Related mechanisms and research progress in straight back syndrome
Mo-Wei Kong,Zhen-Ying Pei,Xiong Zhang,Qiu-Juan Du,Qiang Tang,Jun Li,Guo-Xiang He
Abstract Despite the high prevalence of straight back syndrome (SBS),there is still limited research on this condition,posing challenges for effective diagnosis and treatment.The disease has been known for a long time,but there have been few related studies,which mostly consist of case reports.These studies have not been systematically summarized,making it difficult to meet the current needs of diagnosis and treatment.This article summarized the existing literature and comprehensively reviewed the diagnosis,pathogenesis,treatment,and research status of mitral valve prolapse related to SBS.We specifically emphasized the mechanisms and prognosis of SBS combined with mitral valve prolapse and discussed the latest research progress in this disease.
Key Words: Straight back syndrome;Mitral valve prolapse;Arrhythmia;Review;Diagnosis;Treatment
INTRODUCTION
Straight back syndrome (SBS),also known as flat chest syndrome,is characterized by the disappearance of the normal kyphotic curvature of the thoracic spine,which results in a decrease in the anterior-posterior diameter of the chest and restriction of the heart (Figure 1).It was first reported by Rawlings[1] in 1960.Due to the presence of heart murmurs during cardiac examinations,patients are often referred to the cardiology department for evaluation[2].Mitral valve prolapse (MVP) is a valvular heart disease characterized by soft texture of the mitral valve,which can billow upward and back into the left atrium during heart contraction (prolapse).Prolapse of the mitral valve can lead to mitral regurgitation.If the MVP causes significant enlargement of the atria,arrhythmias may occur.Previously,SBS-associated MVP was considered a “pseudo-heart disease,” and patients may have no symptoms or only mild clinical symptoms such as chest tightness and shortness of breath.Even when severe symptoms occur,they are mostly thought to be due to mechanical and structural changes resulting from compression of the large blood vessels in the heart[3].However,recent studies have found that SBS-associated MVP may be related to the expression of genes,which has attracted wide attention and controversy[2].
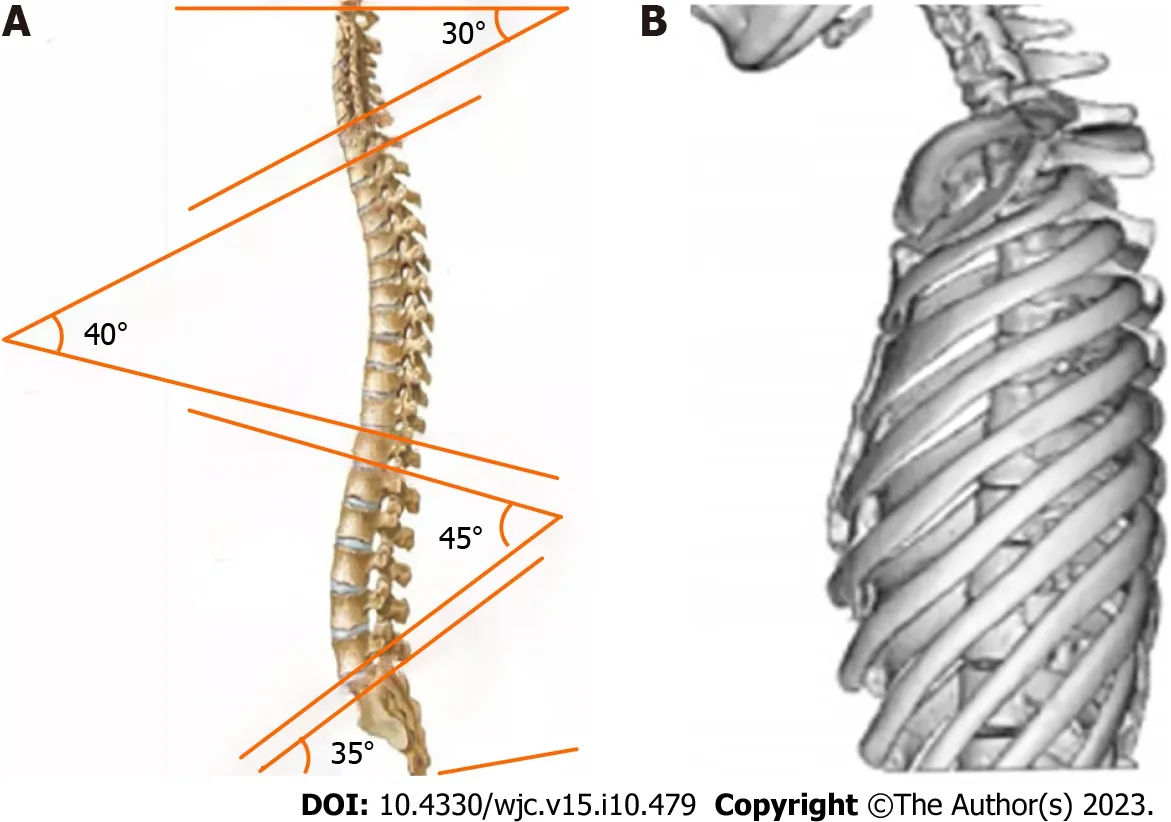
Figure 1 Differentiating between normal individuals and individuals with straight back syndrome with physiological distortion. A: The human spine typically exhibits physiological curvature;B: In individuals diagnosed with straight back syndrome,this curvature is noticeably absent.
In clinical practice,some patients with MVP are often misdiagnosed as having “senile valve disease” or “congenital heart disease” due to the lack of primary disease diagnostic criteria.In the clinic,it is not uncommon for SBS to cause changes in cardiac structure and circulatory function[4].However,the clinical manifestations can vary widely,often leading to misdiagnosis.Unfortunately,despite being discovered over 60 years ago,research on this subject remains scarce and often centers on case reports.Presently,these studies lack systematic summarization and fail to meet the current diagnostic and treatment reference needs.Therefore,this article comprehensively reviewed the literature on the diagnosis,pathogenesis,treatment,and research status of SBS-related MVP and provided clinical assistance by summarizing the latest and most significant achievements in this field.
DIAGNOSIS AND EPIDEMIOLOGY
SBS is a benign thoracic skeletal malformation that is often misdiagnosed as heart disease as it can cause heart murmurs detectable on physical examination[5].Patients are usually asymptomatic,and the most common symptoms are chest pain and palpitations.However,there is a lack of large-scale epidemiological investigations into the incidence of SBS in the general population.A previous study by Jiang and Li[6] reported that among 114 SBS patients,the vast majority of cases (108) occurred in individuals under 40 years of age,with approximately 60% (66 cases) occurring in those aged between 20-40 years.The prevalence in females was higher than that in males.The incidence rate significantly decreased,and symptoms were milder in patients over 40 years of age,which may be due to reduced lung tissue and chest wall elasticity with increasing age,leading to natural alleviation of the condition[7].A study in 2022 found that thoracic vertebrae may undergo degenerative changes with increasing age.In the general population,this change may cause excessive thoracic kyphosis,but in patients with SBS,it may relieve symptoms[8].
In 1956,Deleonetal[9] first proposed a diagnostic criterion for SBS,which was a ratio of anterior-posterior to transverse thoracic diameter less than 1/3 measured at the T8 level.In 1980,Daviesetal[10] revised this criterion and proposed a diagnostic criterion for SBS on lateral chest radiographs,where the distance from the midpoint of the T8 vertebral body to the vertical line connecting the anterior borders of T4 and T12 was less than 1.2 cm (Figure 2).SBS typically occurs in young,lean individuals who lack normal thoracic kyphosis,leading to a decreased sagittal diameter of the thorax[11].Auscultation may reveal splitting of the second heart sound,and a prominent murmur is generally caused by compression of the right ventricular outflow tract by the sternum,which lessens with deep inspiration.X-ray examination is the most important diagnostic method,especially left lateral images,where the spinal thoracic segments appear straight and the sagittal diameter is narrowed.Twiggetal[12] measured the ratio of thoracic anterior-posterior to transverse diameter in 24 patients with SBS and 100 normal individuals,revealing a mean ratio of 37.1%,which is lower than the normal value of 40.0%.Furthermore,X-ray features may include a heart-thorax ratio ≤ 0.5,protruding pulmonary artery segments,leftward shift of the cardiac silhouette,or signs of a pseudo-enlarged heart.In SBS patients,electrocardiography generally demonstrates normal values.In some patients,due to compression of the heart and positional changes,V1 leads may show a rsR’ pattern,avR for the Qr pattern,R/S >1,or R/S=1,and some individual patients may exhibit high voltage of the left ventricle,sinus bradycardia,and incomplete right bundle branch block.
Figure 2 Diagnostic criterion for straight back syndrome on lateral chest radiographs,where the distance from the midpoint of the T8 vertebral body to a vertical line connecting the anterior borders of T4 and T12 is less than 1.2 cm.
In some ultrasonography results of SBS patients,there may be a concomitant finding of MVP.Previous studies have suggested that this may be related to the compression-induced deformation of the mitral valve due to narrowing of the sagittal diameter[13].However,a study conducted in 2020 indicated that both SBS and MVP may be inherited in an autosomal dominant manner[14].It is believed that the vertebral malformation occurs during the 8thwk of gestation before ossification of the spine,and the penetrance is incomplete,with a higher expression frequency observed in female subjects.It has been reported in the international literature that 17%-23% of MVP patients have scoliosis,while 54%-67%of scoliosis patients have concomitant MVP[15].Based on these studies,it can be inferred that the higher incidence of MVP in SBS is not only a result of physical factors but is also related to genetic factors.National literature has not reported such a high incidence rate,which may be due to inadequate knowledge at the time.
SBS WITH COMORBIDITY MECHANISMS
SBS is often associated with several complications,and the underlying mechanisms are still not fully understood.One theory suggests that compression of the thorax could reduce lung capacity and cause hypoxia,leading to various cardiovascular and respiratory problems.Recent studies have shown that compression may also affect the functioning of the autonomic nervous system,leading to abnormal heart rate variability[16].Additionally,the reduced sagittal diameter of the chest could lead to mechanical distortion of the heart and major vessels,causing abnormal blood flow[17].In the study by Grilloetal[18],MVP was the most common associated cardiac disease,occurring in approximately 64% of SBS cases.Some studies suggest that the high incidence of MVP and scoliosis in SBS patients may be related to a common genetic background,but further research is needed to clarify this relationship.
Mechanisms underlying the co-occurrence of MVP in SBS
In several previous studies,there was consistent evidence of the co-occurrence of SBS and MVP[19],but it was not until 2017 that Movahedetal[20] demonstrated a statistically significant association between these two conditions.In their study,77% of SBS patients underwent mitral valve repair or replacement due to severe mitral regurgitation caused by MVP.There are two hypotheses regarding the mechanism of SBS co-occurring with MVP.The first hypothesis,proposed by Chenetal[3],suggests that scoliosis and MVP are both manifestations of a more common disease,which is inherited in an autosomal dominant manner with incomplete penetrance.Family studies have found a tendency towards clustering of MVP and scoliosis,indicating that these diseases may have a genetic basis.
Additionally,a number of genome-wide association studies have identified several genetic variations associated with SBS,some of which may affect the susceptibility of MVP and scoliosis.For example,one study found that theLRP2gene located at 9q22.32 was associated with the susceptibility of scoliosis and MVP[21],whereas another study found a close relationship between theEFEMP1gene located at 5q35.3 and both scoliosis and MVP[22].Furthermore,recent research has suggested that the MVP comorbidity in SBS may be related to the TGF-β signaling pathway,which induces cardiomyocyte apoptosis and promotes fibroblast proliferation[23].
It has also been discovered that SBS and floppy mitral valve disease are both inherited in an autosomal dominant manner with a significant family history,and three possible gene loci (16p12.1-p11.2,11p15.4,and 13p31.3-p32.1) have been reported[24].Floppy mitral valve causes enlargement of the mitral valve orifice area,elongation or rupture of the chordae tendineae,and enlargement of the mitral annulus,ultimately leading to MVP.It is believed that these malformations occur during the 8thwk of gestation before ossification of the spine,with incomplete penetrance and higher expression frequency in female subjects[4].These studies suggest that genetic variations and molecular mechanisms may play important roles in the high incidence of MVP and scoliosis in SBS patients.However,the notion of a “more common disease” proposed by Chenetal[3] has yet to be proven.
Another hypothesis suggests that physical compression is a primary factor leading to the co-occurrence of MVP in SBS.The thoracic spine of SBS patients loses its normal posterior convex curvature,resulting in a decreased distance between the sternum and spine.This leads to compression of the heart and torsion of the major vessels,ultimately leading to distorted mitral valve morphology due to pressure[25].The study by Chenetal[3] indirectly supported this view using cardiac magnetic resonance imaging to identify a correlation between the site of cardiac compression in SBS patients and the occurrence of arrhythmia.This evidence indicated that some,if not all,of the cardiac alterations seen in SBS are influenced by physical compression factors.
Mechanisms underlying the cardiac morphological changes and arrhythmia comorbidity in SBS
The causes of cardiac morphological abnormalities in SBS may include: (1) Differing degrees of cardiac pulsation restriction due to varying degrees of front-to-back narrowing of the thoracic cavity caused by flat thoracic vertebrae;(2)Left atrial compression,which increases pulmonary circulation resistance and reduces returning blood volume;and (3)Long-term frequent rapid arrhythmia causing cardiac enlargement and decreased cardiac function.In the study by Chenetal[3] among SBS patients with cardiac morphological abnormalities,54.2% showed a consistent location between arrhythmia origin and the cardiac compression site,suggesting that mechanical compression of the heart may lead to enhanced aberrant electrophysiological activity in the heart through the activation of self-regulatory mechanisms.
Previous understanding of SBS was limited to chest wall deformities,which may cause mild symptoms such as palpitations,chest tightness,and chest pain,while severe cases may have morphological functional changes in the heart chambers,valves,and major vessels.However,recent studies have compared cardiac magnetic resonance imaging,electrocardiogram,and electrophysiological data of 43 SBS patients and found a relationship between the cardiac compression site and arrhythmia occurrence in addition to the morphological and functional changes caused by flat chest walls[3].This study proposed that arrhythmias are the result of cardiac compression.However,a study conducted in 2013,found that MVP comorbid with various arrhythmias is very common,with ventricular arrhythmias being the most frequent,potentially due to increased sympathetic nervous activity and stimulation of the myocardium by prolonged chordae tendineae[26].Given that MVP frequently co-occurs with SBS,it may be one of the reasons for the frequent occurrence of arrhythmias in SBS.This view has been partially confirmed by recent research.In the study by Xiaetal[27],8.3% of MVP patients were found to have preexcitation syndrome,and follow-up results showed a generally good prognosis for SBS comorbid with MVP.
SBS comorbid with cardiac murmurs
In SBS patients,cardiac murmurs may sometimes be heard on auscultation,but detailed examination often reveals no cardiac abnormalities.There have been reports of SBS being misdiagnosed as cardiac diseases with abnormal heart sounds,such as atrial septal defect,pulmonary valve stenosis,or mitral regurgitation[3].The mechanism underlying cardiac murmurs in SBS has not been well studied,but a more credible explanation is that the loss of thoracic physiological kyphosis in SBS patients causes displacement of the heart and great vessels.This leads to the right ventricle and pulmonary artery being closer to the posterior sternum,increasing the contact area between the sternum and the posterior heart margin,resulting in a “strengthened” jet effect of blood flow,almost invariably causing grade I-III systolic murmurs in the pulmonary valve auscultation area of each patient[28].Due to prolonged compression of the heart on the dorsal margin of the sternum,the heart is subjected to long-term overload,causing hypertrophy of cardiomyocytes characterized by increased cell volume,diameter,and length.When hypertrophy reaches the critical limit,it increases the systolic wall tension of the ventricular wall,leading to the parallel proliferation of myocardial fiber cells,followed by thickening of the myocardial fibers.As a result,the thickness of the ventricular wall increases and the cavity does not expand significantly,leading to outflow tract obstruction and the production of murmurs (Figure 3).
Figure 3 Comparison of right ventricular outflow tract between normal patients and patients with straight back syndrome. A: Right ventricular outflow tract patency in a normal individual;B: Right ventricular hypertrophy and narrowing of the right ventricular outflow tract in a patient with straight back syndrome.
RESEARCH STATUS
It has been over 60 years since SBS was first reported in 1960[1].Despite the long history,the literature on this topic remains relatively scarce,with case reports dominating the available data (Figure 4).According to recent statistics,the past 10 years have seen the greatest SBS-related research activity,and significant breakthroughs have been made.

Figure 4 Distribution of straight back syndrome publications from 1953 to 2022.
Xuetal[29] conducted a retrospective analysis of 16 cases of misdiagnosed situs inversus totalis and summarized the clinical features of SBS patients.The authors emphasized the importance of chest X-ray lateral films and concluded that echocardiography is an effective diagnostic tool for SBS.Another study conducted in the same year also highlighted the importance of chest X-ray lateral films as a key diagnostic feature of SBS (Figure 5)[30].
Houetal[31] investigated a method for diagnosing SBS by combining the ratio of the distance from the anterior edge of the T8 vertebral body to the posterior edge of the sternum to the anterior-posterior diameter of the thorax with the ratio of the distance from the anterior edge of the T8 vertebral body to the posterior edge of the sternum to the transverse diameter of the thorax and the curvature arc height from T3 to T12.Recently,studies have also shown that this diagnostic method was more reliable for diagnosing SBS[32-35].In a recent study of 1569 randomly selected patients who underwent 64-row chest computed tomography (CT) scans,it was found that CT could identify signs that were not visible on X-ray films,leading to a more accurate diagnosis of SBS and a better correlation of clinical symptoms with imaging findings,as has also been reported in studies overseas.Matsumotoetal[33] recently used echocardiography and right ventricular angiography to uncover the mechanism underlying the change in heart murmur with respiration,which they found to be due to variation in the diameter of the right ventricular outflow tract during respiration.It was not until 2017 that Marbellaetal[34] investigated the statistical correlation between SBS and MVP and revealed that 27% of patients with severe mitral valve regurgitation caused by MVP also had SBS.
In terms of treatment,Betzetal[35] reported the successful case of a 19-year-old patient with SBS who presented with spinal pain and exertional dyspnea.The patient’s symptoms were relieved by a 12-wk course of treatment involving corrective exercises,traction,and posture adjustment.In another study,the use of 3D printing technology to simulate chest wall replacement tissue was reported to alleviate severe compression symptoms in an SBS patient[17].Another complication of SBS is tracheomalacia caused by chronic compression of the trachea and main bronchi,resulting in decreased mediastinal diameter.In 2021,Schmidetal[36] successfully cured a 36-year-old female patient suffering from this condition by proximal aortic,brachial artery,sternoplasty,and anterior tracheal fixation surgery.
SBS is a benign thoracic skeletal abnormality that is usually associated with MVP and a heart murmur often detected during physical examination.Patients are usually asymptomatic,with chest pain and palpitations being the most common symptoms.X-ray examination is the most important diagnostic tool,showing a straightened thoracic spine and a decreased anterior-posterior diameter in the thoracic segment.Recent studies have confirmed the importance of echocardiography and CT in the diagnosis of SBS.The mechanism of SBS with MVP may be due to genetic or physical factors (compression),and the mechanism of heart murmur may also be due to physical factors or indirect causes following MVP.
Although SBS was discovered some time ago,there have been relatively few related studies.Recent studies have explored the importance of diagnostic tools in SBS and reemphasized the clinical features of SBS to avoid misdiagnosis.Some studies have also identified the mechanisms of arrhythmia in patients with SBS and the possible mechanism of heart murmur changing with respiration.Progress has been made in the treatment of SBS in some patients with severe compression symptoms through corrective exercise,traction,3D printing technology,and surgery.
All these studies have achieved success under the development of new equipment and technologies,which may be one of the reasons for the rapid increase in related research in the past decade.Looking back on the development of medicine in recent years,adjusting or updating existing diagnostic and treatment methods to improve the quality of life of SBS patients and reduce or eliminate serious complications caused by diseases is the focus of clinical physicians and an important direction of academic research.We hope that this article will serve as a stimulus for future research and provide new ideas.
CONCLUSION
SBS is a thoracic skeletal malformation often accompanied by MVP.Diagnostic criteria include the ratio of anteriorposterior to transverse thoracic diameter and specific X-ray features.The co-occurrence of SBS and MVP may be due to genetic variation and molecular mechanisms or physical compression.Cardiac morphology and arrhythmia in SBS may be caused by restricted cardiac pulsation,left atrial compression,and sympathetic activity.Recent studies have emphasized the importance of chest X-rays,echocardiography,and CT scans for diagnosing SBS.New methods for diagnosing SBS with MVP have been proposed.Treatment options include exercise,traction,posture adjustment,3D printing,and surgical interventions.
ACKNOWLEDGEMENTS
We would like to express our gratitude to Li-Gang Xiao and Chun-Jiao Zhao for their valuable feedback and constructive suggestions on the manuscript.We also thank Yun-Bo Lai,Wen-Qing Zhang,and Li Xu for their efforts in translating and polishing the manuscript.
FOOTNOTES
Author contributions:He GX and Li J provided crucial suggestions and guidance for the writing;Kong MW wrote the manuscript;Pei ZY,Zhang X,Du QJ,and Tang Q reviewed and revised the manuscript;All authors read and approved the final manuscript.
Conflict-of-interest statement:The authors declare that they have no competing interests.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Mo-Wei Kong 0000-0002-1214-164X.
S-Editor:Lin C
L-Editor:Filipodia
P-Editor:Yuan YY
 World Journal of Cardiology2023年10期
World Journal of Cardiology2023年10期
- World Journal of Cardiology的其它文章
- Value of cardiac magnetic resonance on the risk stratification of cardiomyopathies
- Integrated analysis of comorbidity,pregnant outcomes,and amniotic fluid cytogenetics of fetuses with persistent left superior vena cava
- Establishment of a prediction model for prehospital return of spontaneous circulation in out-of-hospital patients with cardiac arrest
- Cardiovascular complications following medical termination of pregnancy: An updated review
- Do cardiopulmonary resuscitation real-time audiovisual feedback devices improve patient outcomes? A systematic review and metaanalysis
- Systemic right ventricle complications in levo-transposition of the great arteries: A case report and review of literature
