Garlic extract addition for soil improvement at various temperatures using enzyme-induced carbonate precipitation (EICP) method
Hengxing Wang,Xiaohao Sun,Linchang Miao,Ziming Cao,Xin Guo
Institute of Geotechnical Engineering,School of Transportation,Southeast University,Nanjing,210096,China
Keywords:Enzyme-induced carbonate precipitation(EICP)Urease activity Garlic extract addition Sand solidification
ABSTRACT Enzyme-induced carbonate precipitation (EICP) is an emerging technique to improve the soil and most studies are carried out at room temperature.However,considering some foundations are in hightemperature environments (>40 °C),the higher urease activity at high temperature results in the solidification inhomogeneity,limiting the application of EICP.The higher urease activity at high temperature hampers the application of EICP because of solidification inhomogeneity.The garlic extract has been used as a type of urease inhibitor in medical science and food engineering.Here,we propose to use it to control urease activity for sand solidification at high temperature.The effects of garlic extract addition on urease activity and precipitation rates for calcium carbonate (CaCO3) were studied.Extra tests were conducted to study the effect of garlic extract addition on the solidification homogeneity.The results showed that the garlic extract addition significantly decreased urease activity.To reduce the rate of CaCO3 precipitation at different temperatures,a suitable concentration of garlic extract was necessary to obtain a suitable urease activity.In the sand solidification test,garlic extract addition resulted in a smaller difference in sonic time values or CaCO3 contents at different parts of samples.The improved solidification homogeneity can achieve higher strength.The correlation between sonic time values and CaCO3 content was higher than that between CaCO3 content and strength.Appropriate concentrations of garlic extract were obtained at 35 °C,40 °C,45 °C,50 °C,and 55 °C.The proposed garlic extract addition method was significant to improve the homogeneity of solidified soil in practical engineering applications.
1.Introduction
In engineering practices,several materials (e.g.cement,lime)are frequently injected to improve the strength or stiffness of soils to meet the requirements of the application.But the commonly used materials may negatively impact the environment (DeJong et al.,2010).Recently,some environmentally friendly techniques,such as microbially induced calcite precipitation (MICP) and enzyme-induced carbonate precipitation (EICP),have been widely studied to improve soil properties(Whiffin et al.,2007;Ivanov and Chu,2008;Hamdan,2015).In MICP and EICP,the induced calcium carbonate (CaCO3) precipitation can fill voids within soils and cement loose soil particles(De Muynck et al.,2010;Sun et al.,2018).The cemented soils can be used to replace cement for grouting.The MICP and EICP techniques have promising application potential in geotechnical engineering,for example soil improvement (Whiffin,2004;Van Paassen,2009;DeJong et al.,2011;Almajed et al.,2019;Cui et al.,2021;Gowthaman et al.,2021;Sharma et al.,2021a),liquefaction reduction (Sasaki and Kuwano,2016;Simatupang et al.,2018;Sun et al.,2021a;Sharma and Satyam,2021;Zamani et al.,2021;Sharma et al.,2022a,2022b),soil permeability reduction(Chu et al.,2014;Meng et al.,2021;Sharma et al.,2021b),erosion mitigation(Hamdan et al.,2017;Wang et al.,2018;Zomorodian et al.,2019;Miao et al.,2020;Sun et al.,2022;Dagliya et al.,2022a,2022b,2023),and heavy metal immobilization(Mwandira et al.,2017;Sharma et al.,2020,2022c).
Most studies on MICP or EICP are carried out at room temperature(about 25°C)(Harkes et al.,2010;Al Qabany et al.,2012;Chu et al.,2014).For application of the MICP or EICP at low temperat-ures,the bacterial domestication method was proposed to improve the bio-cementation effects by Sun et al.(2019).However,the practical application environments were not limited to room temperature,and fewer studies focused on high-temperature environmental conditions.Much of application fields have a higher environmental temperature(30°C-60°C),such as oil fields,mining areas,deserts hot springs.(Jin et al.,2010;Cable et al.,2011;Xue et al.,2021;Hu et al.,2022;Wang et al.,2022a).The urease enzyme has been demonstrated to be effective at higher temperatures(Illeová et al.,2003;Elias et al.,2014).In addition,compared with MICP,EICP avoids the complicated culture and storage process of bacteria,using solubilized urease enzymes with smaller sizes(Hamdan,2015).However,urease activity significantly increases at a higher temperature (Ivanov and Chu,2008;DeJong et al.,2010;Van Paassen et al.,2010),leading to massive precipitation near the injection point (Cheng et al.,2019).The heterogeneity in biocementation also limits the extensive application of the EICP technique.Therefore,it is significant to propose an effective method to decrease urease activity at high temperature.Several methods have been proposed to decrease urease activity and to resolve the issue of solidification inhomogeneity,such as low-pH environment and low-concentration urease (Cheng et al.,2019;Cui et al.,2021;Wang et al.,2022b).These studies achieved a better solidification homogeneity at room temperature.Considering the effect of high temperature on low-pH environment and urease,this method cannot be effectively applied at high temperature.Thus,a method that can adapt to various temperatures is needed to promote the efficiency of EICP at high temperature.
Researchers generally use urease inhibitors to decrease urease activity in soil(e.g.Manunza et al.,1999;Krajewska,2009a,b;Engel et al.,2015).There were two ways for using urease inhibitors: the first is binding with Ni2+in urease enzyme to lower urease activity(Bachmeier et al.,2002;Modolo et al.,2015)because urease itself is a Ni-containing metalloenzyme and the Ni2+is quite important in the process of urea hydrolysis.The second one is binding with sulfydryl(-SH)in the urease enzyme to form a disulfide bridge(-SS-) (Huey et al.,2019),which changes the microcosmic spatial structure of the urease enzyme.Garlic is a common plant and garlic extract is rich in thiosulfinates (Zaborska et al.,2009;Olech et al.,2014).The thiosulfinates can bind with sulfydryl of cysteine in the urease enzyme(Juszkiewicz et al.,2004).The garlic extract has been used as a type of urease inhibitor in medical science and food engineering (Mansor et al.,2016;Mathialagan et al.,2017;Salehuddin et al.,2020).
In other words,garlic extract addition has the potential to decrease urease activity for improvement of the solidification homogeneity at various temperatures.In this study,the EICP technique was applied to sand solidification at various temperature,and the effects of garlic extract addition on urease activity and precipitation rates for CaCO3were studied.Next,the sand columns with garlic extract addition were prepared and the sonic time values,unconfined compressive strength (UCS),and CaCO3contents at different parts were measured to investigate the effect of the proposed method on solidification at high temperature.Finally,the scanning electron microscopy (SEM)was used to study the microstructure of treated samples (Sun et al.,2021d).
2.Material and methods
2.1.Urease enzyme
The suspension of soybean powders(100 g/L)was centrifuged at a speed of 3000 r/min for 15 min to obtain a urease enzyme solution(Miao et al.,2020;Sun et al.,2021b).The pH value of the urease solution was adjusted to 7 for subsequent studies.The urease activity of the urease solution was obtained by measuring the change in ammonium concentration generated from urea hydrolysis(Knorst et al.,1997;Whiffin et al.,2007;Stabnikov et al.,2011).Previous studies also used this method to measure the ammonium concentration of the solution(e.g.Cheng,2012;Yang et al.,2019).In this study,the concentration of urea was 1 mol/L,and the urease activity of the urease solution was 16 mmol/L urea hydrolysed/min at 25°C.
2.2.Effect of temperatures on urease activity
A water bath(HH501,Xinbao Company,Changzhou,China)was used to stabilize the temperature during testing.The temperature was controlled at 30°C-60°C.To understand the change in urease activity at various temperatures,the urease solution was mixed with urea solution at 0.75 h,1.5 h,2.25 h,3 h,4 h,5 h,6 h,8 h,10 h,and 12 h.
2.3.Effects of garlic extract addition on EICP
In reference to the method in Juszkiewicz et al.(2004),garlic cloves were crushed and blended with 5 mL of distilled water per gram of garlic.The homogenate was then shaken for 20 min and filtered using a 0.2 μm filter.The aqueous extracted garlic sauce was standardized for its thiosulfinate concentration,using the spectrophotometric method (Han et al.,1995).The total thiosulfinate concentration of garlic extract was determined to be 5.2 mmol/L(22 μmol TS/g wet mass).The pH value of garlic extract was adjusted to 7 for subsequent studies.
The garlic extract with various concentrations(0 g/L,5 g/L,10 g/L,15 g/L,and 20 g/L)was added to the 20 mL of urease solution to obtain a modified urease solution.The urease activity was obtained to study the effect of the garlic extract addition on urease activity.Similarly,the temperature varied at 30°C,35°C,40°C,45°C,50°C,55°C,and 60°C.A sample without garlic extract was prepared at 25°C for comparison to obtain the concentrations of garlic extract needed for decreasing urease activity at various temperatures.The test was conducted three times to obtain average levels.
Urease solution and cementation solution were mixed with equal volume(20 mL+20 mL)and the pH value was adjusted to 7 to study the effect of precipitation rates for CaCO3.The cementation solution obtained 1 mol/L of urea and 0.75 mol/L of calcium acetate.Both the garlic extract concentration and temperature varied,as shown in Table 1.The garlic extract concentration was determined according to the results of the above-mentioned urease activity tests.The precipitation rate for CaCO3(the ratio of the actual produced amount of CaCO3to the theoretically calculated amount)was obtained using the acid dissolving method at 1 h,2 h,3 h,6 h,8 h,12 h,and 24 h (Whiffin et al.,2007;Chu et al.,2014).

Table 1Sample arrangement for the effect of garlic extract on precipitation rate for CaCO3.
2.4.Sand solidification tests
2.4.1.Setup and sands
Sands sampled from the Yangtze River were used for solidification tests.The particle size distribution of the sand is shown in Fig.1,and its physical properties are summarized in Table 2.Weathering and deposition lead to a high content of small particles(<0.25 mm) and high specific gravity.According to the USCS classification system,the sand used belonged to SP(García-Gaines and Frankenstein,2015).Sands were sterilized at 120°C and confinement of 0.25 MPa to remove the influence from other bacteria before being added to PVC cylinders.Mass of 210 g of sand was loaded in polyvinyl chloride (PVC) cylinders (4.6 cm in diameter,15 cm in height) to obtain an 8-cm specimen with an initial dry density of 1.59 g/cm3.

Fig.1.Grading curve of Yangtze River sand.
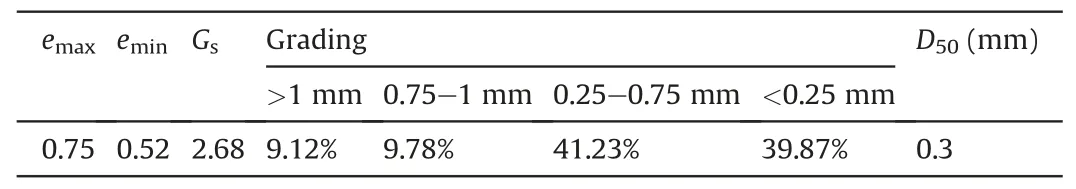
Table 2Physical properties of Yangtze River sand.
The specimens were divided into seven groups at various temperatures,as shown in Table 3.There were four samples in each group because of different garlic extract concentrations.Urease solution and cementation solution were mixed in equal volumes(30 mL+30 mL) and the pH value was adjusted to 7 for curing samples.The cementation solution obtained 1 mol/L of urea and 0.75 mol/L of calcium acetate.The mixed solution was injected from the bottom of samples with a speed of 10 mL/min using a pump,which could let the sand columns be kept fully saturated throughout the treatment (see Fig.2).The samples were cured for 24 h after injection.The above-mentioned steps were an EICP treatment cycle.After six EICP treatment cycles,the samples were dislodged from the cylinders for subsequent tests.Each group contains three parallel patterns.

Fig.2.Schematic set-up of curing sample tests.
2.4.2.Treatment effect evaluation
Samples with good curing have lower sonic time values because the pores are filled by produced CaCO3and the air state of pores is transferred to a solid state (Li et al.,2011;Sun et al.,2021c).The ultrasonic testing detector type I-rock parameter test (RPT) was used to measure the sonic values of samples.The method of measure refers to as the method used in Van Tittelboom et al.(2010).The diameter of the probe is about 3 cm;however,the ultrasonic waves travel through the central point of the probes.Hence,the curved surface measurement had a marginal impact on testing results.To obtain the sonic time values at the bottom part,middle part,and top part of the samples,the measuring points were chosen at 2 cm,4 cm,and 6 cm from the bottom of the samples (see Fig.2).
Next,we obtained the UCS of the samples.In this study,specimens were compressed at a constant rate(1 mm/min)until failure using an electric strain-controlled UCS meter.The UCS was then calculated by the peak load.
After the UCS test,the fractions of the treated sand columns at the bottom part,middle part,and top part of specimens were collected to obtain the amount of produced CaCO3using the acid dissolving method (Chu et al.,2014).All tests were repeated three times for reliability.
Moreover,SEM was carried out on the treated samples with or without garlic extract addition.
3.Results
3.1.Effect of temperatures on urease activity
Without the garlic extract,the urease activity significantly increases with higher temperatures(see Fig.3)with bacterial urease activity,which is the same as the findings in Whiffin (2004) and Van Paassen(2009).The urease activity at 60°C was four times that at 25°C.The urease activity almost did not change at 25°C-40°C during the tests.However,the urease activity gradually decreased from 42 to 31 mmol/Lurea hydrolysed per min at 45°C after 12 h.At 45°C-55°C,the decreasing ranges of urease activity in 12 h were larger.Furthermore,the urease activity decreased at 60°C,as the urease enzyme deteriorated gradually at high temperature(Cowan,1995).

Fig.3.Changes in urease activity at various temperatures.
3.2.Effects of garlic extract addition on EICP
Previous studies found that temperature has a negligible influence on the activity of garlic extract(Durairaj et al.,2009;Sah et al.,2012;Bar et al.,2022).Fig.4 also shows the decreased urease activities resulted from garlic extract addition.With the garlic extract addition,the initial urease activity clearly decreased,regardless of temperature.The urease activity further decreased with increased garlic extract concentrations.A smaller decrease in urease activity was in the samples at lower temperatures.If the urease activity at 25°C was used as the benchmark activity,the garlic extract concentration to reach the benchmark activity was smaller at lower temperatures,as shown in Fig.5.The suitable concentrations of garlic extract were below 6 g/L between 30°C and 40°C.When the temperature increased to 55°C,the concentration of garlic extract increased to 8 g/L.The needed concentration of garlic extract would be over 8 g/L,but still less than 10 g/L at 60°C.Consequently,the concentration of added garlic extract varied at different temperatures in subsequent calcification tests and sand solidification tests.

Fig.4.Effect of the garlic extract addition on urease activity.

Fig.5.Empirical correlation between the concentration of garlic extract and temperature.
The garlic extract addition indeed affected the precipitation rate for CaCO3.Before 24 h,the precipitation rate for CaCO3of the sample without the garlic extract(C30-0)was always the largest at 30°C because of the largest urease activity(see Fig.6a).The garlic extract addition decreased the precipitation rate.Nevertheless,the precipitation rate for CaCO3still reached 1 at 12 h for sample C30-2 because of a smaller concentration of garlic extract.The decreasing range of precipitation rate for CaCO3became larger as garlic extract concentration increased.For sample C30-6,the 6 g/L of garlic extract resulted in the precipitation rate for CaCO3being only 0.85 at 12 h.However,the eventual precipitation rate for CaCO3increased to 1 at 24 h.The precipitation rate for CaCO3of samples without the garlic extract addition increased at a higher temperature.
The precipitation rates for CaCO3of sample C35-0 and C40-0 reached 1 at 8 h and 4 h,respectively (see Fig.6b and c).Compared with the sample C30-6,the decreasing ranges resulted from the garlic extract addition were smaller for the samples C35-6 and C40-6.The urease activity of the sample with 2 g/L of garlic extract was still larger at 45°C;therefore,the smallest concentration of garlic extract increased to 6 g/L (see Fig.6d).For the eventual precipitation rate for CaCO3for C45-10,it was smaller than 1 at 24 h due to a larger concentration of garlic extract.
With 10 g/L of garlic extract,the decreasing ranges for precipitation rates for CaCO3were smaller at 50°C and 55°C (see Fig.6e and f).Nevertheless,the eventual precipitation rate for CaCO3was still below 1 at 24 h.The smallest concentration of added garlic extract increased to 8 g/L at 60°C (see Fig.6g).The garlic extract addition resulted in smaller eventual precipitation rates for CaCO3.The precipitation rates for CaCO3did not increase from 12 h to 24 h because the urease activity was quite small after 12 h at 60°C.
3.3.Sand solidification tests
3.3.1.Effects of garlic extract addition on sonic time values of specimens
The solidification effects can be evaluated effectively by sonic time value.Fig.7a shows that the difference in sonic time values at different parts of specimen S30-0 was larger than the specimens with the garlic extract addition at an identical temperature.When the concentration of garlic extract was higher,the precipitation rate for CaCO3decreased;thus,more pores were not filled with precipitation,which increased the sonic time values(Sun et al.,2021d).The sonic time value at the bottom of S30-0 was the smallest(only 22 μs).The differences in sonic time values at different parts for S30-2 and S30-4 were much smaller,especially the specimen S30-2.For specimens S30-4 and S30-6,the bottom part had smaller sonic time values than the top part again,even reaching 1.6%for the difference between the top part and bottom part.The reason might be that the higher concentration of garlic extract led to a much smaller precipitation rate,and the mixed solution had better liquid fluidity.A large amount of CaCO3precipitation was produced in the static process between the two treatment cycles instead of the injection process;however,these CaCO3precipitation crystals would sink to the bottom part because of gravity.The sonic time values of S30-6 were relatively larger than other specimens at 30°C,indicating that the S30-6 had larger pores (Sun et al.,2021d) and a relatively smaller amount of CaCO3precipitation.The results were different from the results of precipitation tests (see Fig.6).In the precipitation tests,the calcium ions in the solution can completely bind with carbonate ions to reach an eventual precipitation rate of 1 in 24 h,despite the garlic extract of 6 g/L.However,the transport of ions would be hampered by sand particles,inhibiting the production of precipitation (Zhang et al.,2010;Yoon et al.,2012) and resulting in smaller eventual precipitation rates for CaCO3than in precipitation tests.
Higher temperature increased the urease activity,causing higher precipitation rates and obtaining smaller sonic time values.However,the difference in sonic time values from the bottom to up of the sample was large,which resulted from the larger precipitation rate.The smallest difference in sonic time values was achieved at the garlic extract of 2 g/L at 35°C,4 g/L at 40°C,6 g/L at 45°C,and 8 g/L at 50°C and 55°C,respectively.Additionally,with garlic extract of 6 g/L,the increase in temperature decreased the difference in sonic time values for the specimens.It was noted that the garlic extract addition significantly increased sonic time values because of a shorter maintenance period of urease activity at 60°C(see Fig.3).The 10 g/L was the optimum concentration of garlic extract for the homogamous solidification (see Fig.7g).However,the sonic time values were much larger than the specimens with most homogamous solidification at other temperatures.
3.3.2.Effects of garlic extract addition on UCS of specimens
The loose sand grains could be cemented to a whole sand column by sand solidification test (DeJong et al.,2010;De Muynck et al.,2010).The strength of samples without the garlic extract decreased gradually with increasing temperatures (see Fig.8)because of worse solidification homogeneity (Shahrokhi-Shahraki et al.,2015).With the garlic extract addition,the samples S30-2,S35-2,S40-4,S45-6,S50-8,and S55-8 had the largest strength than their rivals at an identical temperature.It was also due to the homogeneous distribution of CaCO3precipitation;the results were consistent with that of sonic time values.With increasing temperatures,the needed concentration of garlic extract increased to achieve better solidification homogeneity and higher strengths.The solidification homogeneity affected the failure mode.For nonhomogeneously solidified specimens,the strength of the sample depends more on the strength of the weak part (such as S45-0 in Fig.9a).The garlic extract addition improved the solidification homogeneity,and the specimens reached brittle failure and the failure crack ran through the whole specimen (such as S35-2 in Fig.9b).The S30-2 had smaller average sonic time value than the S30-4,and the difference of sonic time value for S30-2 was also smaller than that for S30-4.Both of them resulted in a larger strength for S30-2.

Fig.8.UCS of solidified sand columns: (a) 30 °C,(b) 35 °C,(c) 40 °C,(d) 45 °C,(e) 50 °C,(f) 55 °C,and (g) 60 °C.

Fig.9.The failure mode of solidified sand columns: (a)Sample S45-0,and (b)Sample S35-2.
At 60°C,the S60-0 had the largest strength (UCS of 660 kPa).With increasing concentration of garlic extract,the strength of specimens decreased,which was different from the result at other temperatures.The strength of the sample S60-10 was only 450 kPa,due to the degradation of urease protein and decrease of urease activity at a higher temperature.The decreasing range was larger at a higher temperature.At 30°C,the slight decrease in urease activity might be due to measurement accuracy (see Fig.3).Therefore,the amount of precipitation significantly decreased with the temperature increasing to 60°C,especially for the samples with the garlic extract addition.With the garlic extract addition,homogeneous solidification can be achieved;however,the strength (UCS)decreased.
3.3.3.Effects of garlic extract addition on CaCO3 contents of specimens
The bottom part always had a larger CaCO3content because of clogging,regardless of temperatures(see Fig.10).The difference in CaCO3contents in specimens without garlic extract was larger.The garlic extract addition resulted in a homogeneous distribution of CaCO3content,thus obtaining a smaller difference in sonic time values.However,with a temperature below 45°C,the CaCO3contents of samples with the garlic extract were still close to that of samples without garlic extract.It indicated that a small garlic extract concentration marginally affected the production rates for CaCO3in 24 h.However,the urease activity gradually decreased with time at a higher temperature (over 45°C).The eventual production rates for CaCO3decreased with more concentration of garlic extract added.Therefore,the CaCO3contents of C45-10,C50-10,and C55-10 were relatively smaller than others at the identical temperature.There was no significant difference between the CaCO3contents of C50-8 and C50-10,because the values of CaCO3content were small,resulting in smaller variation.However,the more homogeneous solidification and smaller sonic time values for C50-8 can be observed;therefore,the C50-8 had a larger strength than C50-10.Additionally,the uniformity of C50-10 was better than that of C50-0;however,the CaCO3contents of C50-0 were much larger than C50-10,due to the slightly lower UCS for C50-10.At 60°C,the specimens with the garlic extract addition had much smaller CaCO3contents than C60-0,because the urease activity significantly decreased in a short time.The garlic extract addition dramatically decreased the initial urease activity;therefore,the produced amount of CaCO3precipitation was much smaller,especially the specimen C60-12.
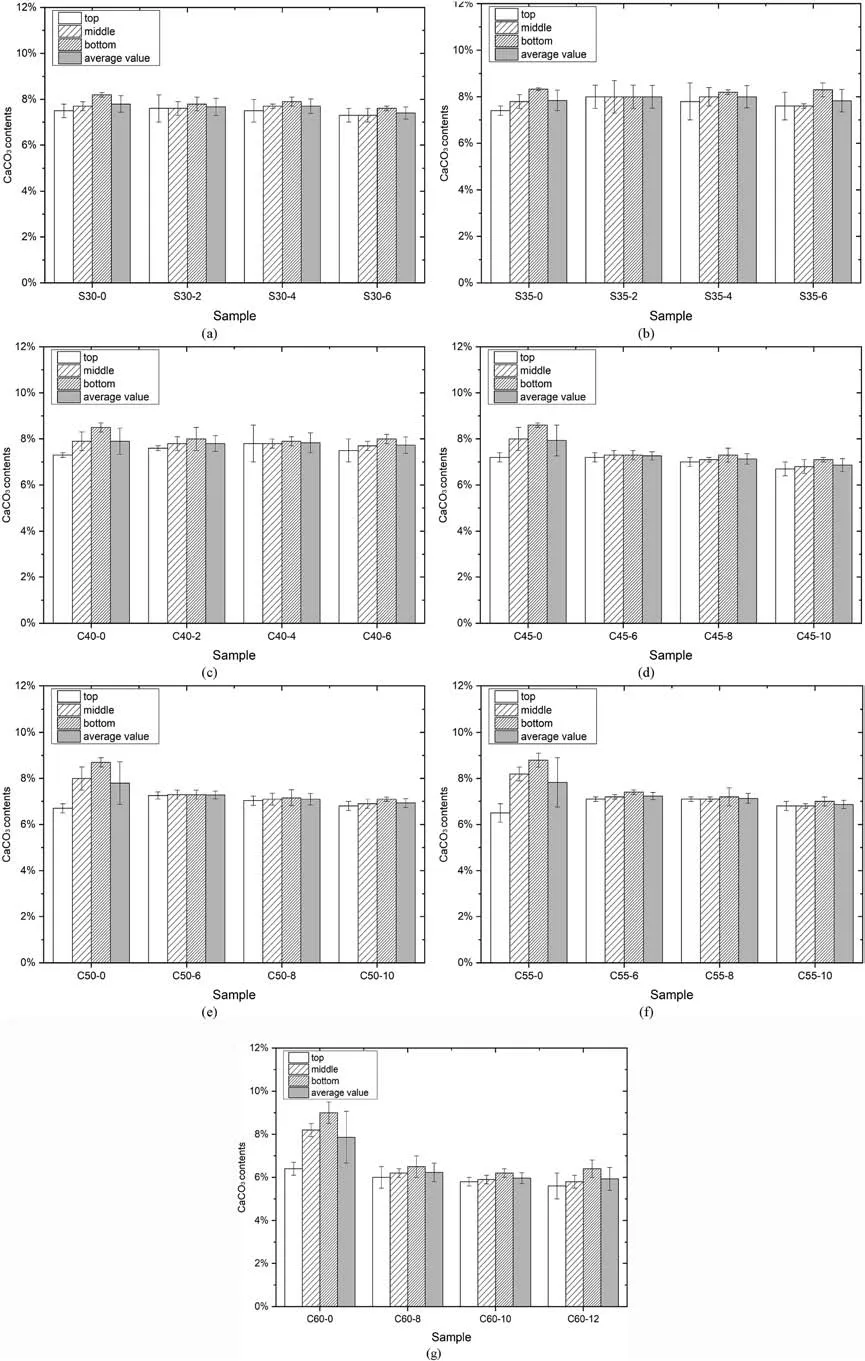
Fig.10.CaCO3 contents of different parts in solidified sand columns: (a) 30 °C,(b) 35 °C,(c) 40 °C,(d) 45 °C,(e) 50 °C,(f) 55 °C,and (g) 60 °C.
The CaCO3content was always related to the compactness and the strength of cemented soils(Van Paassen et al.,2010;Chu et al.,2014;Sun et al.,2021d).The CaCO3contents in Fig.10 are averaged,and the correlation between UCS and CaCO3content is shown in Fig.11a.For the samples without garlic extract,the UCS was not highly correlated to the CaCO3content,which indicated that the solidification inhomogeneity had a more significant impact on the strength than CaCO3content.The garlic extract addition improved the solidification homogeneity of sand columns.Therefore,the correlation between UCS and CaCO3content was higher.The UCS exponentially increased with increasing CaCO3contents.In contrast to UCS,the sonic time values were highly correlated to CaCO3contents,regardless of different parts (see Fig.11b).With increasing CaCO3contents,the sonic time values linearly decreased.Fig.11 shows a higher correlation between sonic time value and CaCO3content than the correlation between UCS and average CaCO3content.

Fig.11.Correlation between (a) strength and CaCO3 content;and (b) sonic time value and CaCO3 content.
3.3.4.Microscopic characteristics of precipitation
The SEM-photograph of carbonate minerals precipitated in the sand columns S45-0 and S45-6 is shown in Fig.12.From Fig.12a,precipitation crystals were found on particle surfaces and between particle contacts.The presence of a moderate amount of precipitation crystals allowed for small voids in the treated sand column.The garlic extract addition did not change the precipitate patterns(see Fig.12c).Fig.12b shows that the precipitated mineral in samples S45-0 seems to be spherical.From several previous studies,the CaCO3precipitated via EICP was also mainly spherical (Fujita et al.,2000;Hoang et al.,2019;Nafisi et al.,2019).With garlic extract addition,the CaCO3crystals were still spherical.Nevertheless,the sizes of crystals in S45-6 were greater than that in S45-0 (see Fig.12d).The reason was that the garlic extract addition decreased the urea hydrolysis rate,which leads to a smaller amount of crystals but larger crystal sizes.The influence of decreased urea hydrolysis rate on crystal growth was also observed by Xu et al.(2021).Nevertheless,they found that the crystals formed were much smaller with N-(N-butyl)-thiophosphoric triamide added into the MICP process.

Fig.12.SEM photos of the sand column solidified without garlic extract at 45 °C(S45-0): (a)800×,(b)5000 ×and the sample solidified with garlic extract at 45 °C(S45-6): (c)800 ×,and (d) 5000 × .
4.Discussion
4.1.Influence of garlic extract addition on EICP treatment
The cysteine was in the flap region of the urease enzyme.With the garlic extract addition,the sulfhydryl(-SH)of the cysteine will bond with the thiosulfinates in garlic extract (Juszkiewicz et al.,2004),affecting the microspatial structure of the urease enzyme(Olech et al.,2014;Modolo et al.,2015)(see Fig.13).Therefore,the initial urease activity decreased,especially the sample with increased garlic extract concentrations (see Fig.4).The garlic extract addition also affected the precipitation rate for CaCO3before 24 h,because of decreased urease activity.The decreasing range of precipitation rate for CaCO3became larger as garlic extract concentration increased (see Fig.6).The needed garlic extract concentration was higher at higher temperatures.As a result,the concentration of added garlic extract varied at different temperatures in subsequent sand solidification tests.

Fig.13.Schematic set-up of GE addition for decreased urease activity.
In the sand solidification experiment,the EICP treatment indeed resulted in uneven distribution of precipitation.Clogging generally existed near the injection point.The strength of samples without the garlic extract decreased with increased temperatures (see Fig.8) because of worse solidification homogeneity (Shahrokhi-Shahraki et al.,2015).A method of garlic extract addition was presented to address inhomogeneity solidification,which indeed led to a smaller precipitation rate,and the mixed solution had a better liquid fluidity during injection.A large amount of CaCO3precipitation was produced in the static process between the two treatment cycles instead of the injection process,which mitigated the clogging around the injection point.The specimens with garlic extract addition had smaller differences in CaCO3contents at different positions than that without garlic extract at the identical temperature.Moreover,better homogeneity could be found in the samples with the garlic extract of 2 g/L at 35°C,4 g/L at 40°C,6 g/L at 45°C,8 g/L at 50°C,and 8 g/L at 55°C,respectively(see Fig.7).When the concentration of garlic extract was lower,it had a smaller impact on precipitation rate.Nevertheless,the solidification homogeneity was significantly improved,which affected the failure mode.Eventually,these samples (S30-2,S35-2,S40-4,S45-6,S50-8,and S55-8) had the largest strength(UCS).
Additionally,the garlic extract addition resulted in larger CaCO3crystals,which was also contributing to the higher strength.The contribution percentages to the strength from the solidification homogeneity or crystal size should be identified in the future.Moreover,the high concentration of the garlic extracts significantly decreased urease activity and precipitation rates;thus,using a much higher concentration of the garlic extract resulted in much smaller CaCO3contents.The solidification homogeneity was also improved;however,the much smaller CaCO3contents contributed to the lower strengths.
Lower urease activity can be achieved with a lower urease concentration under identical environmental conditions (e.g.temperature,pH).However,a much lower urease concentration was needed to eliminate the plugging at a higher temperature,which might result in smaller CaCO3contents (Cheng et al.,2019;Wang et al.,2022b).The garlic extract addition significantly decreased urease activity but slightly impacted the eventual production rate probably due to the subsequent degradation of garlic extract.The low-pH method(Cheng et al.,2019;Cui et al.,2021)is effective to achieve better solidification homogeneity Nevertheless,the tests were conducted at room temperature.Based on the results of this study,the garlic extract addition method can adapt to various temperatures.
Garlic is a common plant and the garlic extract is commonly used as a urease inhibitor in agriculture science,medical science,and food engineering (Papu et al.,2014;Modolo et al.,2015;Mathialagan et al.,2017;Matczuk and Siczek,2021).The garlic extract was easily obtained with water in this study.The garlic extract addition method has been demonstrated to be costeffective for applications (Von White et al.,2012;Perez-Ortiz et al.,2020).Therefore,the proposed garlic extract addition method for the control of urease activity at various temperatures(30°C-60°C)has promising potential to improve the solidification homogeneity of soils in practical applications.
4.2.Urease enzyme application of the combined EICP and garlic extract method
EICP could be used to solidify desert sands for sandstorm control(Miao et al.,2020;Sun et al.,2021e).However,the curing effect could be reduced because of the higher surface temperatures and the small particles of sand,which easily caused the clogging of surface sands and thinner crust layers.The garlic extract addition method proposed herein is beneficial to decrease the calcification rate and to increase the thickness of the crust layer,achieving better wind-erosion resistance.
In parallel with sandstorm control,another application related to sand solidification is to prevent mine cave collapse in several mine cave fields and the EICP technique was also proposed for it(Martín and Goldenfeld,2006;Onac et al.,2007;Pedley,2009).However,many fields with higher temperatures had smaller treated strength after EICP treatment due to inhomogeneous solidification Similarly,the proposed garlic extract addition can ensure homogeneous spatial CaCO3distribution to improve the strength of solidified soils.
In oil engineering,the integrity of the wellbore would gradually become worse,which could affect the efficiency of oil recovery and cause the waste of oil and gas(Mitchell and Casman,2011).EICP can be used for crack repair and pore plugging to enhance oil recovery(Yao et al.,2012;Kirkland et al.,2020;Wang et al.,2022c).However,the higher temperature in the field similarly resulted in a worse solidification,which can be mitigated or even solved by the garlic extract addition.With the proposed garlic extract addition method,the decreased calcification rate can eliminate the plugging and enables injected solution to reach the target area,which could be beneficial to eliminate the gap between wellbore cement and soils.(Kolawole et al.,2021;Wang et al.,2022b).
In addition,the EICP technique is an emerging tool to prevent leakage in the applications of CO2sequestration to achieve the aim of carbon neutrality (Phillips et al.,2015;Paul et al.,2017).The garlic extract addition provided a method to avoid inhomogeneous remediation at high temperature and to improve the security of subsurface storage sites,which is beneficial to better achieve carbon neutrality.
4.3.Limitations of garlic extract addition for sand solidification
In this study,despite of the effectiveness in demonstrating the benefits of the proposed method for homogenous solidification in sand columns,it is still unknown how using the proposed method for extensive soil improvement will turn out in future studies.Furthermore,at 60°C,the garlic extract addition significantly increased sonic time values because of a shorter maintenance period of urease activity (see Fig.3).A lower garlic extract concentration marginally affected the production rates for CaCO3in 24 h.However,when the concentration of garlic extract was higher,the eventual production rates for CaCO3decreased.The 10 g/L was the optimum concentration of garlic extract for homogamous solidification.However,the specimens with the garlic extract addition had much smaller CaCO3contents than C60-0,causing smaller strength,despite better solidification homogeneity.For practical applications,it is necessary to balance the solidification homogeneity and shear strength.The maintenance of urease activity at 60°C should be further studied in the future to obtain both homogeneous solidification and higher strengths.
5.Conclusions
(1) The garlic extract addition method with promising application potential was proposed to decrease urease activity at higher temperatures,to modify the homogeneity of treatment.The higher urease activity at high temperature hampers the application of EICP because of solidification inhomogeneity.The decreasing ranges of urease activity were larger with a higher concentration of garlic extract,which result in slower precipitation rates for CaCO3.
(2) More garlic extract was needed with increased temperatures.The garlic extract addition could be used to eliminate the plugging and reduce the difference of the sample,which resulted in higher strength of treatment at temperatures(30°C-55°C).
(3) The maintenance of urease activity still needs to be further studied in the future to obtain both homogeneous solidification and higher strengths.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the valuable comments from the reviewers.This study was funded by the National Natural Science Foundation of China (Grant No.51578147),the Science and Technology Department of Ningxia (Grant No.2020BFG02014),and the Transportation Department of Ningxia (Grant No.202000173).
 Journal of Rock Mechanics and Geotechnical Engineering2023年12期
Journal of Rock Mechanics and Geotechnical Engineering2023年12期
- Journal of Rock Mechanics and Geotechnical Engineering的其它文章
- The formation of orthogonal joint systems and cuboidal blocks: New insights gained from flat-lying limestone beds in the region of Havre-Saint-Pierre (Quebec,Canada)
- Numerical analysis of the effects of vesicle distribution characteristics on the engineering properties of volcanic rocks
- A hybrid attention deep learning network for refined segmentation of cracks from shield tunnel lining images
- 3D limit analysis of rock slopes based on equivalent linear failure criterion with tension cut-off
- Mutual impact of true triaxial stress,borehole orientation and bedding inclination on laboratory hydraulic fracturing of Lushan shale
- Unloading-induced permeability recovery in rock fractures
