Gut microbiome supplementation as therapy for metabolic syndrome
Mc Anto Antony,Aniqa Chowdhury,Dinesh Edem,Rishi Raj,Priyanshu Nain,Mansi Joglekar,Vipin Verma,Ravi Kant
Abstract The gut microbiome is defined as an ecological community of commensal symbiotic and pathogenic microorganisms that exist in our body.Gut microbiome dysbiosis is a condition of dysregulated and disrupted intestinal bacterial homeostasis,and recent evidence has shown that dysbiosis is related to chronic inflammation,insulin resistance,cardiovascular diseases (CVD),type 2 diabetes mellitus (T2DM),and obesity.It is well known that obesity,T2DM and CVD are caused or worsened by multiple factors like genetic predisposition,environmental factors,unhealthy high calorie diets,and sedentary lifestyle.However,recent evidence from human and mouse models suggest that the gut microbiome is also an active player in the modulation of metabolic syndrome,a set of risk factors including obesity,hyperglycemia,and dyslipidemia that increase the risk for CVD,T2DM,and other diseases.Current research aims to identify treatments to increase the number of beneficial microbiota in the gut microbiome in order to modulate metabolic syndrome by reducing chronic inflammation and insulin resistance.There is increasing interest in supplements,classified as prebiotics,probiotics,synbiotics,or postbiotics,and their effect on the gut microbiome and metabolic syndrome.In this review article,we have summarized current research on these supplements that are available to improve the abundance of beneficial gut microbiota and to reduce the harmful ones in patients with metabolic syndrome.
Key Words: Gut dysbiosis;Metabolic syndrome;Diabetes mellitus;Prebiotics;Probiotics;Postbiotics
lNTRODUCTlON
The gut microbiome is defined as an ecological community of commensal symbiotic and pathogenic microorganisms that exist in the body[1,2].Gut microbiome dysbiosis is defined as dysregulated and disrupted intestinal bacterial homeostasis[2,3].Recent evidence has shown that dysbiosis is related to chronic inflammation,insulin resistance,type 2 diabetes mellitus (T2DM),cardiovascular diseases (CVD),and obesity[2].It is known that obesity,T2DM and CVD are caused or worsened by multiple factors like genetic predisposition,environmental factors,unhealthy high calorie diet,and sedentary lifestyle[4-6].However,recent evidence from human and mouse models suggests that the gut microbiome is also an active player in the modulation of these diseases[7].Host and gut microbiome dysbiosis can influence local or systemic immunity and inflammation by regulating intestinal barrier permeability or by triggering the innate immune system as seen in obesity and T2DM[7].AkkermansiamuciniphilaandFaecalibacteriumprausnitziiare among the protective bacteria that play a significant role in maintaining this intestinal barrier[7].Hyperglycemia in T2DM can disrupt this intestinal barrier,which causes gram negative bacterial products like lipopolysaccharides (LPS) to enter the systemic circulation,leading to endotoxemia,and further local and systemic inflammation[3,7].
Metabolism is the process used by the body to create energy from the food we eat,and metabolic diseases,such as type 2 diabetes and obesity,occur due to metabolic dysregulation.Metabolic syndrome refers to a set of risk factors including hyperglycemia,dyslipidemia,and obesity that increases the risk for CVD,T2DM,non-alcoholic fatty liver,and other diseases[8].Animal studies have shown a causal link between the gut microbiome profile and metabolic syndrome[8].A study in mice fed a 30-d high fat diet showed significant increase of bacteria of the phylumFirmicutesandProteobacteriawith reduction ofBacteroidetesandVerrucomicrobia[9].Another study with high-glucose or high-fructose fed mice showed the gut microbiome of these mice to be significantly altered with an increase inProteobacteriaand decrease inBacteroidetes[10].
The gut microbiota consume the host's diet and produces certain metabolites which act on the host receptors and exert their endocrine effects,leading to hormone secretion,inflammation,and insulin resistance[11].Studies have shown that these microbiota are sensitive to the host's diet composition and the microbiome diversity changes with animal vs.plantbased diets[1].The gut microbiome produces certain beneficial metabolites like short chain fatty acids (SCFA)[2],such as butyrate which promotes colonic health and is protective against T2DM and CVD[1,3,7,11];propionate which promotes the release of glucagon like peptide-1 and peptide YY,which improves insulin sensitivity and weight[2].Secondary bile acids are converted from primary bile acids by the gut microbes,which activate takeda G protein coupled receptor 5,increasing cyclic adenosine monophosphate production,improving insulin sensitivity,interacting with the farnesoid X receptor,pregnane X receptor and vitamin D receptor to regulate lipid metabolism and glucose metabolism,subsequently slowing the progression of CVD and T2DM[7,11].Other favorable metabolites are esculin,anthocyanin,urolithin A,and enterolactone[3].
Research has also revealed some unfavorable metabolites produced by the gut microbiome,of which trimethylamine is the most prominent.Trimethylamine is oxidized to trimethylamine N-oxide which works by increasing low-density lipoprotein uptake in cells,reducing cholesterol excretion,and promoting recruitment of activated leukocytes and platelet aggregation,and thus,trimethylamine promotes atherosclerosis,thrombosis,CVD and diabetes[3,12].In patients with chronic kidney disease (CKD),studies have shown that high indoxyl sulfate levels predict major adverse cardiac events,high P-cresyl sulfate levels correlate with CVD and all-cause mortality,and phenylacetylglutamine is associated with overall mortality and CVD[13,14].Since all of the above mentioned metabolites are uremic toxins,CKD can increase the buildup and worsen the effects of these metabolites resulting in CVD progression[3].
Current research is directed towards finding treatment options for improving the number of beneficial gut microbes and to reduce the harmful ones with the use of probiotics and prebiotics[7].Efforts are also underway to identify novel gut microbiome-host interactions,their associations and mechanisms leading to T2DM,CVD and to design new therapies to modulate these disease processes[11].The purpose of this article is to summarize the use of microbiome supplementation to improve the abundance of the beneficial gut microbes and to reduce the harmful ones in patients with diabetes mellitus and metabolic syndrome.
PREBlOTlC,PROBlOTlC,SYNBlOTlC AND POSTBlOTlC SUPPLEMENTATlON
Microbiome supplementation has been found to alter the composition of the gut microbiome and possibly have effects on human health and diseases[15].There are four types of supplements that are studied.In 2016,the International Scientific Association for Probiotics and Prebiotics (ISAPP) defined prebiotics as a substrate utilized selectively by microorganisms in the host and conferring a benefit to the host's health[16].The widely accepted scientific definition of probiotics as defined by an expert panel convened by the ISAPP in 2013 is “Live microorganisms that,when administered in adequate amounts,confer a health benefit on the host”[17].In 2019,the ISAPP convened and defined synbiotics as,“a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host”;thus,synbiotics are a combination of prebiotics and probiotics[18].Lastly,in 2019,ISAPP defined postbiotics to be inanimate microorganisms with or without their components that confer a health benefit to the host[19].This section will review the current data for each of these supplements and directions for future research.Table 1 summarizes the definitions and examples of the each of these supplements and we have summarized the similarities and differences between each supplements' influence on metabolic syndrome in Table 2 and presented the same in a figure format in Figure 1.
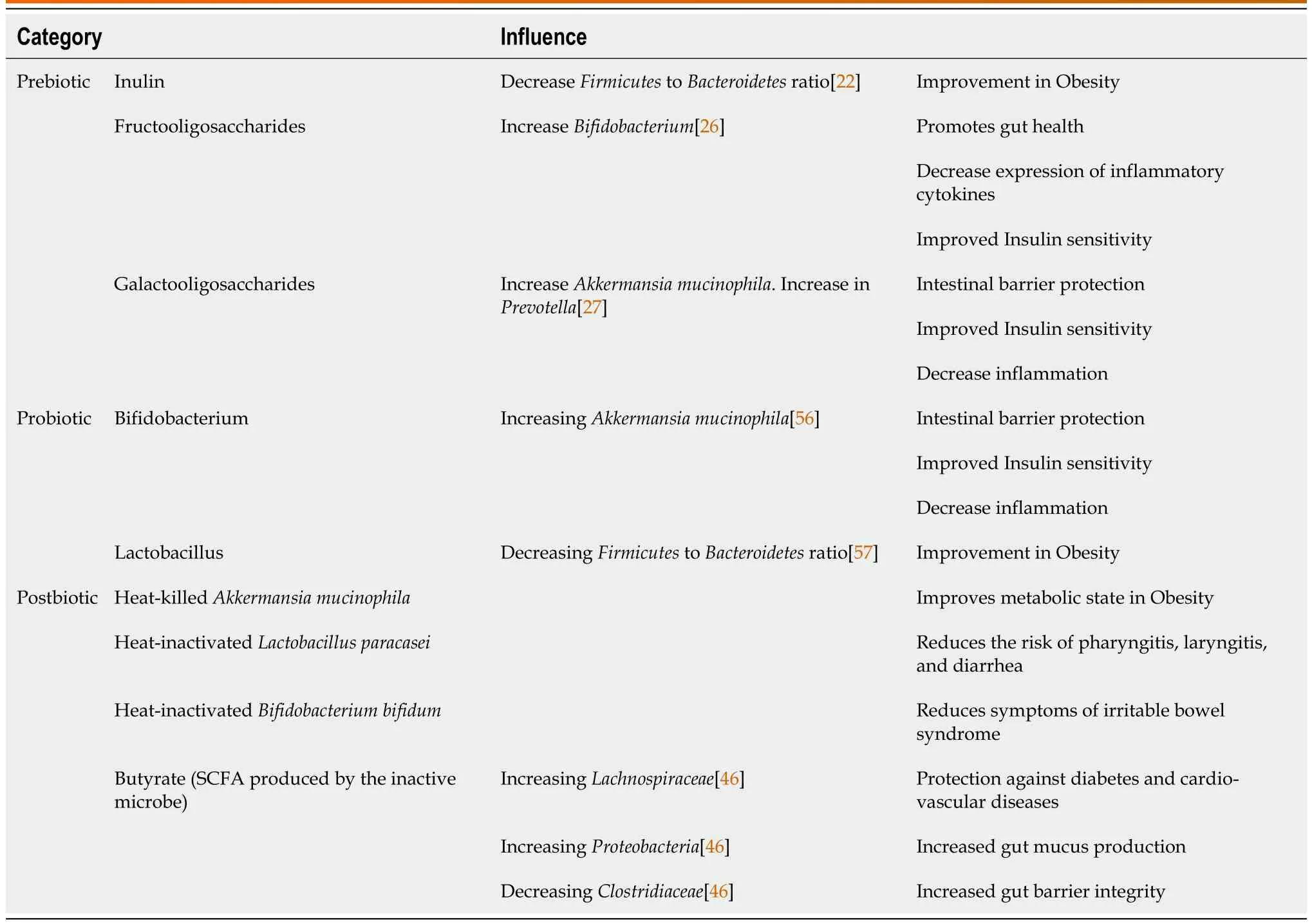
Table 2 The influence of prebiotics,probiotics,and postbiotics on metabolic syndrome
Prebiotic
Prebiotics are consumable substances selectively utilized by microorganisms in the host and confer a benefit to the host[16].Prebiotic effects have been studied in several metabolic diseases with animal studies,but few studies have been done on humans.Inulin,lactulose,fructooligosaccharides (FOS),and galactooligosaccharides (GOS) are the most widely known prebiotics[20].Other prebiotics include human milk oligosaccharides,polydextrose,pectic oligosaccharides,arabinoxylans,and xylooligosaccharides[21].Prebiotics confer a wide range of health benefits,including immune modulation through increased production of interleukins and immunoglobulins with reduction of pro-inflammatory interleukins and production of SCFAs,such as butyrate and acetate[21].SCFAs indicate bacterial fermentation in the gastrointestinal tract and improve the health of the gut through mucus production,protecting against inflammation,and promoting the intestinal barrier integrity[21].Production of SCFAs also results in a reduction on intestinal pH,inhibiting the growth of pathogenic bacteria[21].
Bacteria that promote gut health,such asBifidobacteriumandLactobacillus,proliferate upon consumption of prebiotics[11].Inulin oligofructose supplementation in mice fed a high fat diet showed a reduction in theFirmicutestoBacteroidetesratio[22],and reduction in theFirmicutestoBacteroidetesratio has been the hallmark of obesity[23,24].A study done with a group of 10 elderly women over 19 days of inulin supplementation showed an increase inBifidobacteriaand decrease inEnterococciandEnterobacteriaceae[21],which is associated with a decreased risk of inflammatory bowel disease[25].FOS supplementation in a rat model improved the gut microbiome by increasingBifidobacterium[26].Additional,fermentation of FOS generates SCFAs,decreasing the luminal pH and increasing the bioavailability of minerals in the gut[21].GOS supplementation for prolonged periods in mice fed with a western diet led to increased abundance inAkkermansia mucinophilaandPrevotella[27].In another study,the activity of GOS was analyzed in sequencing fecal samples from humans after GOS administration,and the data showed an increased inFacecalibacteriumprausnitziiandBifidobacteriawith a decrease inBacteroides[21].Facecalibacteriumprausnitziiproduces the SCFA butyrate and is associated with reduced inflammation[28].Bifidobacteriapromotes gut health,decreases expression of inflammatory cytokines,and improves insulin sensitivity[29].Decrease inBacteroidesis beneficial to humans,as it is a pathogen common in anaerobic infections with significant antibiotic resistance[30].
Additional studies found that treatment with prebiotics improved glucose homeostasis and increased leptin sensitivity[31].It also decreased inflammation by improving gut barrier integrity,thus decreasing the number of endotoxins able to leak from the gut lumen into the bloodstream[32].They have also been shown to have a regulatory effect on metabolic disorders,especially those associated with obesity such as dyslipidemia,hypertension,diabetes,and liver steatosis[33].Thus,severalinvivoandinvitrostudies have shown that prebiotics have beneficial impact on diabetes and obesity.Many prebiotics cause an increase in the growth ofLactobacillusandBifidobacterium,but it is not fully understood how prebiotics cause these changes in the gut microbiome[21].It is well-known that prebiotics are fermented by gut microbiota,leading the production of SCFAs,lowering the pH of the colon[21].Figure 2 summarizes the beneficial effects of prebiotics leading to improved glucose homeostasis and reduced inflammation.Further research is necessary to elucidate the impact of prebiotics on the gut microbiome and the molecular signaling mechanisms of SCFAs.
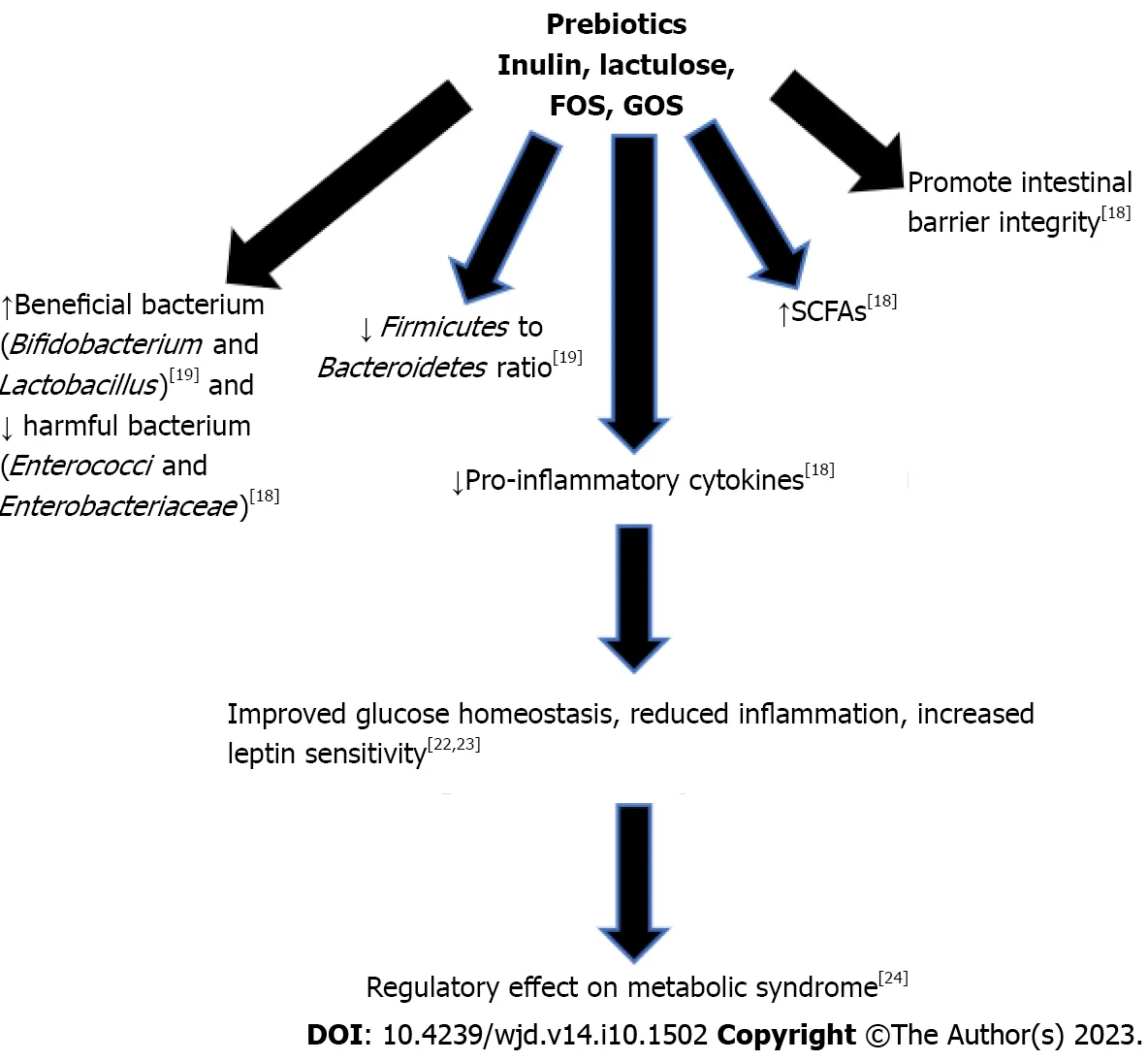
Figure 2 The beneficial effects of prebiotics on metabolic syndrome. FOS: Fructooligosaccharides;GOS: Galactooligosaccharides;SCFAs: Short Chain Fatty Acids.
Synbiotic
Synbiotics are combinations of prebiotics and probiotics,consisting of a combination of live microorganisms and substances that are selectively utilized by the host microbiota to confer a benefit to the host[18].The benefits of synbiotics are thought to come from the initial selection of beneficial commensal microbiome species and aiding these species in subsequent food processing and fermentation[34].These include reduced oxidative stress on intestinal cells and overalldecreased inflammation,thus maintaining the gut barrier[35].Studies show that supplementation with synbiotics or probiotics may lead to beneficial reduction in fasting blood glucose (FBG),although the impact on FBG was more pronounced when multispecies probiotics were used instead of single species probiotics[36].Current synbiotics include the most well-studied probiotics,includingBifidobacteriumandLactobacillus,which ferment indigestible sugars,such as FOS[34].Thus,synbiotic administration of probiotics with FOS aims to increase the abundance of FOS fermentation products in the gut,such as lactic acid[34].
Many species rely on products from other species for survival,for example some species require lactic acid for substrate production and thus rely on lactic acid-producing species.This suggests that synbiotics will need to become more complex and involve multiple strains rather than just one,in addition to the prebiotics required for survival[34].The effects of these supplements may also be altered by the individual's characteristics[37].Person-to-person variation in gene expression was shown in one study to be the main determinant for differences in transcriptomes created postsupplementation[37].This may be a species-specific phenomenon or even location specific,i.e.,in the duodenum but not in the jejunum;thus,further trials are required.
Postbiotic
Postbiotics consist of inanimate microorganisms with or without their components and metabolites that confer a health benefit to the host[38].Contrasting with probiotics,which consist of live microorganisms,postbiotics consist of microorganisms that are no longer alive,such as heat-killedAkkermansiamucinophila[38].There are several challenges to the survival of probiotics during production and storage of food,such as reactions with chemical compounds,acidity,and storage temperature[38].It has long been known that non-viable microbes in addition to their components and metabolites can have significant impact on health[38].In one clinical trial,heat-inactivatedBifidobacteriumbifidumwas found significantly alleviate the symptoms of irritable bowel syndrome[39].In a similar study,Akkermansiamuciniphilawas found to improve the metabolic state of obese and overweight participants in both its living and inactive forms[38].In another systematic review,postbiotics were studied for the prevention and treatment of infectious diseases in children under five years of age,revealing treatment with heat-killedLactobacillusacidophilusreduced the duration of diarrhea and heat-inactivatedLactobacillusparacaseireduced the risk of pharyngitis,laryngitis,and diarrhea[40].
Postbiotics are promising for the development of food supplements with longer stability in comparison to prebiotic supplements[38].Additionally,postbiotics have the potential to broaden the spectrum of microbes used for supplementation,as microbes that could not be administered live due to safety concerns can be administered in the inanimate form.The mechanism of action of postbiotics is due to both the components of the inactivated microbes and the metabolites produced by the microbes,such as SCFAs[38].
An inverse relationship between decline in anti-inflammatory microbiome species and abnormal SCFA production has been demonstrated[41].SCFAs,such as butyrate and propionate,are among the metabolites produced from inactive microbes.Gut microbiome produced SCFA have been shown to have strong effects on metabolic and cardiovascular health through a variety of tissue-specific mechanisms including appetite regulation,glucose homeostasis and metabolism,proper gut barrier and colonocyte maintenance and function,and immunomodulation[41].For example,butyrate is integral in colonocytes for energy production and expanding the regulatory T cell population in the immune system,while propionate is suggested to have a role in gluconeogenesis[42].However,increase in acetate production has been shown to activate glucose-stimulated insulin secretion,increase ghrelin secretion and hyperphagia,leading to obesity and related diseases[43].This suggests that to develop a treatment protocol,the specific proportion of postbiotics in a patient will need to be examined to allow for appropriate adjustment.Increased production of acetate has been found in obesity and decreased production of butyrate and propionate is seen in T2DM[44,45].In mice studies,butyrate was shown to be associated with increased production ofLachnospiraceaeandProteobacteriaand decreased production ofClostridiaceae[46].
However,the mechanism directly responsible remains elusive,and some results from animal models conflict with results from human trials.Many metabolic diseases are associated with a chronic state of low-grade inflammation.The maintenance of the gut barrier is also critical for reducing the amount of pro-inflammatory bacterial byproducts that can cross into the bloodstream,thus potentially decreasing the level of inflammation in the body.Specifically,a high fat diet reduces expression of tight junction genes,thus leading to a leaky barrier[47].This allows inflammatory bacterial byproducts,such as LPS,to circulate in the body leading to an inflammatory response[48].Hyperglycemia can also increase this leakiness and cause hyperpermeability,leading to a similar inflammatory phenomenon[49].However,these findings are mainly in animal studies and further studies in humans are required.Additionally,there is a need for studies on the impact of inanimate microbes on the host without associated metabolites to determine the extent that health benefits are conferred.There is also need for additional research on the mechanisms that are driving the benefits of postbiotics.Figure 3 summarizes the beneficial effects of postbiotics,leading to improved glucose homeostasis and reduced inflammation.
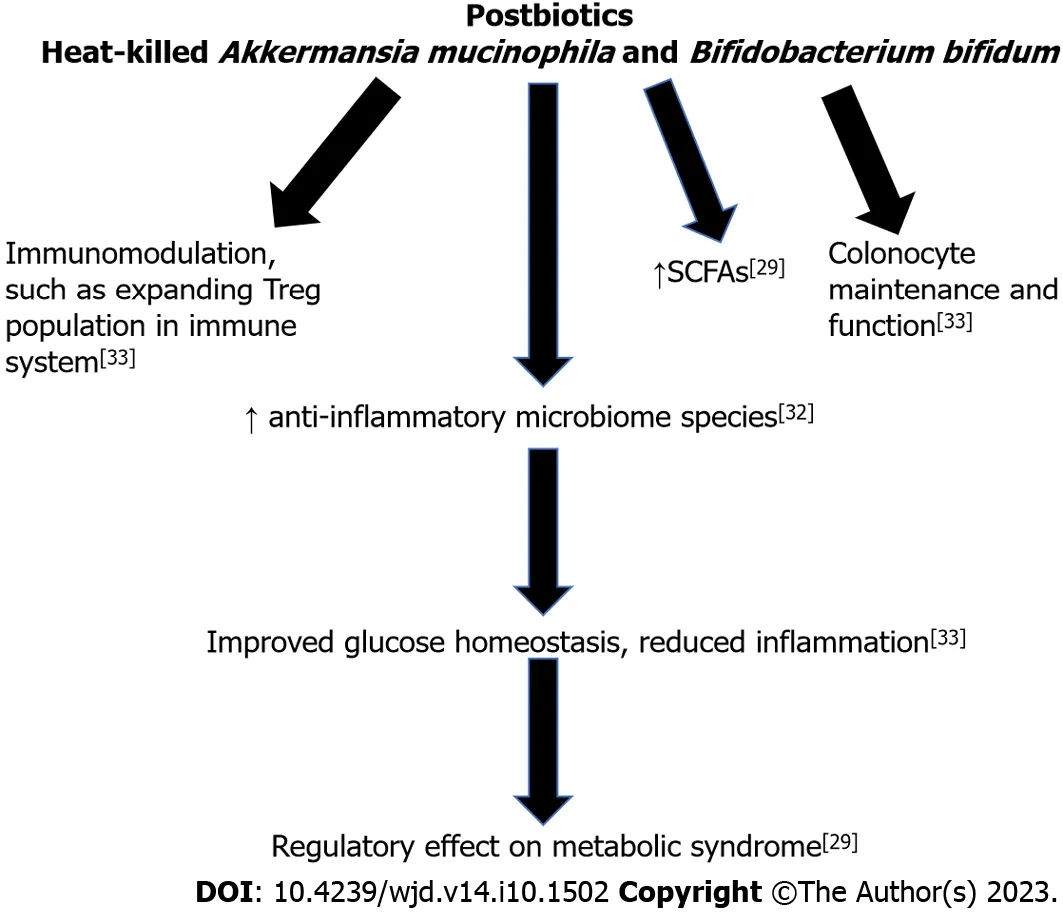
Figure 3 The beneficial effects of postbiotics on metabolic syndrome. Treg: Regulatory T cell;SCFAs: Short Chain Fatty Acids.
Probiotic
Probiotics are live microorganisms that confer a benefit to the host when administered[17].BifidobacteriumandLactobacillusare the two most widely known probiotics.Various studies in animals have shown their benefits in improving gut microbiome composition[50,51].Probiotic administration has been shown to be potential therapeutic target for metabolic syndrome prevention and treatment[52].There is a fine balance between the host's immune system and gut microbiome,and imbalance can lead to systemic inflammation through passage of bacteria and bacterial fragments,such as LPS,through the gut barrier and into systemic circulation[52].Chronic systemic inflammation can lead to the development of insulin resistance and obesity[52].
Additionally,many diseases have been found to have microbial dysbiosis either from an overgrowth of pathogenic species or a loss of microbiome diversity[53].However,it should be noted that there is not a clear definition of a healthy gut microbiome composition,so the term “dysbiosis” is inherently vague[45].This change in microbial composition is found to be associated with increased inflammation,specifically in obese patients since low-grade inflammation is a common finding in many metabolic disorders[45].This suggests that microbiome composition affects the inflammatory state of people.An increase of pro-inflammatory bacterial species has also been found in patients with T2DM,especially with a decrease of anti-inflammatory species[41].
The microbiome present in obese individuals has been found to be different from that of lean individuals though specific differences are difficult to qualify[54].In a study with obese and lean adolescents,it was found that a lower amount ofBacteroidetesand higher proportion ofFirmicuteswas associated with obesity[55].The microbiome of obese individuals has been shown in animal models to extract more energy from the diet,and this phenomenon still occurs when the microbiome from obese mice is transplanted into lean mice[54].Bifidobacteriumsupplementation in diet-induced obese and insulin resistant mice showed an increase inAkkermansiamucinophila[56].In another study,Lactobacillussupplementation in high-fat diet induced hypertensive mice showed a reduction in the ratio ofFirmicutestoBacteroidetes[57].
Probiotics have been found to have an influence on the expression of inflammation-related genes and proteins[58].Many animal studies have shown interactions between the gut microbiome and the immune system[45].These studies reduced the gene expression of immune system components with known or theorized links to metabolic dysfunction.Researchers then studied the effects or interactions of these mutation with the gut microbiome.Knock out of toll-like receptor 5 (TLR5) in mice was found to cause the hallmark features of metabolic syndrome in correlation with changes to the gut microbiome and findings of colitis[59].Upon transfer of the microbiome from the knock-out mice to wild-type germ-free mice,metabolic dysfunction was also transferred,leading to hyperlipidemia,hypertension,and insulin resistance.Food restriction was able to prevent obesity but had no effect on insulin resistance,suggesting that the TLR5 and subsequent microbiome changes have a metabolic effect.Additionally,deletion of myeloid differentiation factor 88 and a high fat diet induced hyperglycemia,leading to metabolic syndrome in knock-out mice along with an increase in bacterial translocation across the intestinal barrier[29].The mice were then givenBifidobacteriumanimalissubsp.lactis 420 (B420) as a probiotic.The result was a general normalization of gut microbiome composition,a decrease in the expression of major inflammatory cytokines,and a complete normalization of insulin sensitivity and levels,although glucose metabolism was only moderately affected[29].However,data on specific benefits conflict from study to study.Improvements in glucose metabolism is more significant in patients with T2DM,and some studies report little effect on cholesterol and lipid levels[45].Supplementation ofAkkermansiamuciniphilahas been found in rodents and humans to improve insulin sensitivity and decrease inflammation[60].The findings are less prominent in humans but indicate the supplement's clinical potential.Another study with 40 participants with insulin resistance were placed in a double-blind trial and given eitherAkkermansiamuciniphilaor a placebo,and the study showed reduction in inflammatory markers and improved insulin sensitivity in theAkkermansiamuciniphilagroup[61].
Only a small number of studies have been conducted to analyze the effect of probiotic administration on weight and glycemic control in humans.In one study,87 subjects with higher body mass index (BMI) (24.2-30.7 kg/m2) were randomly assigned to a group received fermented milk containingLactobacillus(LG2055) or fermented milk withoutLactobacillusfor 12 wk.It was found that the group receiving the milk containing LG2055 experienced a significant reduction in abdominal visceral and subcutaneous fat and BMI[62].Another study showed probiotic yogurt consumption reduced FBG and HgbA1c in patients with type 2 diabetes[63].A double-blind trial with 21 participants showed administration ofLactobacillusreuteriimproved insulin and incretin secretion[64].Thus,the results from clinical experiments are encouraging,but larger trials are needed to confirm the effect of probiotics on improving insulin sensitivity and weight loss[65].Figure 4 summarizes the beneficial effects of probiotics,leading to improved glucose homeostasis and reduced inflammation.
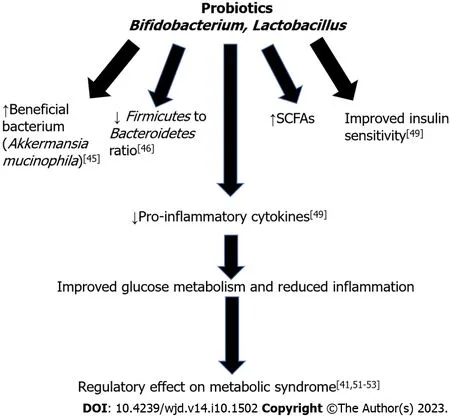
Figure 4 The beneficial effects of probiotics on metabolic syndrome. SCFAs: Short Chain Fatty Acids.
Future directions
Future research is needed to confirm the clinical efficacy of microbiome supplementation.Several times,studies have reached opposing or ambiguous conclusions,even though the research was of high methodological quality[58].This may be due to several factors including data collection methods,different analysis parameters and metrics,and varied methods of interpretation[58].Some studies used self-reporting surveys to measure patient quality of life and psychosocial effects.Others looked at lab values that may not necessarily have significance clinically,such as inflammatory markers or glucose metabolism protein levels in generally healthy individuals.Some studies had animal models while others involved human subjects and may have been observational or randomized with placebo controls.There is also the question of funding and conflicts of interest,as some studies are funded or linked to probiotic companies.While that does not necessarily bias the project,independent research should be a focus in the future.
Additionally,research on the effects of specific strains is lacking and may even vary from stain to strain,thus weakening the argument for the use of specific strains in a project[58].One study found that both mice and humans had colonization resistance to probiotics based on the current composition of their gut microbiome,and that in humans this resistance varies from person to person because microbiome composition is individualized based on person-specific needs,geographic region,and diet among other factors[66].In fact,many of the live probiotic strains were found to still be viable in stool samples after passage through the GI tract[66],and it remains unclear if the colonization that does occur persists after supplementation ceases.This contradictsinvitrostudies in which probiotics were able to adhere to human GI mucosal cells[67],indicating that lab-based work may be a poor predictor of efficacy in human subjects[58].Invitrostudies in general may be poor models for this topic of research since it does not includeinvivosignaling and factors that may play an important role in colonization and efficacy[58].This could also contribute to conflicting results and would require furtherinvivostudies.
Additional studies have also looked at whether the effects of probiotics change depending on a person's specific microbiome composition and have found that it does make a difference.Songetal[68] classified fifty obese but otherwise healthy subjects based on the ratio of two bacterial species,PrevotellaandBacteroidetes,two of the major enterotypes[68].The administration of probiotics improved obesity-related markers,but the efficacy was greater in thePrevotelladominant enterotype.This,along with colonization resistance,could explain why previous studies have found such varied results and accounting for these differences could help reconcile conflicting data[66].This highlights the need for a patient-centered protocol rather than general supplementation.
Postbiotics is the newest area of research and thus will require the most work in future studies.There is the potential to alter bacteria to produce new biological compounds.In one study using a mouse model of alcoholic liver disease,Lactobacillusreuteriwas engineered to produce interleukin-22 (IL-22),an anti-inflammatory cytokine,after it was determined that chronic alcohol use reduces intestinal production of IL-22[69].IL-22 has been found in previous studies to protect against atherosclerosis and CVD[70] as well as protect against beta cell stress and normalize hyperglycemia and insulin levels[71].The increased levels of IL-22 allowed for increased expression of the regenerating islet-derived genes (REG3-gamma gene),which creates a protein that prevents bacterial translocation across the gut barrier.This reduced ethanol-induced steatohepatitis,a direct hepato-protective effect made possible by genetically altered probiotic supplementation.Through this,we have found that it is possible to manipulate commensal bacteria to fit the roles needed in the patient and can treat an enormous variety of metabolic diseases.
Meta analyses may help resolve some of the ambiguity but are not impervious to biases[58].They may include studies that involve different strains of bacteria and thus are difficult to compare.They may also include outlier studies that skew the data and conclusions or be diluted by papers without significant findings.Therefore,efforts should be placed in developing randomized,large-scale,and high-quality experiments and clinical trials to assess the use of prebiotics,probiotics,and postbiotics to modify the gut microbiome and affect various metabolic syndromes.
CONCLUSlON
The relationship between human health and the microbiome has piqued researchers' curiosity in the last decade.Our knowledge of the gut microbiome's composition and functions has considerably improved over the past several years due to rapid advancements in metagenomic sequencing techniques.As a result,it is evident that almost no area of host physiology is fully immune to the effects of gut microbes and their products.Indeed,the gut microbiota's influence extends beyond the gastrointestinal tract's traditional digestion function to include altering the physiology of other organ systems such as the liver,adipose tissue,lung,and brain.With better insight into the interactions between the host and microbiota,human gut microbiome supplementation has emerged as a promising novel therapeutic target.Current research is directed towards finding treatment options for improving the number of beneficial gut microbiota and to reduce the harmful ones with the use of prebiotics,probiotics,synbiotics,and postbiotics.Efforts are also underway to identify novel gut microbiota-host interactions,their mechanisms,and associations with T2DM,CVD and to design new therapies to modulate these disease processes.
FOOTNOTES
Author contributions:Antony MA and Kant R designed the outline,performed the writing,prepared the figure,and edited the paper;Edem D,Raj R,Verma V,Nain P,Chowdhury A,and Joglekar M performed the writing,and prepared the table and figure;Antony MA and Kant R provided the input in writing the paper,performed the writing and edited the paper.
Conflict-of-interest statement:The authors have nothing to declare.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORClD number:Mc Anto Antony 0000-0002-2232-0078;Dinesh Edem 0000-0002-4151-3246;Ravi Kant 0000-0002-9599-8082.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
 World Journal of Diabetes2023年10期
World Journal of Diabetes2023年10期
- World Journal of Diabetes的其它文章
- lndirect comparison of efficacy and safety of chiglitazar and thiazolidinedione in patients with type 2 diabetes: A meta-analysis
- Characteristics of glucose change in diabetes mellitus generalized through continuous wavelet transform processing: A preliminary study
- Analysis of influencing factors and interaction of body weight and disease outcome in patients with prediabetes
- Establishment and evaluation of a risk prediction model for gestational diabetes mellitus
- Effects of insulin aspart and metformin on gestational diabetes mellitus and inflammatory markers
- Effects of vitamin D supplementation on glucose and lipid metabolism in patients with type 2 diabetes mellitus and risk factors for insulin resistance
