Supercapacitive properties of MnNiSx@Ti3C2Tx MXene positive electrode assisted by functionalized ionic liquid
Pengcheng Hu,Ruimin Chai,Ping Wang,Jinke Yang,Shufeng Zhou
College of Chemical Engineering, Huaqiao University, Xiamen 361021, China
Keywords:Supercapacitor Transition metal sulfide MXene Structure characterization Electrochemical performance
ABSTRACT MnNiSx@Ti3C2Tx as the positive electrode of supercapacitor was successfully prepared by hydrothermal method with the assistance of amino-functionalized ionic liquids.The micromorphological structures of MnNiSx@Ti3C2Tx were analyzed using X-ray diffraction,scanning electron microscope,X-ray photoelectron spectroscopy,transmission electron microscope,and energy dispersive spectrometer to reveal the synergistic effect between MnNiSx and Ti3C2Tx MXene.MnNiSx grew into a three-dimensional coral-like structure on the surface and between layers of Ti3C2Tx nanosheets.This structure alleviated the collapse and stacking of Ti3C2Tx,increased the specific surface area of Ti3C2Tx,and promoted the charges transfer on the surface of Ti3C2Tx.The electrochemical performances of MnNiSx@Ti3C2Tx positive electrode,such as cyclic voltammetry,galvanostatic charge/discharge,and electrochemical impedance spectroscopy,were investigated.The synergistic effect between MnNiSx and Ti3C2Tx MXene improved the specific capacitance and the capacitance retention of the MnNiSx@Ti3C2Tx electrode.An asymmetric solid-state supercapacitor (ASC) assembled using MnNiSx@Ti3C2Tx as cathode material had the power density of 816.34 W·kg-1,and the energy density of 35.11 Wh·kg-1.The capacitance retention of ASC reached 98% after 5000 cycles at a current density of 5 A·g-1.
1.Introduction
A supercapacitor is an energy storage device between capacitors and batteries.It had the characteristics that conventional capacitors can be quickly charged and discharged,as well as the energy storage characteristics of batteries [1-3].It can potentially be applied to the hybrid vehicles,wind and solar power generation,industrial control,and other industries.The electrode materials are important parts of supercapacitors,they are the key factors that determine the electrochemical performance of supercapacitors,and are widely studied [4-6].
MXene is a two-dimensional material with a unique layered structure.It is a very promising electrode material for supercapacitors because of its high electrical conductivity,high volume capacitance,and large specific surface area [7,8].Lukatskayaet al.[9]studied the supercapacitive properties of MXene for the first time.A flexible electrode of sheet-like structure Ti3C2with a specific surface area up to 98 m2·g-1was prepared by filtering and layering Ti3C2Tx.The specific capacitance of the Ti3C2Txelectrode reached 450 F·cm-3at a scan rate of 2 mV·s-1in KOH electrolyte.This value hardly decreased after 10000 cycles at a current density of 1 A·g-1.Its capacitance retention rate reached more than 99%.MXene electrode exhibited good specific capacitance and cycling performance,but layer stacking and collapse inevitably occurred between MXene nanosheets,which increased the electrical resistance,affected its supercapacitive properties,and reduced electrochemical utilization[10].Therefore,MXene electrode needs modification,moreover,it needs to be doped or synergistically compounded with other materials to prevent the stack and collapse of the internal structure,as well as to improve its supercapacitive properties[11].
Transition metal sulfide (TMS) has abundant active sites,good electron transport ability,and easily tunable morphology and structure,these are the reasons for its excellent electrochemical activity [12,13].TMS has become a research hotspot for electrode materials because of its high specific capacitance and cycle stability [14,15].Functional ionic liquids (FILs) are often used as templates to prepare TMS nanomaterials with special morphology,because the tunable physicochemical properties of FILs can influence the initial crystal structure and crystal growth direction of TMS nanoparticles.Salomeet al.[16] successfully prepared NiS nanoparticle with submicron structures by chemical sonication using [Bmim][BF4] as a template.Luoet al.[17] reported the method for the IL-assisted synthesis of Ni3S4@MoS2composite nanoparticles with heterostructures.The specific capacitance of Ni3S4@MoS2reached 985.21 F·g-1at a current density of 1 A·g-1.After 20000 times of charge-discharge cycles,Ni3S4@MoS2still maintained a capacity of 573 F·g-1at a current density of 10 A·g-1.The use of IL-assisted synthesis of TMS nanomaterials with special morphologies was among the mothods that can be used to improve the supercapacitive properties of electrode materials.
In this paper,amine-based IL was used as soft templating agent to prepare transition bimetallic sulfide with special morphology and that contained nickel and manganese.It combined the high specific capacitance of nickel sulfide and the high cycling stability of manganese sulfide.The above-mentioned transition bimetallic sulfide was embedded on the surface and interlayer of conductive carrier Ti3C2TxMXene.The synergistic effect between transition bimetallic sulfides with special morphology and Ti3C2TxMXene was studied to improve the overall electrochemical performance of electrode materials.
2.Experimental
2.1.Materials and instruments
All reagents were used as received without further treatment unless stated otherwise.Nickel acetate (99%),manganese acetate(99%),thiourea (99%),ethanol (99%),polyvinyl alcohol (99%),and potassium hydroxide (99%) were purchased from Sigma-Aldrich(China).Polyvinylidene fluoride (PVDF,99%),acetylene black(99%),activated carbon(99%),and nickel foam(NF,99%)were purchased from Sinopharm Chemical Reagent Company(China).[EDA][PF6] ionic liquid (99%) and Ti3C2TxMXene (99%) were purchased from Beijing Beike New Material Technology Co.,Ltd (China).
2.2.Synthesis and characterization of MnNiSx@Ti3C2Tx
The 20 mg of Ti3C2TxMXene was dissolved in 5 ml of anhydrous ethanol.It was uniformly dispersed by ultrasonic treatment for 10 min,and was used as solution A.The 0.6 mmol of nickel acetate and 0.2 mmol of manganese acetate were dissolved in 10 ml of ultrapure water,magnetic stirring was performed for approximately 30 min until these were completely dissolved.The solution was denoted as solution B.The A and B solutions were mixed and stirred for 30 min.Then,1 mmol of [EDA][PF6] IL and 1 mmol of thiourea were slowly added to the above mixed solution.The mixture was transferred to a 30 ml high-pressure hydrothermal reactor and sealed,and the hydrothermal reactor was placed in a muffle furnace at 180 °C for 12 h.Then,the mixture was cooled to room temperature naturally.The mixture was centrifuged,washed with ethanol and ultrapure water for 5 or 6 times,and vacuum dried at 70°C for 12 h to obtain the MnNiSx@Ti3C2Txproduct.
The morphologies and microstructures of product were performed by using scanning electron microscope (SEM,SU5000) at 30 kV and transmission electron microscope (TEM,FEI Talos F200S) at 100 kV.X-ray diffraction patterns (XRD) of the sample were measured using Cu-Kα radiation(λ=0.15406 nm)on a Smart La instrument.The average specific surface area of the sample was determined with Micromeritics 3 FLEX instrument at 77 K by nitrogen adsorption using the Brunauer-Emmett-Teller (BET) method.XPS analysis was performed using a Sigma Probe spectrometer with a high-performance Al monochromatic source operated at 15 kV.
2.3.Electrochemical measurements
Cyclic voltammetry measurements (CV) and electrochemical impedance spectrum(EIS)were performed with a CHI660E electrochemical workstation.Galvanostatic charge/discharge (GCD) measurements were performed between 0.01 and 3.0 V on the Land BT2000 battery tester.The electrochemical measurements were performed in 3 mol·L-1KOH electrolyte solution by using a three-electrode system at room temperature.For single electrode measurement,the active material MnNiSx@Ti3C2Txserved as a working electrode,saturated calomel Ag/AgCl served as a reference electrode,and platinum sheet electrode (1 cm × 1 cm) served as the counter electrode.For double electrode measurement,MnNiSx@Ti3C2Txserved as a working electrode,commercial activated carbon served as a counter electrode,and PVA-KOH served as the solid electrolyte.The loading mass of MnNiSx@Ti3C2Txon the working electrode was 2 mg·cm-2,and the thickness of the working electrodes was 1 mm.CV and GCD tests were conducted at different scan rates and current densities.
3.Results and Discussion
3.1.Structure characterization
The XRD patterns of Ti3C2TxMXene,MnNiSxnanoparticles and MnNiSx@Ti3C2Txcomposite are shown in Fig.1(a).The main XRD diffraction peaks for every sample were narrow,manifesting good crystallinity[18].The diffraction peak at 60.10°of MnNiSx@Ti3C2Txcorresponded to(1 0 9)crystal face of Ti3C2Tx.The diffraction peaks at 31.36°,38.02°and 50.10°corresponded to the(110),(021)and(211) crystal faces of Ni3S2(JCPDS71-1682),respectively.The diffraction peaks at 27.32° and 60.89° corresponded to (202) and(002) crystal faces of MnS (JCPDS40-1289),respectively.The synthesized transition bimetallic sulfide was in the form of MnS and Ni3S2,and named MnNiSx.The results indicated that the MnNiSx@Ti3C2Txcontained the diffraction peaks of Ti3C2Txand MnNiSx.XPS characterization was used to further confirm the synthesis of MnNiSx@Ti3C2Tx,as shown in Fig.1(b)-(g).Full spectrum of XPS from Fig.1(b) showed that MnNiSx@Ti3C2Txcontained the characteristic peaks of Mn,Ni,S,C,Ti,and O elements.Fig.1(c) showed that the two satellite peaks of Ni 2p were located at 861.1 and 880.1 eV,respectively.The spin-orbit spectrum of Ni 2p split into Ni 2p3/2and Ni 2p1/2at 856.5 eV and 873.9 eV,which corresponded to the divalent Ni2+and trivalent Ni3+,respectively[19,20].Fig.1(d)showed that the 2p orbital of Mn element had two characteristic peaks at 654.1 eV and 641.9 eV corresponding to Mn 2p1/2and Mn 2p3/2of MnS,respectively[21].Fig.1(e)showed that the characteristic peaks at 465.6 and 458.9 eV corresponded to the Ti 2p3/2of Ti—O and Ti 2p1/2of Ti—C bond,respectively,and the characteristic peak at 474.1 eV corresponded to the Ti—F bond [22-24].Fig.1(f) showed that the characteristic peaks at 168.2 and 162.1 eV corresponded to the S 2p3/2and S 2p1/2of the bond between sulfur and metal,respectively.The results of XRD and XPS characterization mutually verified that the MnNiSx@Ti3C2Txcomposite was successfully prepared.
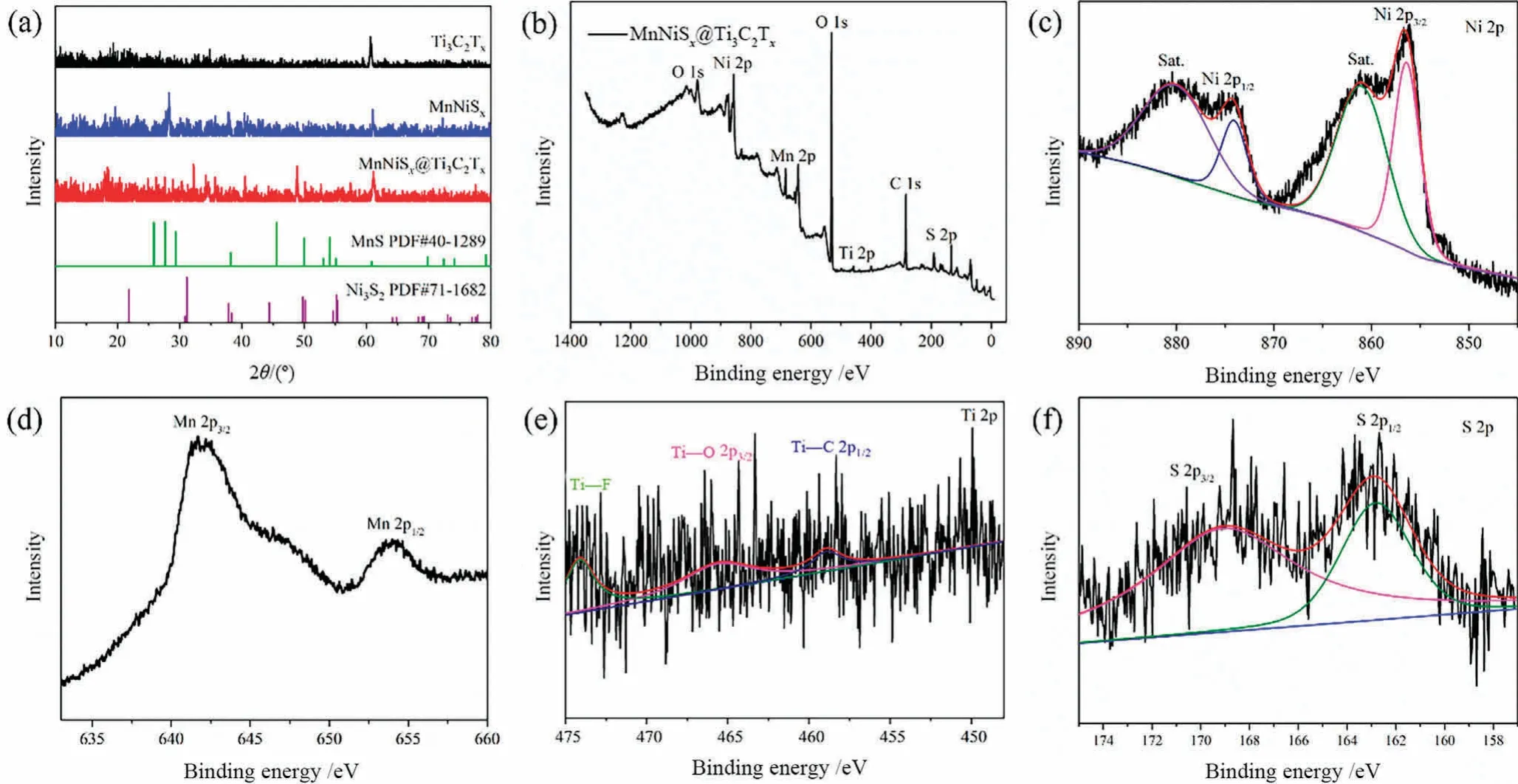
Fig.1.XRD patterns of Ti3C2Tx,MnNiSx,and MnNiSx@Ti3C2Tx (a);XPS spectra of MnNiSx@Ti3C2Tx (b),Ni 2p (c),Mn 2p (d),Ti 2p (e),and S 2p (f).
The SEM images of the Ti3C2Txnanosheets and MnNiSx@Ti3C2Txcomposites were compared and shown in Fig.2(a)-(c).MnNiSx@Ti3C2Txwas a uniformly distributed layered structure.MnNiSx,which has a coral flower-like structure,was heterogeneously nucleated and crystallized on the surface and interlayers of Ti3C2-Txwithout obvious agglomeration.The coral flower-like structure of MnNiSxwas the result of the synergism of Ni2+and Mn2+.Fig.2(d)-(f)were the TEM images of the MnNiSx@Ti3C2Txcomposite at different magnifications,and the coral flower-like structure of MnNiSxwas consistent with the results of SEM analysis.The crystal face diffraction of the selected area electron diffraction pattern from Fig.2(g) corresponded to (111) crystal face of Ti3C2Tx[25],(202) and (002) crystal face of MnS,and (110) crystal face of Ni3S2,respectively [26].HAAD-FSTEM image from Fig.2(h)and element distribution images from Fig.2(i)-(m) showed that the composites contained Ni,Mn,Ti,C,and S elements,and each element was uniformly distributed.These results were consistent with the analysis of XPS.The above results further confirmed that MnNiSxwas uniformly loaded on the surface and interlayers of Ti3C2Tx,and the layered structure of Ti3C2Txwas not damaged.
The specific surface area and pore size distribution of MnNiSx@Ti3C2Txare shown in Fig.3.The specific surface area of MnNiSx@Ti3C2Txwas four and eight times than those of Ti3C2Txand MnNiSx,respectively.The pore size of MnNiSx@Ti3C2Txwas between 3 and 20 nm,which belonged to the mesoporous structure.The pore size and volume of MnNiSx@Ti3C2Txwere also slightly higher than those of single Ti3C2Txand MnNiSx.The synergistic effect between Ti3C2Txand MnNiSxincreased the specific surface area and enriched the pore channels of the composite.
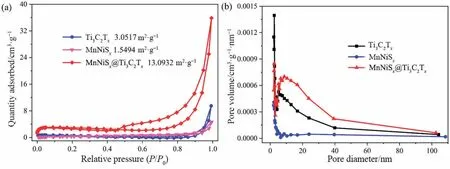
Fig.3.N2 adsorption and desorption isotherms (a) and pore size distributions (b) of Ti3C2Tx,MnNiSx,and MnNiSx@Ti3C2Tx.
3.2.Electrochemical performance analysis
3.2.1.Single electrode measurement
CV curves of Ti3C2Tx,MnNiSx,and MnNiSx@Ti3C2Txat a scan rate of 30 mV·s-1are shown in Fig.4(a).MnNiSx@Ti3C2Txelectrode had the largest area of CV curve,and the area of CV curve was proportional to the specific capacitance.The specific capacitance of MnNiSx@Ti3C2Txelectrode was larger than that of Ti3C2Txand MnNiSx.GCD curves of Ti3C2Tx,MnNiSxand MnNiSx@Ti3C2Txat a current density of 1 A·g-1are shown in Fig.4(b).The broad charge-discharge plateau of MnNiSx@Ti3C2Txelectrode indicated that this was a typical pseudocapacitive behavior,which was consistent with the results from CV curves.MnNiSx@Ti3C2Txelectrode had the longest discharge time and the area of CV curve,indicating that the specific capacitance of MnNiSx@Ti3C2Txwas much higher than those of Ti3C2Txand MnNiSxat the same current density,as shown in Fig.4(c).MnNiSx@Ti3C2Txalso had the largest capacitance retention of 51% when the current density increased from 1 to 10 A·g-1.Nyquist plots of Ti3C2Tx,MnNiSx,and MnNiSx@Ti3C2Txare shown in Fig.4(d).MnNiSx@Ti3C2Txhad the smallest semicircle diameter in the high-frequency region,indicating that MnNiSx@-Ti3C2Txelectrode had the smallest charge transfer resistance (Rct)and the fastest redox reaction rate.The synergistic effect of MnNiSxand Ti3C2Txcould effectively reduce the electrochemical resistance of the composite electrode,and this effect was consistent with its higher specific capacitance [27].
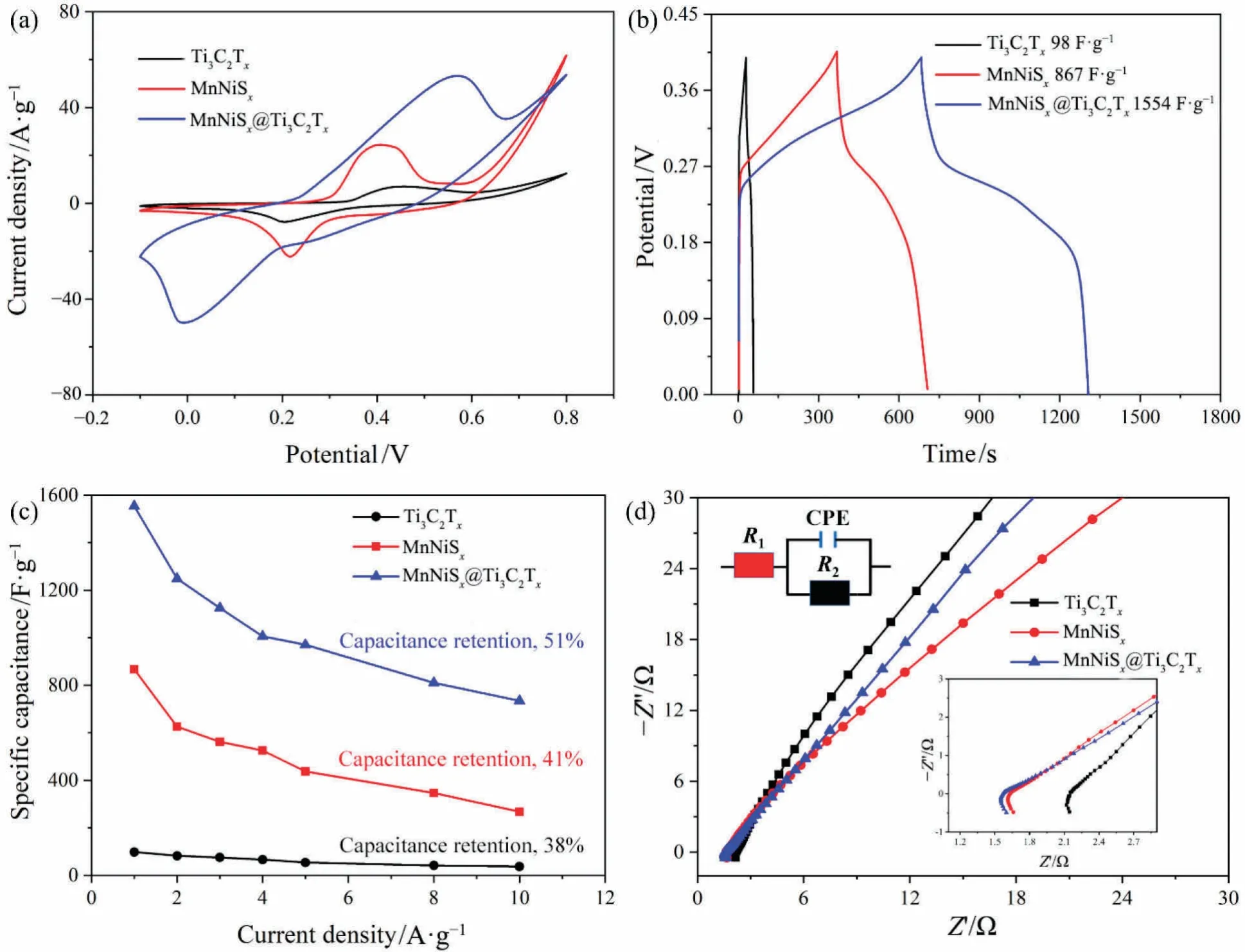
Fig.4.CV curves (a),GCD curves (b),Specific capacitance curves (c),and Nyquist plot (d) of Ti3C2Tx,MnNiSx,and MnNiSx@Ti3C2Tx.
The cycle stability of Ti3C2Tx,MnNiSx,and MnNiSx@Ti3C2Txafter galvanostatic charge-discharge for 5000 cycles is shown in Fig.5.The capacitance retention of MnNiSx@Ti3C2Txelectrode after galvanostatic charge-discharge for 5000 cycles was 99%,which was higher than those of MnNiSx(97%) and Ti3C2Tx(94%).Single MnNiSxexhibited a large fluctuation during the continuous charge-discharge process because of the hindered charge transport.Thus,the cycle stability of MnNiSxwas poor.The good cycle stability of MnNiSx@Ti3C2Txelectrode was attributed to its excellent charge transport ability.
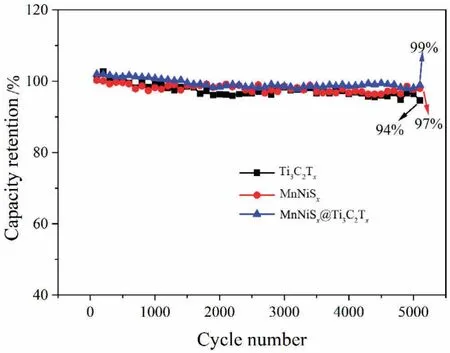
Fig.5.Cycle stability of Ti3C2Tx,MnNiSx,and MnNiSx@Ti3C2Tx after galvanostatic charge-discharge for 5000 cycles at a current density of 5 A·g-1.
Fig.6(a) shows that the CV curve shapes of MnNiSx@Ti3C2Txmaintained a high degree of similarity at high scan rates,indicating that the MnNiSx@Ti3C2Txelectrode had good cycle stability.Fig.6(b) showed that the specific capacitance of MnNiSx@Ti3C2Txelectrode was as high as 1554 F·g-1at a current density of 1 A·g-1.MnNiSxcontributed to the high specific capacitance,and Ti3C2Txcontributed to the excellent cycling stability [28,29].
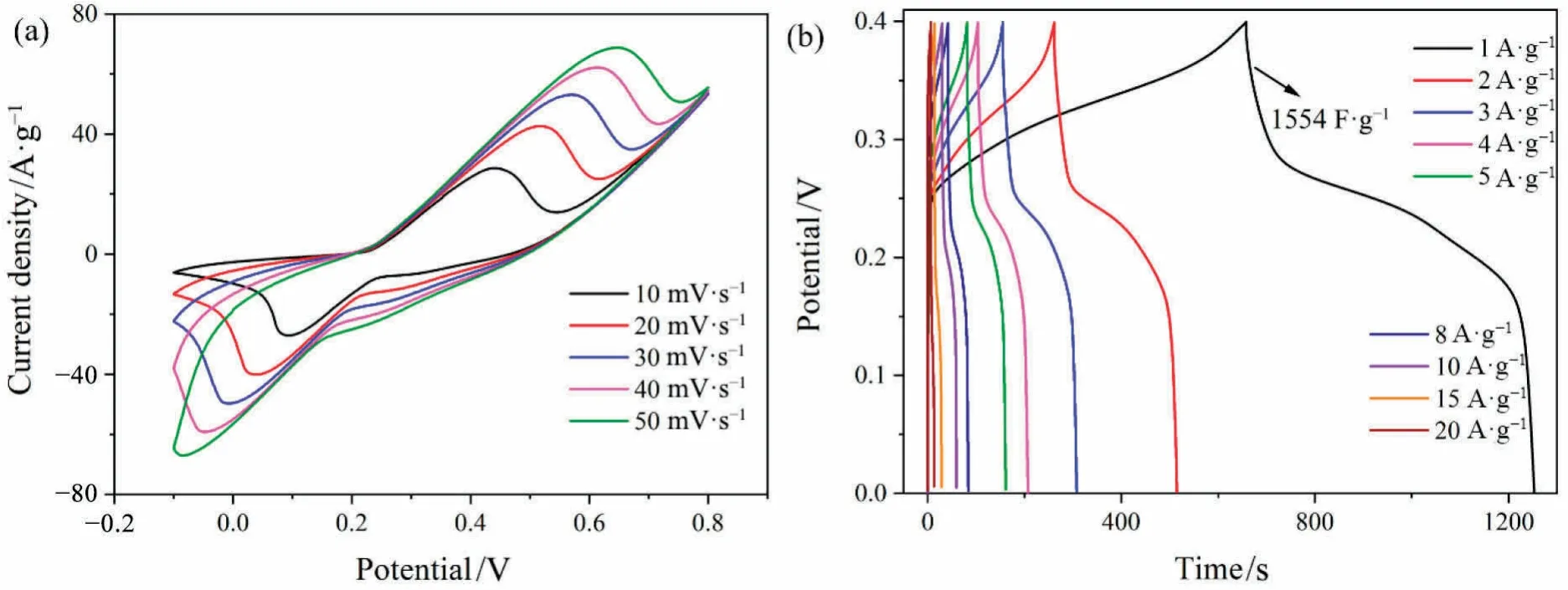
Fig.6.CV curve (a) and GCD curve (b) of MnNiSx@Ti3C2Tx.
3.2.2.Double electrode measurement
To further evaluate the application potential of MnNiSx@Ti3C2Txpositive electrode materials in supercapacitors,asymmetric solidstate supercapacitor (ASC) was assembled with MnNiSx@Ti3C2Txas the positive electrode,activated carbon (AC) as the negative electrode,and PVA-KOH as the solid electrolyte.The electrochemical performance of the assembled solid-state ASC devices was tested.CV curves of the positive and negative electrode at a scan speed of 20 mV·s-1are shown in Fig.7(a).The voltage windows of the positive and negative electrode were 0-0.6 and -1.0-0 V,respectively.Their combination provided a wide voltage window for ASC devices.CV curves of ASC devices at different voltages are shown in Fig.7(b).The oxidation peak of CV curves at 1.7 V began to show a slight polarization,which indicated that the potential voltage window of ASC devices did not exceed 1.7 V.Fig.7(c) showed the GCD curves of the ASC device under different voltage windows at 1 A·g-1.The specific capacitance of ASC device increased gradually with increasing the voltage window.GCD curves were also symmetrical,indicating that ASC device had good electrochemical reversibility.Fig.7(d)showed that the shape of CV curves did not change with increasing scan rate,and ASC device had efficient charge-discharge performance and good matching of positive and negative electrodes.Fig.7(e)showed that the specific capacitance of ASC device reached as high as 98.74 F·g-1at a current density of 1 A·g-1,and this value decreased with increasing current density.MnNiSx@Ti3C2Tx//AC had higher specific capacitance than Ti3C2Tx//AC and MnNiSx//AC at the same current density.The capacitance retention of ASC device still reached 70.78%at the high current density of 10 A·g-1,as shown in Fig.7(f).The above results indicated that ASC device had good rate chargedischarge performance.

Fig.7.CV curves of the negative and positive electrode(a);CV curves of the ASC under different voltage windows(b);CV curves of the ASC under different scan rates(c);GCD curve of the ASC under different voltage windows (d);GCD curve (e) and specific capacitance (f) of the ASC under different current densities.
Fig.8(a) showed the ragone diagram under different current densities.The power density of ASC device at 1 A·g-1reached as high as 816.34 W·kg-1,and the corresponding energy density also reached as high as 35.11 W·h·kg-1,which was attributed to the high specific capacitance of 98.74 F·g-1and the wide voltage window of 1.6 V of the ASC device.Fig.8(b) showed that the energy and power densities of ASC device were higher than those of transition metal sulfide or transition metal oxide reported in literature,such as NiO/NiS@CNT//AC (800 W·kg-1,30 W·h·kg-1) [30],Ni-Mn sulfides//RGO (775 W·kg-1,35 W·h·kg-1) [31],and NiCo2S4@Ni(OH)2@ppy//AC (120 W·kg-1,34 W·h·kg-1) [32].Fig.8(c) showed that the capacity retention and coulombic efficiency of ASC device reached 98% after 5000 cycles at a current density of 5 A·g-1,indicating that the ASC device had good rate charge-discharge performance and reversibility.Two solid-state ASC devices in series could successfully light a red LED with a voltage of 2.2 V and a current of 20 mA,and the brightness could last for more than 5 min.The output voltage of 3.2 V can be achieved by connected two ASCs in series,which was enough to power LED,as shown in Fig.9.This finding showed that the MnNiSx@Ti3C2Txelectrode developed in this paper had certain potential application to practical energy storage.
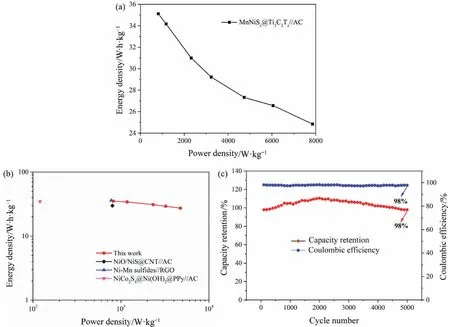
Fig.8.Ragone diagram of the ASC (a);Ragone plots of the ASC in comparison with references (b);Cycle stability (c) of ASC after galvanostatic charge-discharge for 5000 cycles at a current density of 5 A·g-1.

Fig.9.Diagrams of two ASC devices in series lighting up a light-emitting diode (2.2 V,20 mA).
4.Conclusions
MnNiSx@Ti3C2Txelectrode was successfully prepared by hydrothermal method with the assistance of a functionalized ionic liquid.This electrode was formed by thein situgrowth of MnNiSxnanoparticle with unique coral-flower-like structures on the surface and interlayers of Ti3C2Tx.Compared with the single MnNiSxand Ti3C2Tx,MnNiSx@Ti3C2Txhad a large specific surface area and more abundant mesoporous structure,which provided more active sites for metal ions,reduced the resistance of the electrode,and was conducive to the charge transport.The synergistic effect between MnNiSxand Ti3C2Txwas beneficial to the improvement of MnNiSx@Ti3C2Txin electrochemical performance.MnNiSxcontributed to the high specific capacitance,and Ti3C2Txcontributed to the excellent cycle stability.The specific capacitance of MnNiSx@Ti3C2Txelectrode reached 1554 F·g-1at a current density of 1 A·g-1,and the capacitance retention reached 99%.The solid state ASC device assembled with MnNiSx@Ti3C2Txas the positive electrode and activated carbon as the negative electrode achieved a power density of 816.34 W·h·kg-1and an energy density of 35.11 W·h·kg-1at a current density of 1 A·g-1.Two ASC devices in series could easily light up the LED for more than 5 min.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge the financial support of the Scientific Research Funds of Huaqiao University (605-50Y17073),Xiamen,China.
 Chinese Journal of Chemical Engineering2023年9期
Chinese Journal of Chemical Engineering2023年9期
- Chinese Journal of Chemical Engineering的其它文章
- Anti-carbon deposition performance of twinned HZSM-5 encapsulated Ru in the toluene alkylation with methanol
- A highly efficient La-modified ZnAl-LDO catalyst and its performance in the synthesis of dimethyl carbonate from methyl carbamate and methanol
- Studies on polyoxymethylene dimethyl ethers production from dimethoxymethane and 1,3,5-trioxane over /ZrO2-TiO2
- Continuous,efficient and safe synthesis of 1-oxa-2-azaspiro[2.5]octane in a microreaction system
- Closed-loop scheduling optimization strategy based on particle swarm optimization with niche technology and soft sensor method of attributes-applied to gasoline blending process
- Loading CuO on the surface of MgO with low-coordination basic O2- sites for effective enhanced CO2 capture and photothermal synergistic catalytic reduction of CO2 to ethanol
