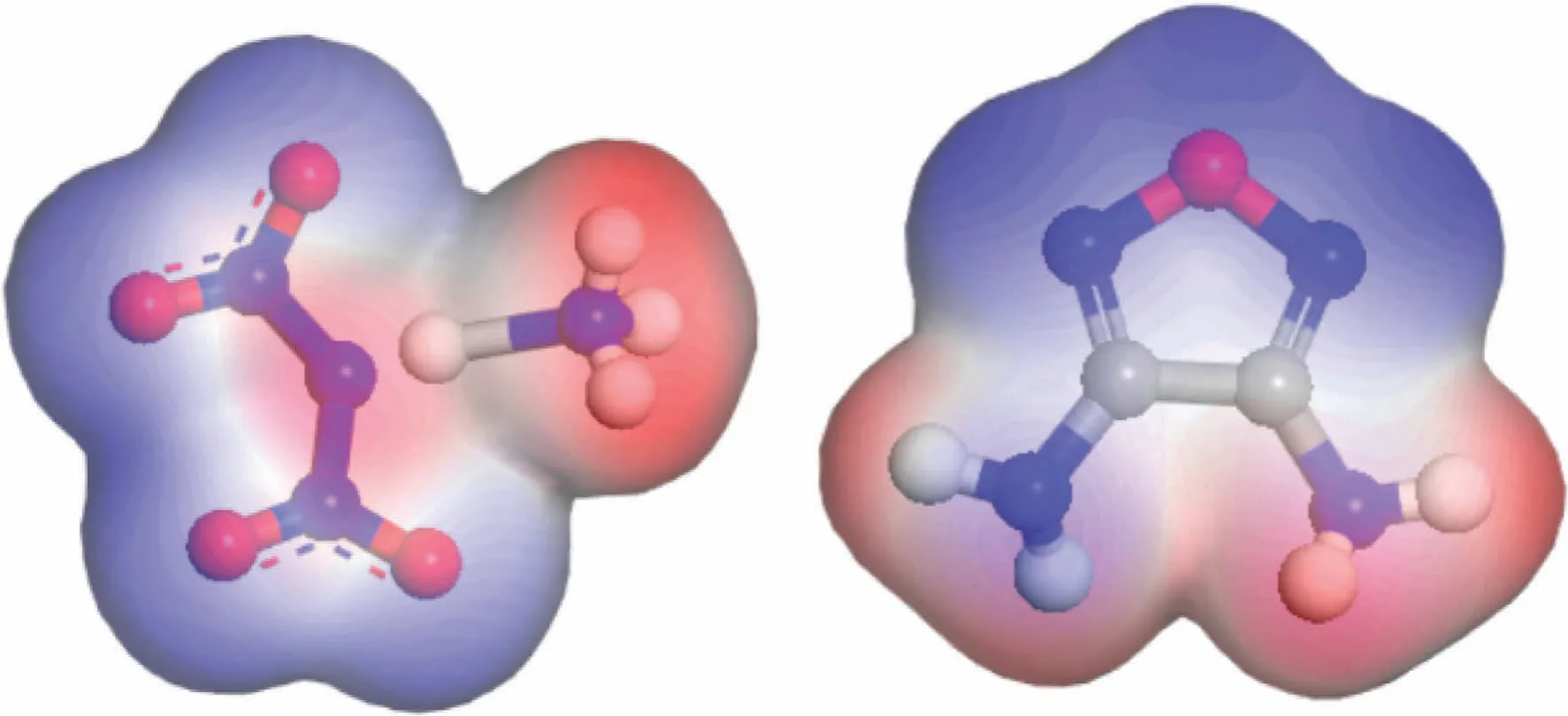
Fig.2.Surface electrostatic potential of ADN and DAF molecules.
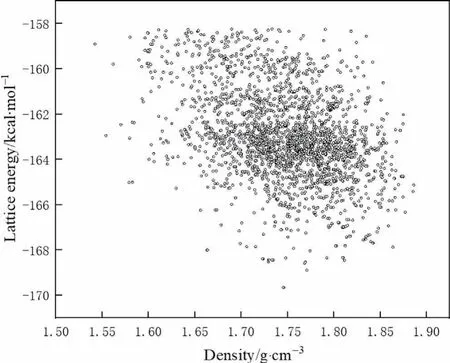
Fig.3.ADN/DAF cocrystal lattice energy and density map (1 kcal·mol-1=4.18 kJ·mol-1).
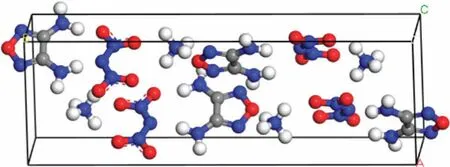
Fig.4.Predicted crystal structure of ADN/DAF co-crystal.
The predicted cocrystal structure parameters were listed in Table 1,mainly including space group,unit cell volume,unit cell parameters,density,hydrogen bond energy,etc.The space group and unit cell parameters reflected the spatial arrangement of molecules in the crystal,and the density and hydrogen bond energy reflected the compactness and interaction energy between molecules.The ADN/DAF cocrystal belonged to the orthorhombic system with a density of 1.75 g·cm-3,indicating that the crystal density decreased slightly after the cocrystal.The reason was that the spatial arrangement of ADN and DAF in the unit cell changed.From the cocrystal density value,DAF had a good application prospect as a cocrystal ligand.The hydrogen bonding interaction of the ADN/DAF cocrystal was larger,which was due to the formation of N-H···O hydrogen bonds between ADN and DAF,which was the main driving force for the cocrystal formation.

Table 1 Predicted crystal structure parameters of ADN/DAF cocrystal
The errors in crystal structure prediction are the applicability of the force field to cocrystal ligands,and there is currently no force field applicable to all substances;the lowest energy point selected is not necessarily the point of cocrystal formation,but the most thermodynamically stable point,which is the point most likely to form a cocrystal.Therefore,there are certain deviations in the crystal structure prediction method.However,due to the difficulty of experimental preparation and determination of single crystals,it is often difficult to obtain or analyze the crystal structure through experimental means.The advantage of the crystal structure prediction method is that it is easier to obtain single crystal date.
4.2.Experimental and computational PXRD spectra
The XRD patterns of ADN,DAF,ADN/DAF cocrystal and cocrystal are presented in Fig.5.The characteristic peaks of ADN with 2θ-values were 14.94°,17.58°,26.96°,27.60°,29.16° and 30.00°,and the characteristic peaks of DAF were 15.18°,17.04°,23.52°,26.76°,28.14° and 30.50°.While the characteristic peaks of ADN/DAF cocrystal were 8.82°,14.80°,16.72°,19.42°,22.88°,27.50°,29.20°,30.82° and 36.88°,and the characteristic peaks of ADN/DAF co-crystal were 15.78°,17.00°,23.52°,26.96°,28.14° and 30.50°.The XRD results indicated that the prepared cocrystal differed obviously from each individual component and was not simple mechanical mixing.The position and intensity of X-ray diffraction peaks are related to the size of the phase and crystal,and the arrangement in the structural unit,etc.The new characteristic peaks and the disappearing characteristic peaks were not the simple superposition of the diffraction peaks of pure ADN and pure DAF,indicating that the ADN/DAF cocrystal was a crystal structure different from pure ADN and pure DAF,which had new lattice parameters.This was mainly because when ADN and DAF formed a cocrystal,the intermolecular hydrogen bonds destroyed the crystal structure and formed a new crystal structure.
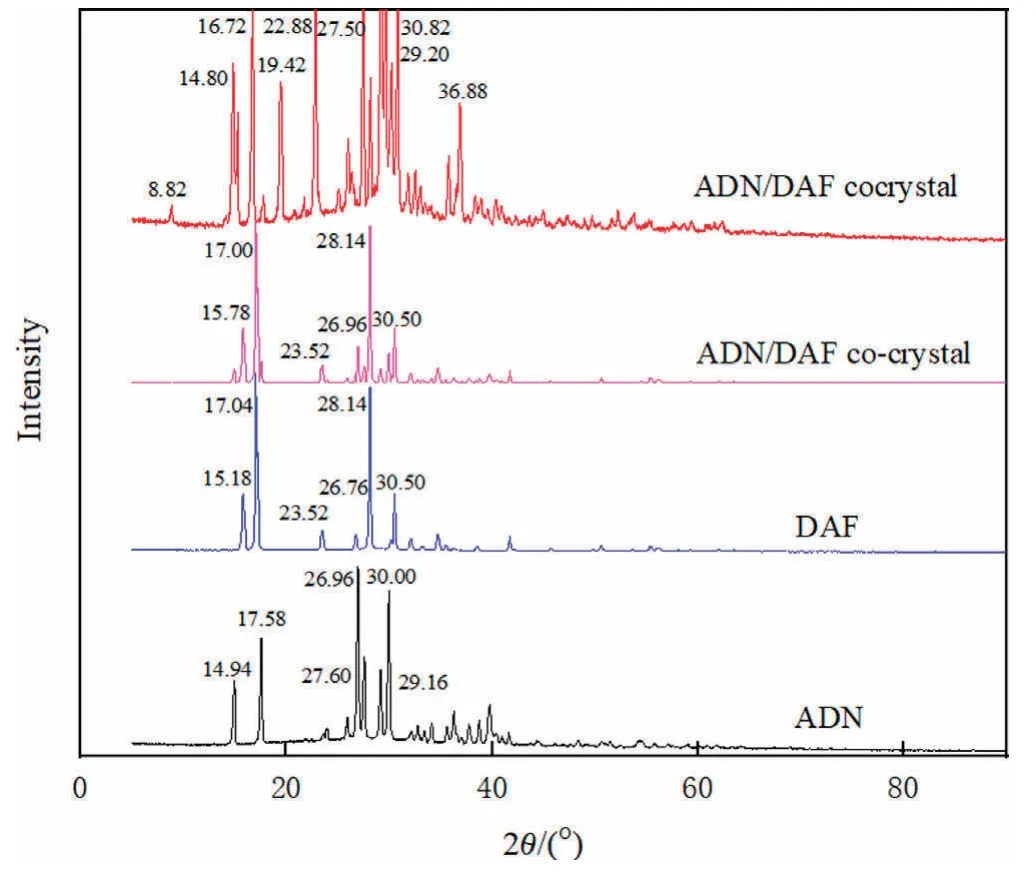
Fig.5.PXRD patterns of ADN,DAF and ADN/DAF cocrystal and co-crystal.
From Reflex module analyzing cocrystal structure,the simulated XRD was obtained for verifying the results of predicted cocrystal structure.Fig.6 was the comparison between the experimental pattern and the simulated pattern of the ADN/DAF cocrystal,which proved that ADN and DAF formed an ADN/DAF cocrystal in the experiment.However,there was a deviation between the experimental XRD pattern and the simulation results,which may be due to the preferred orientation of the crystals.
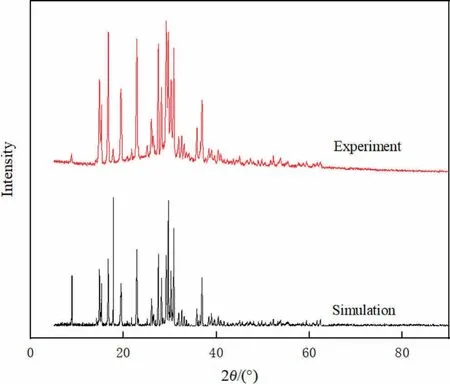
Fig.6.The simulated and experimental PXRD patterns of ADN/DAF cocrystal.
4.3.Experimental and computational crystal morphology
Experimentally prepared ADN crystals usually showed needlelike,lamellate or polyhedral morphology,which is determined by the added conditions of solvents or additives.SEM was used to study the microstructure and morphologies of the cocrystal precursors and cocrystal co-formers,and the results are shown in Fig.7.It can be observed that the morphology of the ADN/DAF cocrystal was obviously different from that of ADN and DAF.The ADN/DAF cocrystal presented cuboid shape,whereas ADN,DAF showed lamellate shape and square shape,respectively.The predicted crystal morphology of ADN/DAF cocrystal was consistent with the observed crystal morphology,which demonstrated accuracy of the crystal structure prediction.The differences in crystal morphology reveals that different microscopic molecular structures will lead to the difference in crystal morphology,and indicates that after the formation of cocrystal,the cocrystal precursors and cocrystal co-formers can be distinguished according to their morphology.
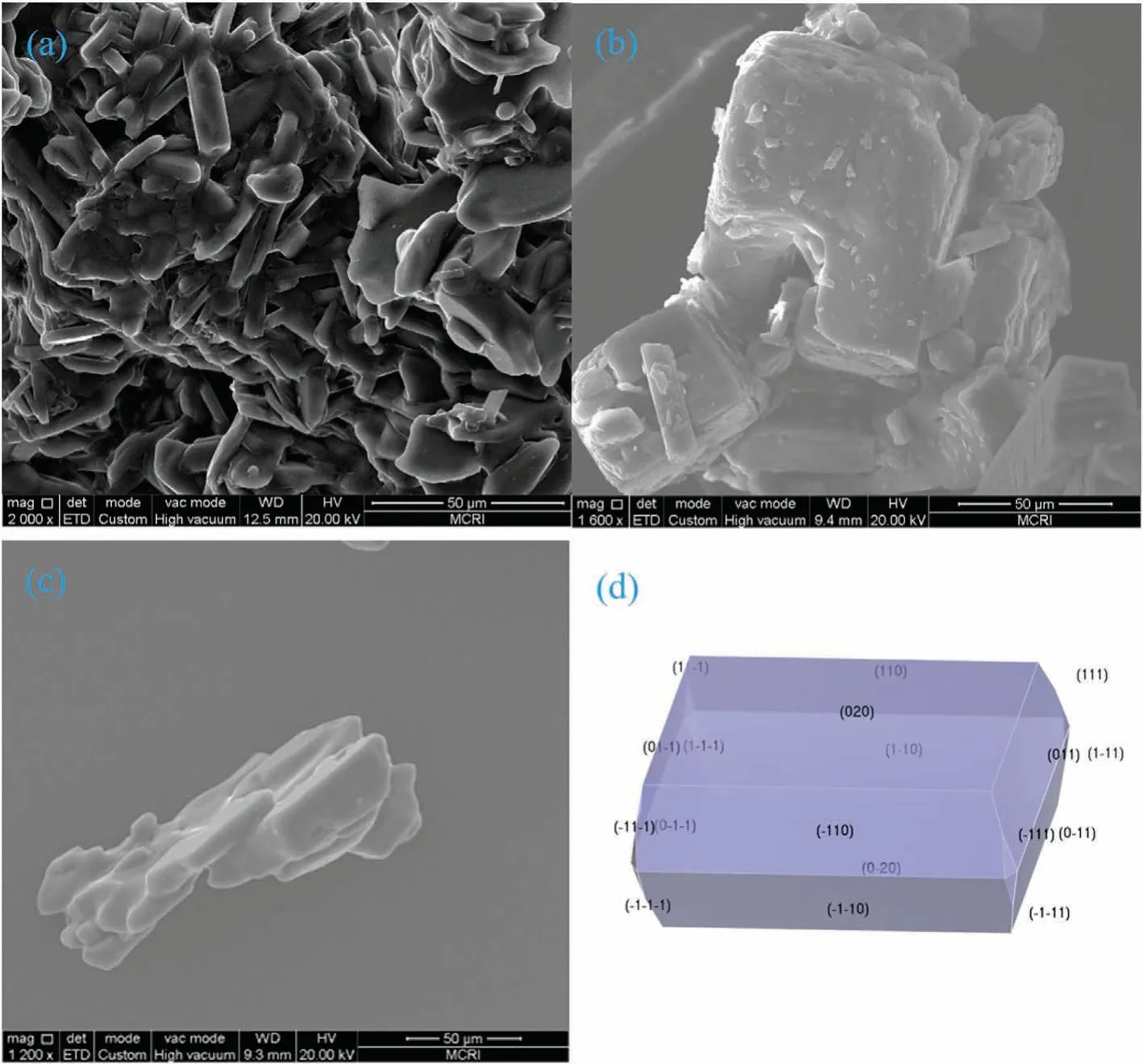
Fig.7.The SEM images of experimental morphologies of ADN crystal (a),DAF crystal (b) and ADN/DAF cocrystal (c),and predicted morphology of ADN/DAF cocrystal (d).
It can be seen from Table 2 that the ADN/DAF cocrystal mainly had four important crystal planes,namely (0 2 0),(1 1 0),(0 1 1)and(0 1 1-),and the crystal planes accounted for a large proportion and the growth rate was slow.

Table 2 Results of ADN/DAF (1:1) crystal morphology by the BFDH method
4.4.Determination of cocrystal formation
4.4.1.Differential scanning calorimetry(DSC)
As shown in Fig.8,the melting points of ADN,DAF and ADN/DAF cocrystal were 88.70,182.96,and 127.12°C,while the decomposition temperatures of ADN,DAF,and ADN/DAF cocrystal were 200.27,259.99,and 205.24°C respectively.After forming a cocrystal,the melting point of the cocrystal was between the two single crystals and it demonstrated a new cocrystal formation changed the melting point and the decomposition temperature.
In the distance I saw some little houses which seemed to be built in a most singular fashion, but as I was by this time very hungry I set out towards them, but before I had walked many steps, I saw that the air was full of shining objects which seemed to be fixed, and yet I could not see what they hung from
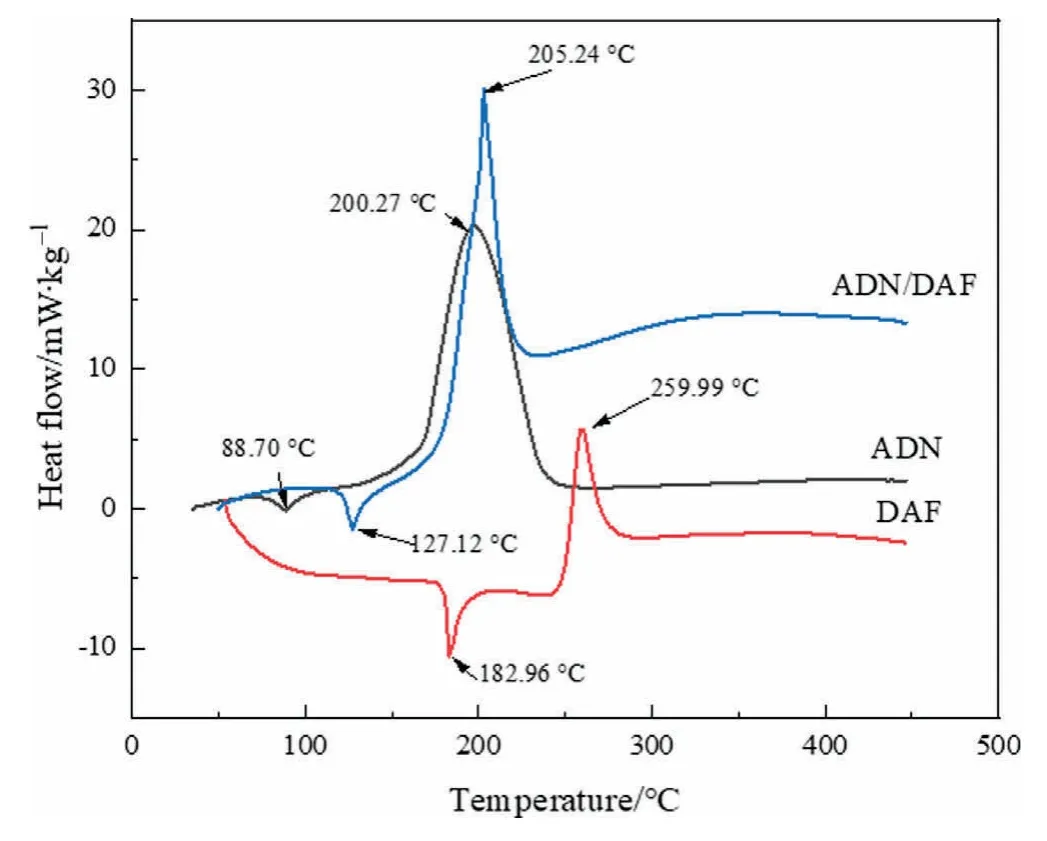
Fig.8.The DSC patterns of ADN,DAF,and ADN/DAF cocrystal.
4.4.2.Infrared radiation(IR)
Infrared spectroscopy can be used to determine whether hydrogen bonds are formed in the molecular structure.Hydrogen bonds are the main driving force for the formation of cocrystals,which are directional and saturated.Compared with covalent bonds,the force of hydrogen bonds is weaker.Therefore,it will not cause the disappearance of the infrared characteristic spectrum of the molecular structure,but will change the position and width of the characteristic peaks,making the characteristic peaks red-shift or blue-shift.
As can be seen from Fig.9,the comparison between the infrared spectra of ADN/DAF cocrystal and raw material ADN and DAF shows that the number and intensity of characteristic absorption peaks have changed after crystallization.In the ADN infrared spectrum,3130 cm-1isvibration absorption peak,while 1538 and 1212 cm-1are the vibration absorption peaks of-NO2.In the ADN/DAF cocrystal infrared spectrum,due to the presence of hydrogen bonds,the peak ofshifts to 3381 cm-1and the peak shape is widened.In addition,the vibration absorption peaks of-NO2shift to 1385 and 1180 cm-1,respectively.This is because the interaction of -NO2···NH2changes the conditions where the -NO2andgroups are located.The results show that there are hydrogen bonds in the ADN/DAF cocrystal structure,indicating that the synthesized product is a cocrystal.
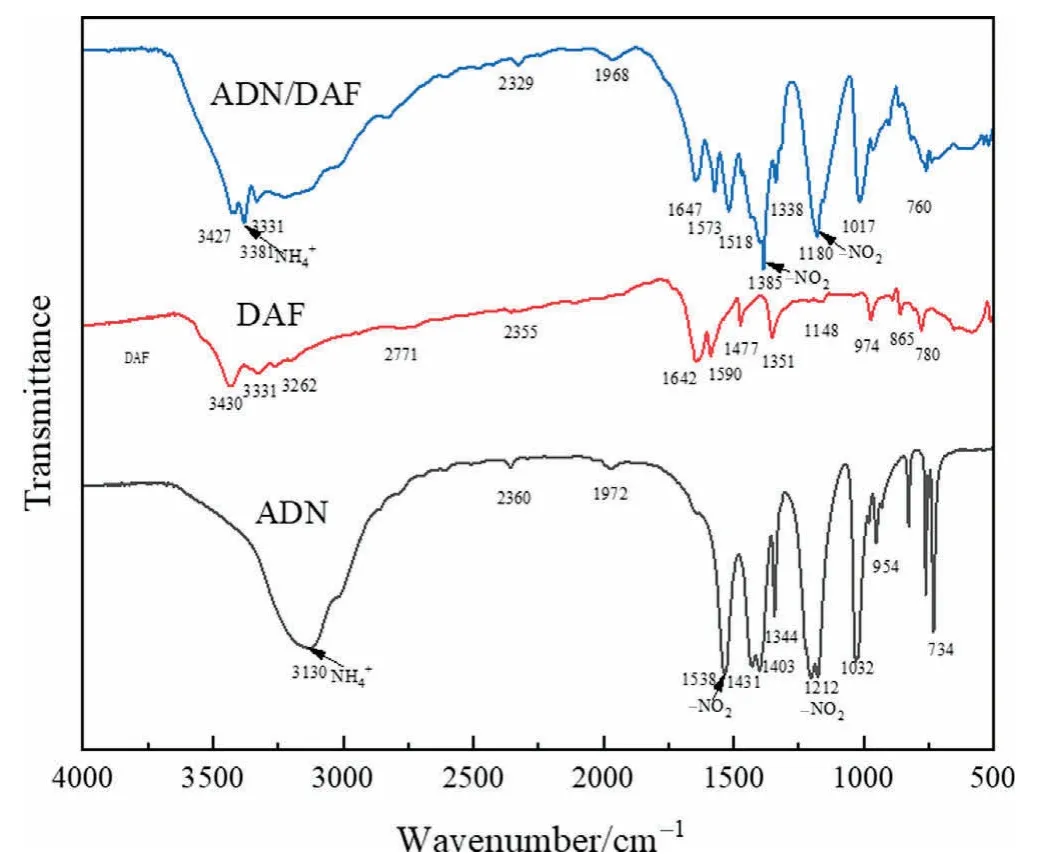
Fig.9.Infrared spectra of ADN,DAF,and ADN/DAF cocrystal.
4.4.3.Raman spectroscopy
As showed in Fig.10,most of the Raman absorption peaks in the raw materials shifted after forming cocrystal.ADN had absorption peaks at 291.50,489.81,742.15,831.03,958.31,1178.18 and 1339.05 cm-1.DAF had absorption peaks at 252.27,292.76,779.44,807.37,1476.81 and 1571.39 cm-1.And after the formation of ADN/DAF cocrystal,absorption peaks appeared at 301.43,477.27,774.38,821.23,967.12 and 1321.12 cm-1.The reason for these shifts may be that the hydrogen bonds formed between the ADN and DAF molecules change the symmetry of the cocrystal structure.
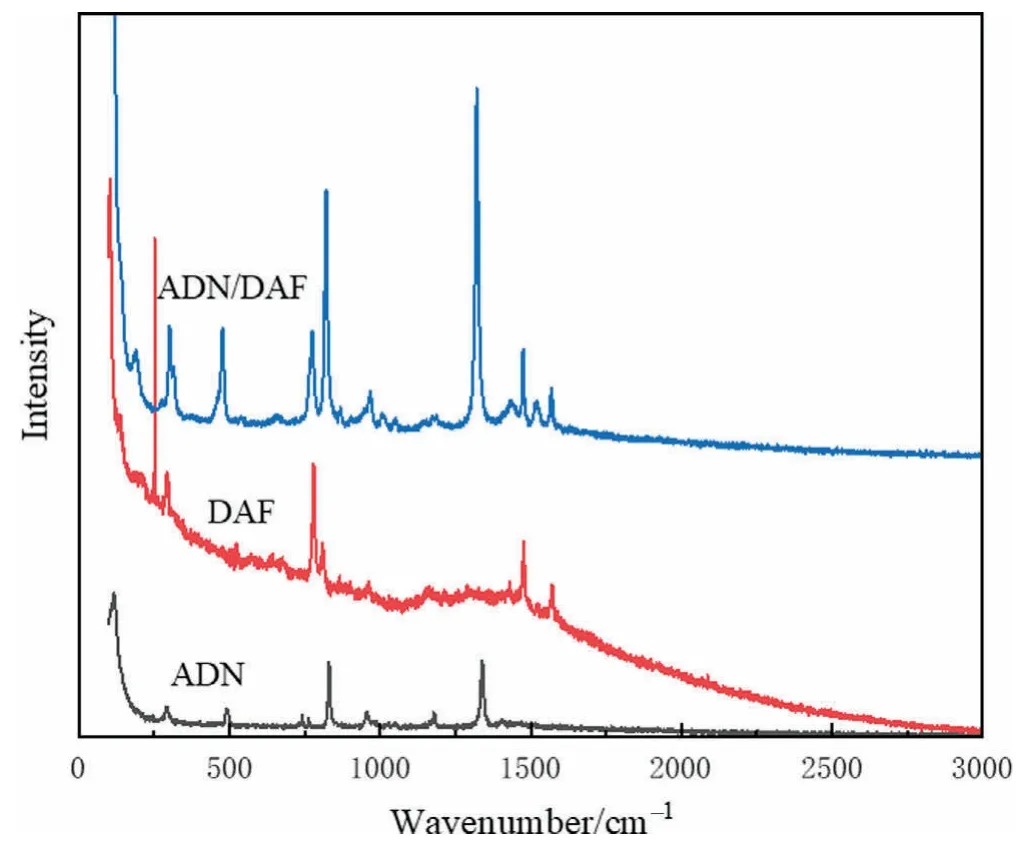
Fig.10.Raman spectra of ADN,DAF,and ADN/DAF cocrystal.
4.5.Water sorption capacity prediction
The hygroscopicity of crystals is mainly determined by the crystal surface properties.As shown in Table 3,the water sorption capacities of the ADN and ADN/DAF important surfaces were predicted.The hygroscopic relationship of the important crystal planes of ADN crystal was(1 1 1-)>(1 1 0)>(0 1 1)>(1 0 0)>(0 2 0),where(0 2 0)and(1 0 0)was the crystal plane with lower water sorption capacity.And the hygroscopic relationship of the important crystal planes of the ADN/DAF cocrystal was(0 1 1)≈(0 1 1-)>(0 2 0)>(1 1 0),(0 2 0)and(1 1 0)crystal planes had lower water sorption capacity.In addition,an important parameter of water adsorption capacity was water adsorption heat.The adsorption heat of water was positive,or the adsorption energy was negative,indicating that the adsorption process of water molecule was an exothermic process.Therefore,it can be seen from Table 3 that both the hygroscopic processes of ADN and cocrystals were exothermic processes.Moreover,the adsorption heat could be used to compare the hygroscopicity capacity of ADN and cocrystals.It can be seen from Table 3 that the values of water adsorption heat of important crystal faces of ADN/DAF cocrystal were lower than those of important crystal faces of ADN.The results showed that co-crystallization could significantly reduce the hygroscopicity of ADN.The water sorption capacity of the ADN/DAF cocrystal was 7.90%.The water sorption capacity of the ADN crystal can be reduced by more than 49% by cocrystallization technology.
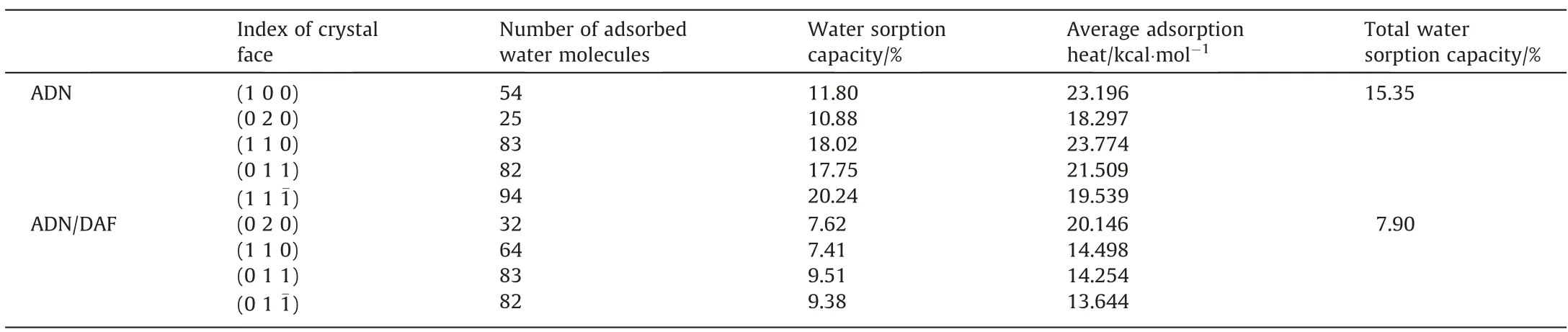
Table 3 Water sorption capacity and adsorption heat on ADN crystal faces and ADN/DAF cocrystal faces (1 kcal·mol-1=4.18 kJ·mol-1)
The main reason for the reduced hygroscopicity of ADN/DAF crystal was that the formation of N-H···O medium-strength hydrogen bonds between the ammonium ion of ADN and the O atom of DAF in the cocrystal,which prevent the binding of water molecules in the air and ammonium ions,reducing the probability of ADN binding to water molecules,and resulting in a reduction in the hygroscopicity of the cocrystal.
5.Conclusions
In this study,a novel energetic cocrystal of ADN/DAF was proposed and successfully synthesized by anti-solvent crystallization method for tuning the hygroscopicity.The formation of cocrystal was verified by tests such as PXRD,DSC,SEM,IR and Raman in experiment.The structure,powder X-ray diffraction,crystal morphology and water sorption capacity of the ADN/DAF cocrystal were predicted by molecular simulation and compared with the experimental results.The results showed that the PXRD and crystal morphology predicted by the simulation were in good agreement with the experimental results.Through the water sorption capacity,it was found that the newly formed cocrystal ADN/DAF had lower hygroscopicity than pure ADN,which reduced the hygroscopicity from 15.35% to 7.90%.The reason for the decrease in hygroscopicity may be the formation of N-H···O mediumstrength hydrogen bonds between the ammonium ion of ADN and the O atom of DAF in the cocrystal,which prevent the combination of water molecules and ammonium ions in the air and reduced the probability of ADN binding with water molecules,resulting in a reduction in the hygroscopicity of the cocrystal.In summary,the newly synthesized ADN/DAF cocrystal is of great significance for the anti-hygroscopicity of ADN crystal,which can effectively reduce the hygroscopicity and serve as potential oxidants for composite solid propellant formulations.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22125802),Beijing Natural Science Foundation(2222017) and National key research and development program(2021YFC2101202).The authors gratefully acknowledge these grants.
 Chinese Journal of Chemical Engineering2023年9期
Chinese Journal of Chemical Engineering2023年9期
- Chinese Journal of Chemical Engineering的其它文章
- Anti-carbon deposition performance of twinned HZSM-5 encapsulated Ru in the toluene alkylation with methanol
- A highly efficient La-modified ZnAl-LDO catalyst and its performance in the synthesis of dimethyl carbonate from methyl carbamate and methanol
- Studies on polyoxymethylene dimethyl ethers production from dimethoxymethane and 1,3,5-trioxane over /ZrO2-TiO2
- Continuous,efficient and safe synthesis of 1-oxa-2-azaspiro[2.5]octane in a microreaction system
- Closed-loop scheduling optimization strategy based on particle swarm optimization with niche technology and soft sensor method of attributes-applied to gasoline blending process
- Loading CuO on the surface of MgO with low-coordination basic O2- sites for effective enhanced CO2 capture and photothermal synergistic catalytic reduction of CO2 to ethanol
