Brain iron deposition increases in the bilateral substantia nigra of patients with medication-overuse headache: a quantitative susceptibility mapping analysis
LI Xin *,ZHAO He *,LIU Mengqi ,CHEN Zhiye
1Department of Radiology,Hainan Hospital of PLA General Hospital,Sanya 572013,China;2Second School of Clinical Medicine,Southern Medical University,Guangzhou 510515,China;3Department of Neurology,4Department of Radiology,First Medical Center of PLA General Hospital,Beijing 100853,China
Abstract: Objective To investigate iron accumulation level over the whole brain and explore the possible neuromechanism of medication-overuse headache(MOH)using quantitative susceptibility mapping(QSM).Methods Thirty-seven MOH patients and 27 normal control subjects were enrolled in the study for examinations with both a multi-echo gradient echo magnetic resonance(MR)sequence and brain high resolution structural imaging.Avoxel-based analysis was performed to detect the brain regions with altered iron deposition,and the quantitative susceptibility mapping values of the positive brain regions were extracted.Correlation analysis was performed between the susceptibility values and the clinical variables of the patients.Results In patients with MOH,increased susceptibility values were found mainly in the bilateral substantia nigra(SN)(MNI coordinate:8,-18,-14;-6,-16,-14)as compared with the normal control subjects(P<0.001),but these alterations in iron deposition were not significantly correlated with the clinical variables of the patients(P>0.05).The susceptibility value in the left SN had an area under curve(AUC)of 0.734,and at the cut-off value of 0.077,its diagnostic sensitivity was 72.97%and its specificity was 70.37%for distinguishing MOH from normal controls;The susceptibility value in the right SN had an AUC of 0.699 with a diagnostic sensitivity of 72.97%and a specificity of 62.96% at the cut-off value of 0.084.Conclusion Increased iron deposition occurs in the bilateral SN of MOH patients,which provides a new insight into the mechanism of mesocorticolimbic dopamine system dysfunction in MOH.QSM technique can be used as a non-invasive means for quantitative analysis of brain iron deposition in migraine neuroimaging.
Keywords:medication-overuse headache;quantitative susceptibility mapping;brain;voxel-based analysis;iron deposition
INTRODUCTION
According to the International Classification of Headache Disorders,medication-overuse headache(MOH) is defined as a headache secondary to a pre-existing primary headache caused by excessive use of acute analgesics(e.g.ibuprofen,triptans,and opioids),occurring on ≥15 days per month for at least 3 months[1].As a common cause of disability worldwide[2],its prevalence ranges from 0.5% to 2.6% in the general population,and can be as high as 11%-70% in people with chronic daily headache[3].The patients with a combination of chronic musculoskeletal complaints,gastrointestinal complaints and psychiatric comorbidities(including anxiety and depression) appear to be especially susceptible to MOH[4].MOH is a chronic condition with difficult treatment,and its complex pathophysiology remains poorly understood.
Over the past several decades,remarkable advances in the neuroimaging techniques have enabled the observation of structural and functional changes of the human brain.Previous studies reported altered brain iron deposition in the periaqueductal gray matter (PAG),red nucleus (RN),globus pallidus (GP) and putamen in patients with episodic migraine (EM) and chronic migraine (CM)[5-8],suggesting the potential pathogenic role of iron deposition in these headaches,which,however,awaits validation with more reliable clinical as well as imaging evidences.
A growing number of advanced magnetic resonance imaging(MRI)studies are capable of showing features of iron deposition,including T2 turbo spin echo sequence,T2 gradient echo imaging,transverse relaxation rates R2,quantitative T2*,and susceptibility weighted imaging(SWI)[8-11],but none of these modalities allow quantitative measurement of iron deposition.Quantitative susceptibility mapping (QSM) is a novel non-invasive MRI technique and can analyze the distribution of magnetic susceptibility throughout the whole brain by using phase images of gradient-recalled echoes.With a high sensitivity and reliability,QSM allows for quantitative estimation of brain iron deposition and thus provides more valuable information for studying iron deposition[12,13].
In this study,we hypothesize that patients with MOH have altered iron deposition in the brain.To test this hypothesis,we carried out QSM examinations in 37 MOH patients and 27 normal control subjects,and voxel-based analysis was applied to evaluate iron deposition level over the whole brain to obtain insights into the pathophysiological mechanism of MOH.
MATERIALS AND METHODS
Subjects
This prospective study was performed under approval by the Ethics Committee of the Chinese PLA General Hospital (S2018-027-02) in line with the ethical guidelines of Declaration of Helsinki.Written informed consents were obtained from all the participants before examination.MOH patients were sequentially recruited from Headache Clinic and the staffs of the hospital,and the age-and gender-matched healthy control subjects were recruited from the hospital staffs and their relatives.The inclusion criteria for MOH patients were determined based on the International Classification of Headache Disorders,third Edition (beta version) (ICHD-3 beta)[1],including(1)presence of both MOH and migraine;(2)the diagnosis of 8.2 MOH,1.1 and 1.2 migraine;and (3)discontinuation of preventive medication for migraine at least 3 months prior to the study.The exclusion criteria included (1) cranium trauma,cerebrovascular diseases,long-standing hypertension,diabetes mellitus,and a history of tumors and brain surgery;(2) substance abuse(such as alcohol and nicotine);(3)other neuropsychiatric disorders,such as chronic pain other than headache,severe anxiety or depression,and other psychiatric disorders;and(4)MRI contraindications including metal clips in the body and claustrophobia.All the normal control subjects were free of primary headache disorders or other types of headaches in the past year,and reported no conditions listed in the exclusion criteria.All the MR examinations were performed in the interictal stage,and alcohol,nicotine,caffeine,and other substances were avoided for at least 12 h before MRI examination.
All the participants underwent assessment with Visual Analogue Scale (VAS) for pain intensity,Hamilton Anxiety Scale (HAMA) for anxiety,Hamilton Depression Scale (HAMD) for depression,and Montreal Cognitive Assessment (MoCA) and Mini-mental State Examination(MMSE)for cognitive functions.
MR data acquisition
For MRI examination,the subjects lied in a supine position with the head immobilized with padding,and MR data were obtained through a conventional 8-channel phased array head coil on a GE 3 Tesla MR scanner(DISCOVERY MR750,GE Healthcare,Milwaukee,WI,USA).QSM images were acquired with a multi-echo gradient echo sequence using the following parameters:first time echo(TE)=4.72 ms,the last TE=22.96 ms,delta TE=3.648 ms,time repetition(TR)=26.7 ms,field of view(FOV)=22×22 cm,matrix=416×320,slice thickness=1.2 mm,slice gap=-0.6 mm,number of averages=1,and flip angle(FA)=15°.An axial 3-dimensional(3D)T1-weighted gradient echo sequence was performed to acquire the brain structure images with the following parameters:TR/TE/time inversion=7/3/400 ms,FOV=25.6×25.6 cm,matrix=256 × 256,slice thickness=1.0 mm,slice gap=-0.6 mm,number of averages=1,FA=12° .All the participants underwent MRI examination using the same imaging protocols.
Imaging analysis
The MR structural image data were preprocessed under MATLAB R2013b (version 8.2.0.701) (The Mathworks,Natick,MA,USA) environment.Image coregistration,normalization and voxel-based analysis were performed with Statistical Parameters Mapping software (SPM)(v12.0) (https://www.fil.ion.ucl.ac.uk/spm/software/).The magnitude and phase images of the QSM images were imported into the STI Suite (V3.0) software (STI.SUITE.MRI@gmail.com),and the magnetic susceptibility images were automatically reconstructed using the default setting.The 3D T1 images were coregistered with the magnitude images (TE=4.72 ms) to generate the warped T1 images,which were normalized with the T1 template (provided by the SPM software) to obtain the normalized parameters for processing the raw quantitative susceptibility images to generate normalized susceptibility images.The normalized images were smoothed by 8 mm full width at the half maximum(FWHM).Two-samplet-test was performed between the MOH patients and the normal subjects with age and gender as the covariances.The susceptibility value of the positive clusters was extracted based on a binary clusters mask obtained from the previous voxel-based analysis.
Statistical analysis
Statistical analysis of the data was performed using MedCalc (V19.0.4).Independent samplet-test was used for comparison of clinical variables between the two groups,and gender distribution was compared with Chi square test.The data with normal distribution are presented asMean±SD,and a significant difference was set at aPvalue of <0.05.Voxel-based QSM analysis was performed using the SPM software,and two-sample t-test was used to investigate the positive regions with the susceptibility value in MOH patients in comparison with the normal controls.APvalue of <0.001 without false discovery rate (FDR) correction was considered to indicate a significant difference.Pearson correlation analysis was performed between the susceptibility values of all the positive brain regions and the clinical variables,and a significant correlation was defined for aPvalue of <0.05.
RESULTS
Demographic and clinical data of the participants
Thirty-seven MOH patients(8 males and 29 females)and 27 normal control subjects(9 males and 18 females)were enrolled in the study,and their demographic and clinical characteristics are shown in Tab.1.HAMA,HAMD,and MoCA scores differed significantly between the two groups (allP<0.05),and gender distribution,age and MMSE were comparable between them(P>0.05).
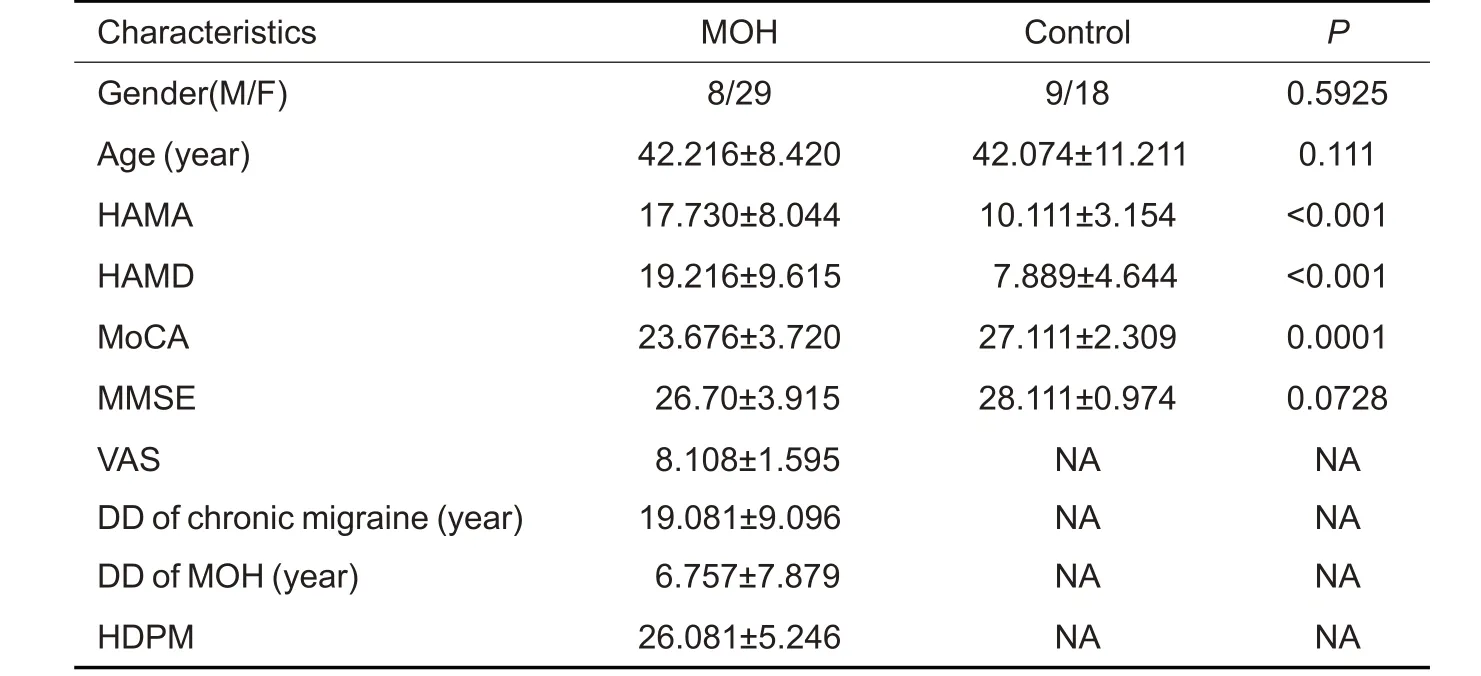
Tab.1 Clinical characteristics of MOH patients and normal control subjects(Mean±SD)
Whole brain susceptibility value increases in MOH patients
Fig.1 shows the brain regions with increased susceptibility value in the bilateral substantia nigra (SN)of MOH patients,and more detailed information of the regions with altered iron deposition over the whole brain in MOH is listed in Tab.2.No brain regions showed significant decrease of iron deposition in MOH patients as compared with the normal control subjects.
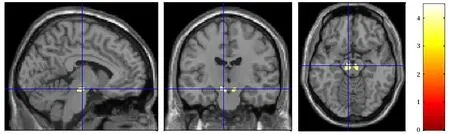
Fig.1 Brain regions(the bilateral SN)with increased susceptibility value over the whole brain in MOH patients.
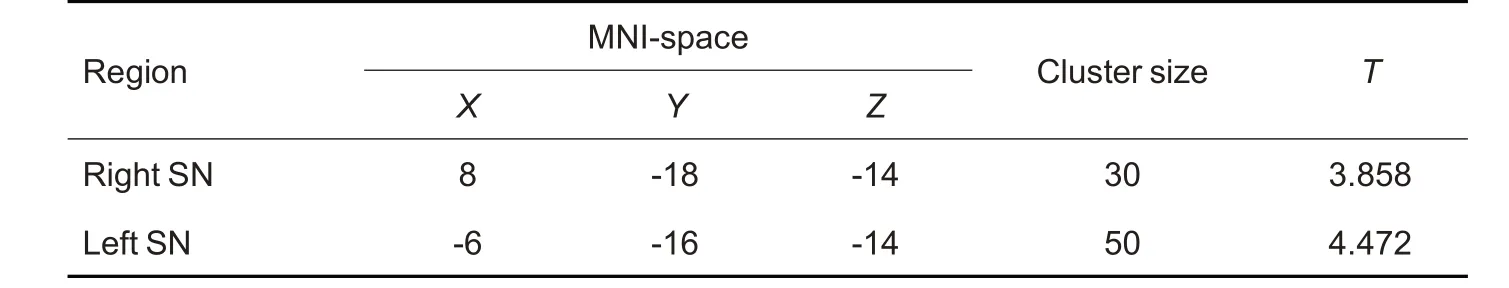
Tab.2 Brain regions with increased susceptibility value over the whole cerebrum in MOH patients
Receiver operating characteristics (ROC) analysis of susceptibility value
We conducted ROC analysis to assess the diagnostic value of susceptibility value of the bilateral SN.The results(Fig.2)showed that the area under curve(AUC)of the left SN was 0.734 (P<0.001,95%CI: 0.609-0.837),and at the cut-off value of 0.077,the susceptibility value had a sensitivity of 72.97% and a specificity of 70.37%for distinguishing MOH patients from normal control subjects.The right SN had an AUC of 0.699 (P=0.003,95%CI: 0.572-0.808),and at the cut-off value of 0.084,the sensitivity and specificity of the susceptibility value was 72.97%and 62.96%,respectively,for distinguishing MOH patients.
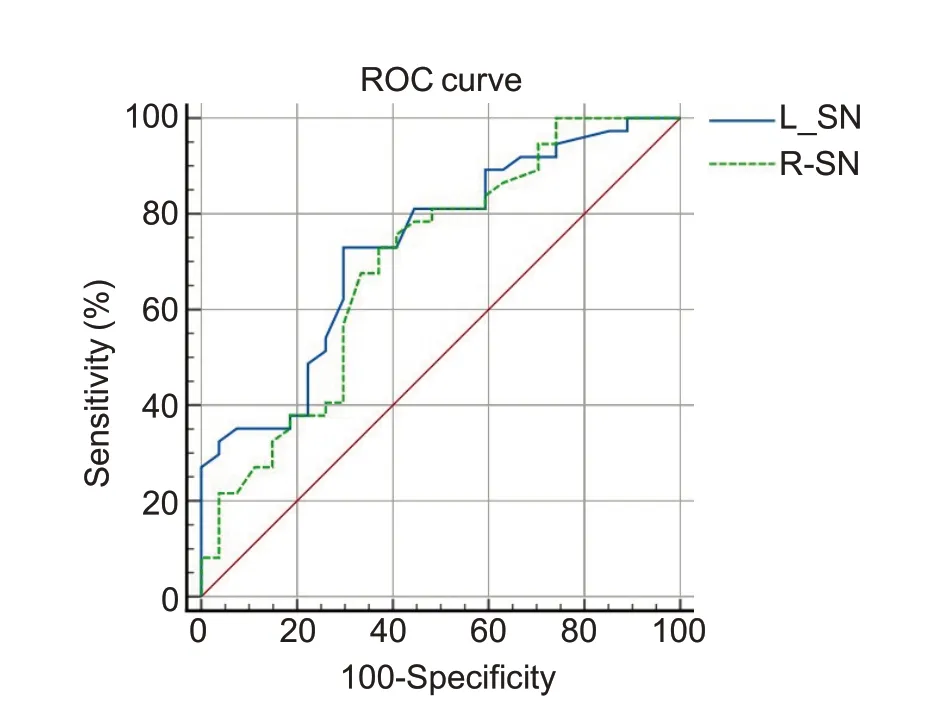
Fig.2 ROC curve of MOH versus normal controls with the AUC of 0.734 in the left SN and 0.699 in the right SN.
Correlation between susceptibility value of positive brain regions and clinical variables
The susceptibility value of the bilateral SN showed no significant correlation with the patients' clinical variables,including HAMA,HAMD,MoCA,VAS,and MIDAS scores,disease duration or headache frequency(P>0.05,Tab.3).
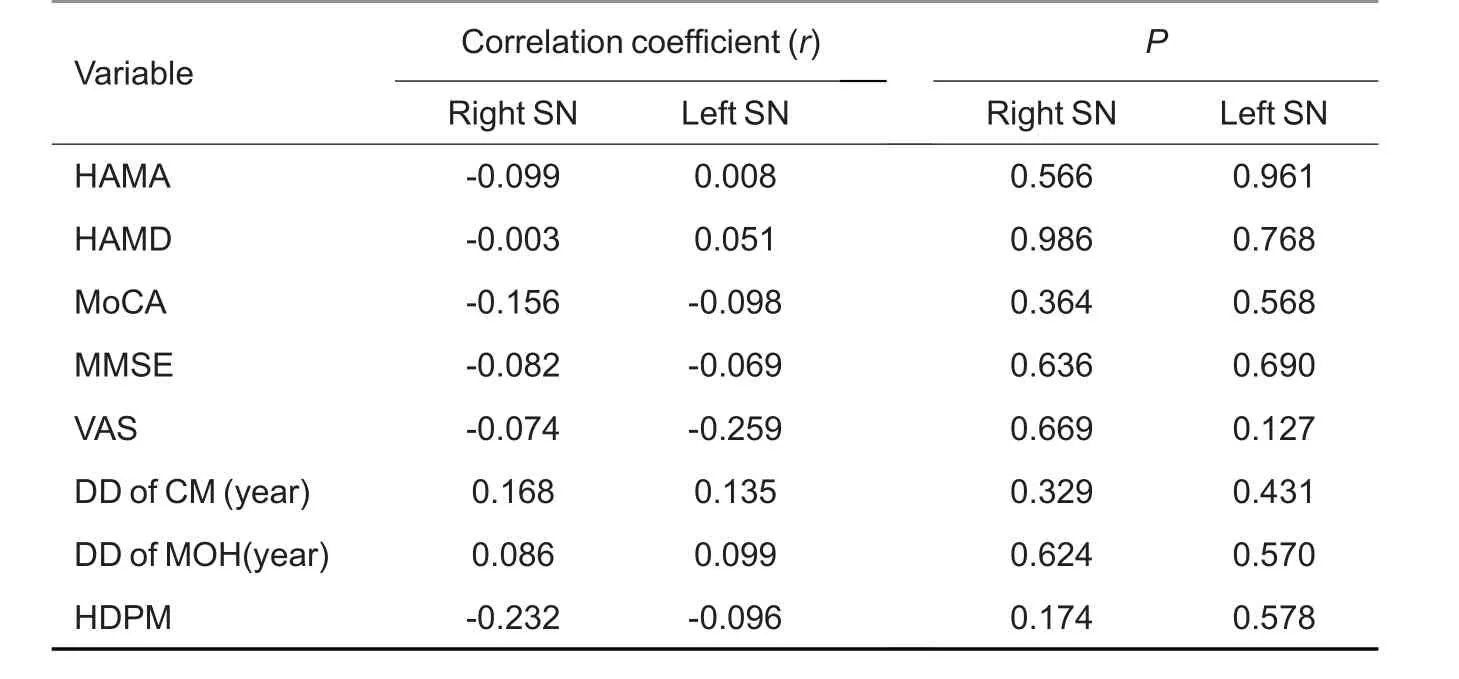
Tab.3 Correlation analysis between susceptibility value of all the positive brain regions and clinical variables
DISCUSSION
Excessive iron content in the brain contributes to the production of free radicals,which may cause oxidative damage of the neurons and other brain cells[14].In this study examined the changes in iron deposition in MOH patients to explore its role in the pathophysiological processes of MOH.
Our psychiatric evaluation demonstrated that the MOH patients had greater anxiety and depression burdens than the normal control subjects,as was consistent with the findings of previous epidemiologic studies[4,15].Sances et al[16]suggested that the presence of psychiatric disorders was an independent modifiable riskfactor for MOH,but whether these psychiatric comorbidities were the triggers or the consequences of MOH had not been elucidated and awaits further longitudinal investigation.A comparative study reported that psychiatric disorders occurred significantly more often before the transformation from migraine into MOH than after that[17].Overall,the treatment of migraine should involve a multidisciplinary approach,stressing to identify and,if necessary,eliminate the possible risks and the comorbidity factors[18].
In this QSM study,significant iron deposition of the SN was observed in MOH patients as compared with the healthy control subjects.This result was consistent with the finding reported by Welch and colleagues in chronic daily headache[6],suggesting the presence of iron metabolism dysfunction in MOH patients.But this result differed from that of a previous study,where iron deposition was not found in the whole brain of MOH patients[8].The discrepancy might be partly due to the technical constraints,such as a poor contrast.Before QSM was used to detect iron deposition in migraine patients,T2 and T2* sequences were more frequently used[7-9]for semi-quantitative assessments[19].QSM can not only quantitatively visualize iron deposition in the brain,but also avoid the influence of magnetically sensitive materials such as calcifications and vessels,thus allowing accurate differentiation of iron deposition foci from others[20].In addition,voxel-based QSM technique is capable of objectively and sensitively detecting iron deposition level in MOH,and its performance is far beyond that of the conventional T2,T2*and SWI sequences.
Compared with chronic migraine,where the susceptibility value increases in multiple brain regions[21],MOH shows only a single brain region involvement,which might be another feature of MOH in association with the consequence of analgesic overuse[22].In fact,analgesic overuse was observed more often in chronic migraine than in other common pain conditions[23].However,the causative role of iron deposition in substance addiction has remained speculative.The SN is a critical structure of the mesocorticolimbic dopamine system,and exposure to addictive drugs can stimulate this crucial circuitry to promote compulsive drug-seeking behavior[24].Ferraro et al[25]observed that MOH patients presented with persistent dysfunctions in the SN/ventral tegmental area complex (SN/VTA) using functional MRI(fMRI),which indicated the possible involvement of the dopaminergic reward system in the mechanism of MOH,similar to that of addiction.In this study we found that iron deposition was increased in the SN of MOH patients,suggesting altered iron metabolism in the mesocorticolimbic dopamine circuit,which may cause volume expansion of the bilateral SN[26].Therefore,iron deposition may potentially serve as a new indicator for evaluating the changes in the mesocorticolimbic dopamine system after medication withdrawal in MOH patients.
We performed ROC analysis of iron deposition in the bilateral SN for assessing its diagnostic value for MOH.The results showed that iron deposition in the left SN had a moderate accuracy (AUC=0.734,P<0.001) for distinguishing MOH from normal controls,while the right SN had a relatively low diagnostic accuracy(AUC=0.699,P=0.003).At the cut-off values of 0.077 and 0.084,respectively,iron deposition in the left and right SN both had good diagnostic sensitivity and specificity,but their AUCs alone were not sufficient to establish a differential diagnosis of MOH against the normal control,which still relies on a comprehensive evaluation of multiple radiographic and clinical evidences.
Although previous studies suggested that MOH patients were vulnerable to stress and negative emotional reactions such as anxiety and depression[17,27],in this study we did not find any significant correlation of the susceptibility value of the SN with the clinical variables of the patients.It is likely that iron deposition participates in the development of MOH as an independent factor,which is not directly related to anxiety,depression,or cognition of the patients.
This study has several limitations: (1) The sample size of MOH patients was relatively small because of the strict inclusion criteria,and further investigation with a larger sample size is warranted to verify the distribution characteristics of iron deposition;(2) This study was a cross-sectional observation,which could not establish a causal relationship between iron deposition and the overuse of analgesics;(3) Although QSM technique detected increased iron deposition in some brain nuclei of MOH patients,whether these alterations cause impairment of the nuclei remains to be investigated.
CONCLUSION
In conclusion,we found in this study that patients with MOH have increased iron deposition in the bilateral SN.The non-invasive QSM technique can provide an effective means for quantitative detection of brain iron deposition in migraine neuroimaging.