Measles, mumps, and rubella revaccination in children after completion of chemotherapy and hematopoietic stem cell transplantation: a single-center prospective efficacy and safety analysis
Yin Wan · Qin uan · PeMn-Fei Den · Yi Fei · Hua Zhan · Fen Zhou · Wen-Juan Chen · Qin Cao ·Jin Chen · Yi-Jin Gao
Keywords Adjuvant · Chemotherapy · Children · Hematopoietic stem cell transplantation · Immunogenicity · Mumps–measles–rubella · Vaccine
tInroduction
Children with malignant and nonmalignant hematologic or inborn errors of immunity (IEI) need to undergo chemotherapy, HSCT, or a combination of both, which greatly enhances overall survival rates [1– 4].However, these therapies or the diseases themselves may lead to prolonged immunosuppression and the loss of seroprotection, increasing the risk of infectious diseases [5– 8].
MMR are infectious diseases that can cause severe symptoms, long-term complications, and death in childhood.Fortunately, the advent of an MMR combined attenuated live vaccine has greatly reduced the incidence of MMR infection [9– 12].To address the immune system complications of chemotherapy and HSCT, MMR revaccination after completion of therapy is necessary.Previous studies have indicated a loss of seroprotection after chemotherapy and HSCT [13– 17].However, the results vary widely due to factors such as the primary disease, vaccination status before therapy, and timing of revaccination.
In the present study, subjects were enrolled for antibody level testing after chemotherapy and HSCT to evaluate the impact of primary disease treatments on immunogenicity to MMR.Then, the patients received a single dose of MMR revaccination.To evaluate the immune response and persistence, at 1, 6, and 12 months after revaccination, antibody levels were tested again.
Methods
Patients and clinical data
A total of 110 children who visited the vaccination clinic of Shanghai Children’s Medical Center between March 2017 and July 2019 were enrolled in this study.All patients had completed chemotherapy, HSCT, or a combination of both.The inclusion criteria were as follows: patients had been diagnosed in our center, the primary disease maintained continuous remission, at least 1 year after f inishing chemotherapy or stopping immunosuppression, serum immunoglobulin levels and T-cell subsets were restored to the normal range.The exclusion criteria were as follows: patients had used intravenous immunoglobulin in the last 6 months,had active graft versus host disease (GVHD), or had severe adverse reactions occurring after previous vaccinations,allergy to vaccine ingredients, or adjuvants.The study was approved by the Ethics Committee of Shanghai Children’s Medical Center (SCMCIRB-K2017028) and registered at www.clini caltr ials.gov (NCT03373656).Informed consent was obtained from the parent or legal guardian of the participants (and themselves in the case of children over 8).
Methods
The sex, age, type of primary disease, and therapeutic schedule of all participants were collected using the hospital information system.History of immunization was obtained from vaccination record books provided by the participants’parents.
Children who matched the inclusion and exclusion criteria were recruited.All participants received a single dose of MMR vaccine (Beijing Institute of Biological Products Co.LTD), except for one who received a dose of leprosy vaccine (Beijing Institute of Biological Products Co.LTD).Two weeks after revaccination, adverse reactions were recorded by the researchers communicating with parents through telephone calls or WeChat group messages.There were four follow-up points for antibody titer detection: before revaccination (baseline) and 1, 6, and 12 months after revaccination.Peripheral venous blood (3 mL) treated with EDTA was collected from the enrolled children at each follow-up point, and plasma was separated for antibody titer analysis by ELISA (Virion-Serion Biotechnology).In our experimental protocol, antibody titers higher than 200 mIU/mL,100 U/mL and 20 IU/mL were def ined as positive for measles, mumps, and rubella, respectively.
Statistical analysis
SPSS version 22 (IBM Corp., Armonk, New York, USA)was used for the statistical analyses.Pearson’s chi-square tests were used to analyze the differences in clinical characteristics between negative and positive antibody titers after primary disease treatment.The titer changes before and after vaccination were analyzed using one-way analysis of variance (ANOVA).Univariable analyses were performed to determine the impact of different factors on the immune response.For multivariable analysis, logistic regression was used, which included all factors that had a signif icant impact on vaccination response in the univariate analysis.Categorical variables are described as numbers and relative frequencies (%), and continuous variables are summarized as averages and medians.Statistical signif icance was set atP< 0.05.
Results
Clinical characteristics
After screening using the inclusion and exclusion criteria,133 children were included in the present study.However,23 children were eliminated, because 15 had no MMR vaccination history before primary disease treatment and eight could not provide a clear vaccination record.Therefore, 110 children were successfully included in the statistical analysis of negative conversion rates before revaccination and relative factors (Fig.1).Among them, 66 (60%) were male and 44 (40%) were female, with a median age at diagnosis of 4.7(range 0.8–14.4) years and a median age at revaccination of 8.1 (range 2.8–21.5) years.
Primary diseases of the participants included both malignant (61.8%) and nonmalignant (38.2%) diseases.The malignant group included 12 patients with acute lymphoblastic leukemia (ALL), 15 with acute myeloid leukemia (AML), 3 with chronic myelogenous leukemia (CML), 4 with myelodysplastic syndrome (MDS), 1 with juvenile monocytic leukemia (JMML), 14 with lymphoma, 4 with neuroblastoma(NB), 3 with hepatoblastoma (HB), 3 with Wilms’ tumor(WT), 7 with rhabdomyosarcoma (RMS), 1 with germinoma,and 1 with Langerhans cell histiocytosis (LCH).The nonmalignant group included 37 patients with aplastic anemia(AA), 1 with congenital thrombocytopenia and 4 with mucopolysaccharide storage syndrome (MPS).There were three therapeutic schedules for primary diseases: chemotherapy,HSCT, or a combination of both, including 48 (43.6%), 42(38.2%), and 20 (18.2%) participants, respectively.Throughout the course of treatment, 33 (30%) patients were treated with radiotherapy, and 77 (70%) were not (Table 1).
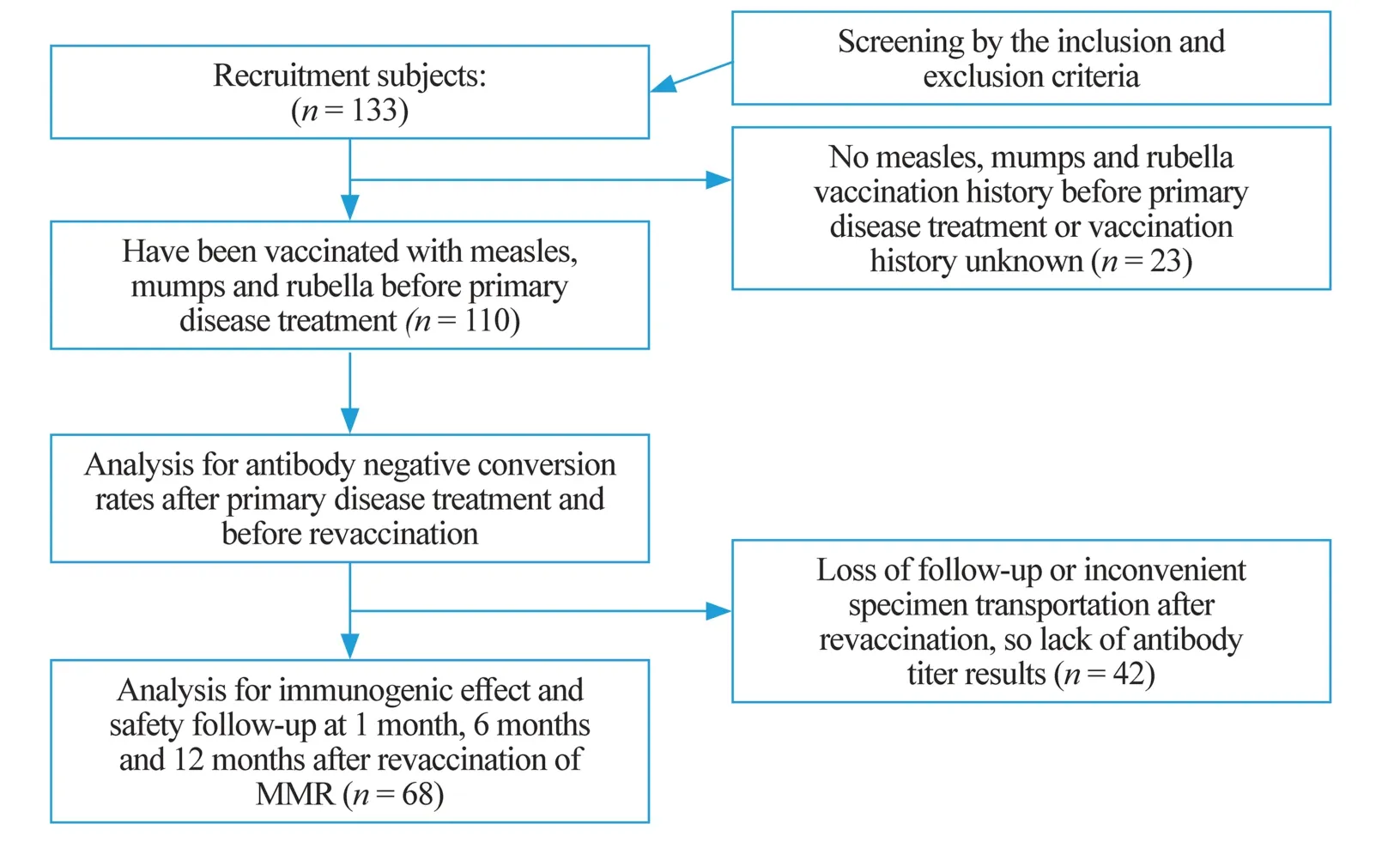
Fig.1 The procedure of participant recruitment and implementation

Table 1 Clinical characteristics of all 110 participants
Rates of lack of protective anti-MMR titer after completing primary disease treatment and before revaccination
Data on negative or positive protective serum antibodies in patients who had undergone a full treatment course are presented in Table 2.The negative rates of protective antibody titers for MMR after therapy were 63.6% (70/110), 75.5%(83/110), and 75.5% (83/110), respectively.
Measles
Antibody titer detection showed that 70 children were seronegative after completing the entire therapy course and before revaccination.Among them, 47 (67.1%) were male and 23 (32.9%) were female.Forty-eight patients(68.6%) were under 7 years old, and 22 (31.4%) were 7 years and older.Thirty-f ive patients (50.0%) had nonmalignant disease and were treated with HSCT, the therapeutic schedule of the present study.Nineteen patients(27.1%) received chemotherapy, and 16 patients (22.9%)received both chemotherapy and HSCT.Pearson chi-square tests showed that sex (χ 2= 4.092,P= 0.043), the type of disease (χ 2= 14.463,P= 0.001) and therapeutic schedule(χ 2= 21.357,P< 0.001) signif icantly affected the measles antibody titer negative rate after the entire therapeutic course for the primary disease.Multivariable analysis for the three factors was performed using logistic regression,which showed that only the therapeutic schedule correlated with the seronegative rate for measles after primary disease treatment (P= 0.008).

Table 2 Antibodies for MMR after treatment and univariate analysis of risk factors
Mumps
Antibody titer detection showed that 83 children were seronegative after completing the entire therapy course and before revaccination.Among them, 52 (62.7%) were male, and 31(37.3%) were female.Fifty-six (67.5%) were under 7 years,and the others were 7 years or older.Forty-nine (59.0%)had malignant diseases, and the others were diagnosed with nonmalignant diseases.Thirty-one patients (37.3%) received chemotherapy, 34 (41.0%) received HSCT only, and 18(21.7%) received both chemotherapy and HSCT.Pearson chi-square tests showed that only the therapeutic schedule(χ 2= 9.14,P= 0.01) signif icantly affected the anti-mumps titer negative conversion rate after the entire therapeutic course for primary disease.
Rubella
Antibody titer detection revealed 83 seronegative participants after completing the entire therapy course and before revaccination.Among them, 61 (73.5%) were male, and 22 (26.5%) were female.Fifty-three (63.9%) were under 7 years, and the others were 7 years or older.Forty-nine patients (59.0%) had malignant diseases, and 34 (41.0%)were diagnosed with nonmalignant diseases.Thirty-two patients (38.6%) received only chemotherapy, 34 (41.0%)received HSCT only, and 17 (20.5%) received both chemotherapy and HSCT.Correlations between the negative antibody rate and sex, age, type of disease, therapeutic schedule and radiotherapy were analyzed and tested by the Pearson chi-square test in SPSS, which showed that only sex was an important factor.
Adverse reactions after revaccination of MMR
Only one participant reported pain at the injection site in the evening following MMR revaccination, and the symptoms spontaneously improved the next day.The rate of adverse reactions was 1/110 (0.9%).rates and the type of disease, therapeutic schedule and revaccination timing showed no statistical signif icance (Table 3).
Immunogenic effect after revaccination of MMR
Sixty-eight patients’ antibody titers were analyzed at given follow-up points.The results are demonstrated in Fig.2.
Measles
The mean measles antibody titers were 254.4 ± 67.46 mIU/mL, 1471.0 ± 210.0 mIU/mL, 1872.0 ± 262.1 mIU/mL, and 1710.0 ± 238.6 mIU/mL at baseline and 1, 6, and 12 months after revaccination, respectively.The differences between baseline and the other three follow-up points were statistically signif icant (P< 0.001).It demonstrated an upward trend from baseline to 6 months and a downward trend thereafter, up to 12 months.However, no signif icant difference was observed between the 1-, 6-, and 12-month follow-up points.
Mumps and rubella
The mean mumps antibody titers were 130.00 ± 35.63 U/mL, 346.10 ± 69.23 U/mL, 456.60 ± 88.03 U/mL,426.50 ± 78.53 U/mL at the four stated follow-up points,respectively.The mean rubella antibody titer was 24.23 ± 5.30 IU/mL, 54.7 ± 8.88 IU/mL, 74.82 ± 9.80 IU/mL, and 64.87 ± 9.26 IU/mL at the four stated follow-up points.Similar results were obtained for mumps and rubella.Compared to baseline, the mumps and rubella antibody titers were signif icantly higher at 1 month (P< 0.05 and 0.01, respectively), 6 months (P< 0.001), and 12 months(P< 0.01).The differences between the 1-, 6-, and 12-month follow-up points were not statistically signif icant, although there was an upward trend from baseline to 6 months and a subsequent pronounced downtrend to the 12-month point,although it was still higher than the 1-month point.
Positive seroconversion after revaccination of MMR
Due to loss of follow-up or inconvenient specimen transportation, 42 out of 110 participants missed all antibody titre results after revaccination.Therefore, the antibody titer levels of the remaining 68 patients at given follow-up points were included for statistical analysis (Fig.1).There were 40,42, and 44 patients whose antibody titers for MMR were negative before revaccination.After revaccination, the positive seroconversion rates were 39/40 (97.5%), 34/42 (81.0%),and 41/44 (93.2%) for measles, mumps, and rubella, respectively.The correlation between the positive seroconversion
Discussion
In the present study, the impact of chemotherapy and HSCT on the antibody titer of the MMR vaccine was demonstrated, as well as the positive seroconversion rates after revaccination.The follow-up point was extended to 1 year after revaccination, so the persistence of antibodies to MMR was also assessed.
Because pretherapy titers were not uniformly obtained,we were not able to assess rates of loss of protectiveantibody titers, which was instead by rates of lack of protective antibody titers.After completion of chemotherapy,HSCT, or combination therapy, the rates of lack of protective antibody titers for MMR were 63.6%, 75.5%, and 75.5%, respectively, indicating that only 36.4%, 25.5%,and 25.5% of the participants remained seropositive after the primary disease treatments.However, a study of over 500 healthy children in Zhejiang Province, China, reported that 3 years after MMR vaccination, the seropositivity rates for measles, mumps, and rubella were 98.1%, 93.4%,and 88.1%, respectively [18].Researchers have conf irmed that the lack of protective antibody titers after therapy is frequent, even if the percentage varies depending on the study, ranging from 25% to 88% for measles [8, 13,16, 19– 24], 26% to 88% for mumps [8, 13, 16, 21– 24],and 19% to 88% for rubella [8, 13, 16, 21– 24].Moreover,we found that in comparison, HSCT was associated with a higher rate of seronegativity for measles than chemotherapy (multivariate analysis,P= 0.008), which may be that HSCT is more intensive than chemotherapy alone.Previous studies have shown that patients with higher treatment intensity have a signif icant loss of preserved immunity ref lected by lower specif ic antibody levels [24,25].It is verif ied again that these therapies did lead to loss of protection for infectious diseases.In addition, univariable analysis results revealed that sex affected the seronegative rate of measles and rubella.We found that 62.1%of the male patients had received HSCT, while in female patients, the percentage was only 47.7%.This may explain why sex was analyzed as a factor impacting the seronegative rate.However, previous studies did not provide similar conclusions.Sample size should be increased in future studies to reduce statistical bias.

Table 3 Rates of positive seroconversion in protective anti-MMR titers after revaccination and risk factors analysis
Our research yielded comparatively ideal positive seroconversion rates after single-dose MMR revaccination, consistent with 90%–95% seroprotection levels in immunocompetent populations.In patients who received chemotherapy, HSCT, or a combination of both, the results ranged between 45.7% and 100% for measles [8, 19, 20,23, 26– 32], 33.3% and 87.5% for mumps [8, 23, 26– 30],and 66.9% and 100% for rubella [8, 23, 26– 32] (Table 4).The earliest revaccination timings were 12.9 months after completion of chemotherapy, 13.4 months after stopping immunosuppressants, and 23.6 months after HSCT in the present study.Immunoglobulin and lymphocyte subsets were quantitatively analyzed for each participant before revaccination.Revaccination was allowed only if the quantitative results reached normal levels or at least 80%.This may explain why we obtained high positive seroconversion rates similar to those in healthy children and higher than those in many other studies.Therefore, to ensure high efficiency, we suggest immunologic detection, which is usually represented by immunoglobulin and lymphocyte subset levels, before revaccination.Of course, we are looking forward to the establishment of more scientif ic and accurate methods of immune function detection.
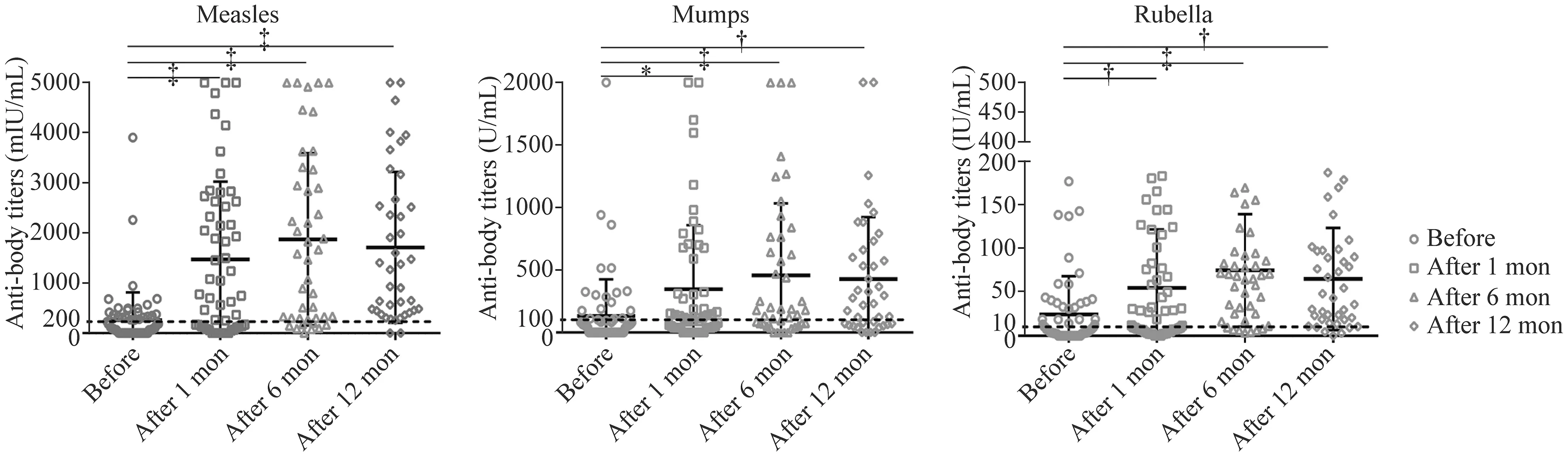
Fig.2 Analysis of antibody titers levels before and after MMR revaccination.* P < 0.05, † P < 0.01, ‡ P < 0.001.Compared with baseline(before), antibody titers increased signif icantly at all three follow-up sites.Compared with each other, the differences among the three follow-up sites were not statistically signif icant

Table 4 Comparison of positive seroconversion cases after revaccination of MMR according to some clinical and laboratory f indings
Compared with the baseline, the antibody titer was signif icantly increased at the 1-month follow-up and continued to increase to a peak at 6 months but began to decrease thereafter.This tendency is consistent with data reported by Aurpibul et al.[33].Some patients’ antibody titers were negative at the 1-month follow-up and turned positive at 6 months, while some did not convert to positive until the 12-month follow-up.In the present study, we noticed that 57.5% of patients’ anti-measles titers turned positive at the 1-month follow-up, 30% at the 6-month followup and 12.5% at the 12-month follow-up.For mumps,the percentages were 61.9%, 26.2% and 11.9%, respectively.For rubella, the percentages were 27.3%, 15.9%and 56.8%, respectively.Spoulou et al.[29] showed that the seropositivity rates at baseline and 12-month followup in 30 vaccinated children who received bone marrow transplantation increased from 13.3% to 23.3% for measles, from 33.3% to 36.6% for mumps, and from 66.6% to 90% for rubella.Even in a healthy population, the levels of antibodies induced by MMR vaccines have been shown to decline over time.However, the downtrend of antibody titer levels (6 months after revaccination) was observed comparatively earlier in waning immunity patients than in healthy children, whose estimated overall annual waning rates were 0.009 for measles, 0.024 for mumps, and 0.012 for rubella [34].Therefore, we recommend a booster vaccination after the initial immunization in these patients,similar to healthy children.However, the optimal timing and schedule require further investigation.
The rate of adverse reactions in the present study was 1/110 (0.9%), which was much lower than the reported rate(5%–19.1%) in patients after either chemotherapy, HSCT,or combination therapy [35– 37].This may attribute to the recovery of immunity when revaccinated, as mentioned above.In addition, adverse reactions were collected from parents through telephone calls or WeChat group messages at regular follow-up visits, and parents' initiative was likely to have a great inf luence on the results.This needs to be improved in follow-up studies.
There have been few previous relevant clinical studies in this f ield in China, and the guidelines lack supporting data.The present study opens research on the revaccination of live attenuated vaccines for children recovering from chemotherapy and HSCT treatment in China and clarif ies its necessity, effectiveness, and safety to provide a scientif ic basis for formulating and optimizing vaccination strategies for this population.Nevertheless, there are limitations to our study.First, antibody titer levels before treatment were not obtained.Therefore, negative seroconversion rates were not available.Second, it was a single-center study with a limited number of enrolled participants who were difficult to follow-up and had an incomplete specimen series due to the loss of follow-up sites.Multicenter studies with close follow-up should be performed to provide stronger evidence.
In conclusion, the rate of decline of protective antibody titers for measles in children who completed chemotherapy,HSCT or combination therapy was affected by the therapeutic schedule for primary disease.MMR revaccination is immunogenic for the population.We recommend booster vaccines and periodic monitoring of all patients.However,the optimal timing and schedule require further investigation.
Acknowledgements The authors appreciate the assistance from the Department of Immunology, Shanghai Pudong New Area Center for Disease Control and Prevention, Shanghai, China and Department of Infectious Diseases, Shanghai Children's Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China.We would like to thankEditage( www.edita ge.cn ) for English language editing.
Author contributions WM and YQ contributed equally to this work.GYJ: conceptualization, writing–review and editing, supervision.CJ:conceptualization.ZH, ZF, CWJ and CQ: data curation.DPF and FY:investigation.WM: formal analysis, writing–original draft.YQ: formal analysis.All authors read and approved the f inal manuscript.
Funding None.
Data availability The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest No f inancial or non-f inancial benef its have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethical approval The study was approved by the Ethics Committee of Shanghai Children’s Medical Center (SCMCIRB-K2017028) and registered at www.clini caltr ials.gov (NCT03373656).Informed consent to participate in the study have been obtained from parent or legal guardian of the participants (and themselves in the case of children over 8).
 World Journal of Pediatrics2023年11期
World Journal of Pediatrics2023年11期
- World Journal of Pediatrics的其它文章
- Efficacy of perampanel as an adjunctive therapy in pediatric focal epilepsy
- Status of the neonatal follow-up system in China: survey and analysis
- Analysis and validation of diagnostic biomarkers and immune cell inf iltration characteristics in pediatric sepsis by integrating bioinformatics and machine learning
- An eighteen-year longitudinal examination of school victimization and weapon use in California secondary schools
- National trends in alcohol and substance use among adolescents from 2005 to 2021: a Korean serial cross-sectional study of one million adolescents
- Medical clowning in hospitalized children: a meta-analysis
