Role of glycolysis in diabetic atherosclerosis
Qian-Jia Liu,Wei Yuan,Ping Yang,Chen Shao
Abstract Diabetes mellitus is a kind of typical metabolic disorder characterized by elevated blood sugar levels.Atherosclerosis (AS) is one of the most common complications of diabetes.Modern lifestyles and trends that promote overconsumption and unhealthy practices have contributed to an increase in the annual incidence of diabetic AS worldwide,which has created a heavy burden on society.Several studies have shown the significant effects of glycolysis-related changes on the occurrence and development of diabetic AS,which may serve as novel therapeutic targets for diabetic AS in the future.Glycolysis is an important metabolic pathway that generates energy in various cells of the blood vessel wall.In particular,it plays a vital role in the physiological and pathological activities of the three important cells,Endothelial cells,macrophages and vascular smooth muscle cells.There are lots of similar mechanisms underlying diabetic and common AS,the former is more complex.In this article,we describe the role and mechanism underlying glycolysis in diabetic AS,as well as the therapeutic targets,such as trained immunity,microRNAs,gut microbiota,and associated drugs,with the aim to provide some new perspectives and potentially feasible programs for the treatment of diabetic AS in the foreseeable future.
Key Words: Atherosclerotic plaque;Hyperglycemia;Trained immunity;microRNAs;Gut microbiota;Drugs
lNTRODUCTlON
The global incidence and prevalence of diabetes are rapidly increasing.Notably,China,the most populous country in the world,bears the heaviest burden of diabetes[1].A recent study revealed that in 2021,the global population of individuals living with diabetes was projected to reach 529 million,with an age-standardized prevalence of 6.1%.It is estimated that by 2050,1.31 billion people will be diagnosed with diabetes worldwide[2].Atherosclerosis (AS) is among the most common complications of diabetes,a well-established independent risk factor for AS[3].AS is classified as a chronic inflammatory disease associated with complex etiopathogenesis.The disease originates from intimal lesions and is characterized by local lipid accumulation,fibrous tissue hyperplasia,and calcareous deposition with formation of plaques that reduce vessel elasticity and cause hardening of the vessel walls[4].The disorder is referred to as AS owing to the yellowish appearance of lipids that accumulate in the arterial lining.AS may result in stroke,heart failure,coronary heart disease,and other serious cardiovascular complications[5].
AS develops earlier and progresses more rapidly in patients with diabetes than in the general population[6].Compared with individuals without diabetes,those with diabetes show coronary plaques with typically larger necrotic cores and more pronounced inflammation,characterized by abundant macrophages[7].The plaque load measured using the mean area and maximum wall thickness,is significantly higher in patients with diabetes than in those without diabetes,with a well-documented higher incidence of vascular calcification[8].Diabetes-associated hyperglycemia (HG) disturbs vascular endothelial function,triggers inflammation,and promotes the formation of advanced glycosylation end-products (AGE) and a series of adverse effects[9,10].Diabetes may also be associated with defective autophagy and destroys the internal homeostasis of smooth muscle cells (SMCs),leading to plaque expansion,core necrosis,and fibrous cap thinning,all of which favor plaque instability and increase the risk of plaque rupture[11].Diabetic AS causes greater blood vessel damage,thereby emerging as the leading cause of disability and mortality in patients with diabetes globally[12].Consequently,it gives rise to a substantial socioeconomic burden on society.Further research is warranted to gain deeper understanding of diabetic AS plaques,with the objective of exploring novel therapeutic approaches.
Endothelial cells (ECs),vascular SMCs (VSMCs),and macrophages play key roles in AS plaque formation,in which glycolysis is also an important contributor[13].Abnormalities at any stage of the glycolytic pathway may promote AS.Numerous studies have investigated the mechanism underlying glycolysis in AS and plaque formation and described the effects of trained immunity,microRNAs,gut microbiota (GM),and other factors on glycolysis.Nevertheless,the comprehensive and precise mechanism of glycolysis in diabetic AS remains incompletely understood.The current findings provide novel concepts and potential strategies for targeted therapy of AS in the future.In this review,we summarize and discuss the role of glycolysis in the promotion,inhibition,and treatment of diabetic AS.
ROLE OF GLYCOLYSlS lN THE DEVELOPMENT OF DlABETlC AS
Glucose is the primary energy source for most body cells,serving as an essential substrate for various physiological and pathological activities.Glycolysis includes a series of biochemical reactions involved in the degradation of glucose by glycolytic enzymes,with the ultimate goal to produce pyruvate and adenosine triphosphate (ATP)[14].Glycolysis is a common pathway in glucose metabolism that,under anaerobic conditions,culminates in the production of lactic acid,which subsequently enters the tricarboxylic acid cycle for oxidative phosphorylation when oxygen is available.However,these processes may differ under specific conditions.In the 1920s,the German scientist,Warburg,discovered significantly higher glycolytic activity in cancer cells than in normal cells[15].Compared with the large amount of energy produced by mitochondrial oxidative phosphorylation,glycolysis generates limited amounts of energy.However,in contrast to normal cells,cancer cells rely on glycolysis for energy even under aerobic conditions,a phenomenon referred to as the Warburg effect[16].Currently,a growing body of evidence suggests that aerobic glycolysis is not unique to tumor cells,and this phenomenon also significantly affects cells associated with AS[17,18].
ECs
The process of AS plaque formation commences with EC injury.Although ECs are in close proximity to oxygenated blood and receive abundant oxygen compared with other cells,they primarily rely on glycolysis for energy[19].Under physiological conditions,glycolysis provides 85% of the ATP required by the entire EC unit[20].ECs depend on glycolysis for energy production based on the following features: (1) The mitochondrial content in ECs is too low to provide sufficient ATP through oxidative phosphorylation[21];and (2) glycolysis is the source of energy for survival and maintenance of the cell itself because the ATP production rate during glycolysis is much higher than that during oxidative phosphorylation[22].HG is an important sign of diabetes[23].Glucose is transported into ECsviathe glucose transporter 1 (GLUT-1),a receptor whose activity is regulated by extracellular glucose concentration independent of insulin[24,25].ECs in patients with diabetes are therefore more vulnerable.An intact vascular barrier composed of quiescent ECs is essential to maintain vascular homeostasis[26],which is a favorable factor against AS.However,in the context of diabetes,HG can trigger overproduction of reactive oxygen species (ROS) in ECs,which disrupts the normal physiological state[27].An increasing body of evidence shows that cardiovascular complications in diabetes mellitus occur secondary to an increase in nitrosative stress.Oxidative and nitrosative stress can lead to DNA injury,subsequently triggering the activation of the ribozyme,and poly polymerase 1 (PARP-1),and thus mediate the onset and progression of diabetic cardiovascular complications[28].This ribozyme is a key enzyme involved in glycolysis in the nuclei of DNAinjured ECs[29].PARP-1 not only slows glycolytic efficiency by facilitating NAD+consumption but also promotes adenosine diphosphate (ADP) ribosylation of proteins.However,glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity decreases after ADP ribosylation.Therefore,GAPDH entry into the nucleus to form a complex with nuclear proteins (ADP ribosylation) inhibits glycolysis.Glycolytic intermediates are also transferred to other pathways (including the hexosamine and polyol pathways).These changes lead to endothelial dysfunction in individuals with diabetes and worsens AS[30-32].Additionally,non-enzymatic glycosylation of proteins or lipids in patients with diabetes leads to the production of AGE,which bind to the receptor for AGE (RAGE) on ECs and produce inflammation and dysfunction of ECs[33-35].Human umbilical vein ECs (HUVECs) treated with AGE reportedly showed a decrease in glycolysis,which leads to the conclusion that AGE inhibits the migration and proliferation of ECs[36].Moreover,HG-induced ROS was shown to increase the expression of RAGE and its pro-inflammatory endogenous ligands[37].Additionally,enhanced endothelial superoxide production in patients with diabetes results in increased AGE accumulation[38].These processes collectively lead to a vicious cycle of endothelial dysfunction.In summary,the hyperglycemic environment itself in people with diabetes,as well as the accompanying oxidative stress,AGEs and other adverse factors interfere with glycolysis,leading to ECs dysfunction.These changes eventually disrupt vascular homeostasis,which leads to AS in people with diabetes.Therefore,a series of internal environmental changes caused by diabetes-related HG may play an important role in diabetic AS by influencing glycolysis,which deserves further study.
However,every situation has dual implications.Inhibit glycolysis disrupt the normal physiological state of ECs,thus destroying vascular homeostasis.Nonetheless,in patients with diabetic AS,glycolysis inhibition could potentially reduce angiogenesis within atherosclerotic plaques and stabilize them to a certain extent.Energy metabolism of ECs,which is intricately associated with their germination,migration,and proliferation,is an important prerequisite for angiogenesis[39].In patients with diabetes,HG can significantly alter EC metabolism,resulting in higher risk of pathological neovascularization in these cells than that in healthy cells under normal conditions[40].Plaque rupture is a primary contributor to acute cardiovascular events.Many studies have shown that plaque angiogenesis promotes AS progression,particularly plaque instability[41].Restricted oxygen diffusion coupled with activation of inflammatory mediators leads to plaque hypoxia,which eventually accelerates neovascularization[42].Newly formed vessels are fragile and highly vulnerable to bleeding or leakage,resulting in increased plaque instability and rupture[43].Excessive or abnormal neovascularization in plaques significantly increases capillary permeability and tissue edema.This,in turns results in a likelihood of bleeding or rupture of diabetic AS plaques[44].In glycolysis,6-phosphofructokinase-2/fructose-2,6-bisphosphatase 3 (PFKFB3) is a key activator that provides active ATP and essential biosynthetic products for angiogenesis[45].Based on these findings,previous studies have shown that the knockdown of PFKFB3-related genes in ECs can lead to defective angiogenesis,and the use of 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO,a PFKFB3 inhibitor) can reduce vascular germinationviainhibiting EC proliferation and migration[46].Notably,3PO does not affect the glycolysis necessary for normal EC to maintain homeostasis but only reduces excess glycolysis required for EC germination[47].Nonetheless,research has shown that 3PO can reduce T cell activation in vitro,which may adversely affect the body's immune suppressive function[48].A recent study reported that partial inhibition of glycolysis prevented plaque angiogenesis without a particularly significant effect on the size,composition,and vulnerability of pre-existing plaques,although it reduced the frequency of plaque formation.The study also reported that 3PO-induced metabolic stress could stimulate autophagosome formation and promote autophagy in ECs[49].Aging of ECs can lead to loss of function and transition to a pro-inflammatory state,which stimulates transformation of mononuclear macrophages into a pro-inflammatory phenotype[50].The simultaneous increase in the expression of adhesion molecules in aging ECs accelerates macrophage migration and activation in plaques[51].The aforementioned factors act synergistically to promote AS plaque progression.The previously described autophagic response can effectively inhibit EC senescence and apoptosis,thereby limiting plaque formation and development.Additionally,another study has shown that PFKFB-3 not only promotes glycolysis,but also directly participates in glycolytic-dependent DNA repair of diabetic ECs under oxidative stress damage,which may play a certain role in vascular protection of diabetes[52].Therefore,the use of the glycolytic inhibitor 3PO may disrupt this protective effect,making the application of glycolytic inhibitors uncertain.In conclusion,although a substantial body of evidence suggests that targeting glycolysis inhibition can effectively improve plaque stability in patients with diabetic AS and prevent plaque rupture and bleeding by limiting EC metabolism and preventing plaque neovascularization,the safety and efficacy of this approach require further elucidation and warrant further research (Figure 1).
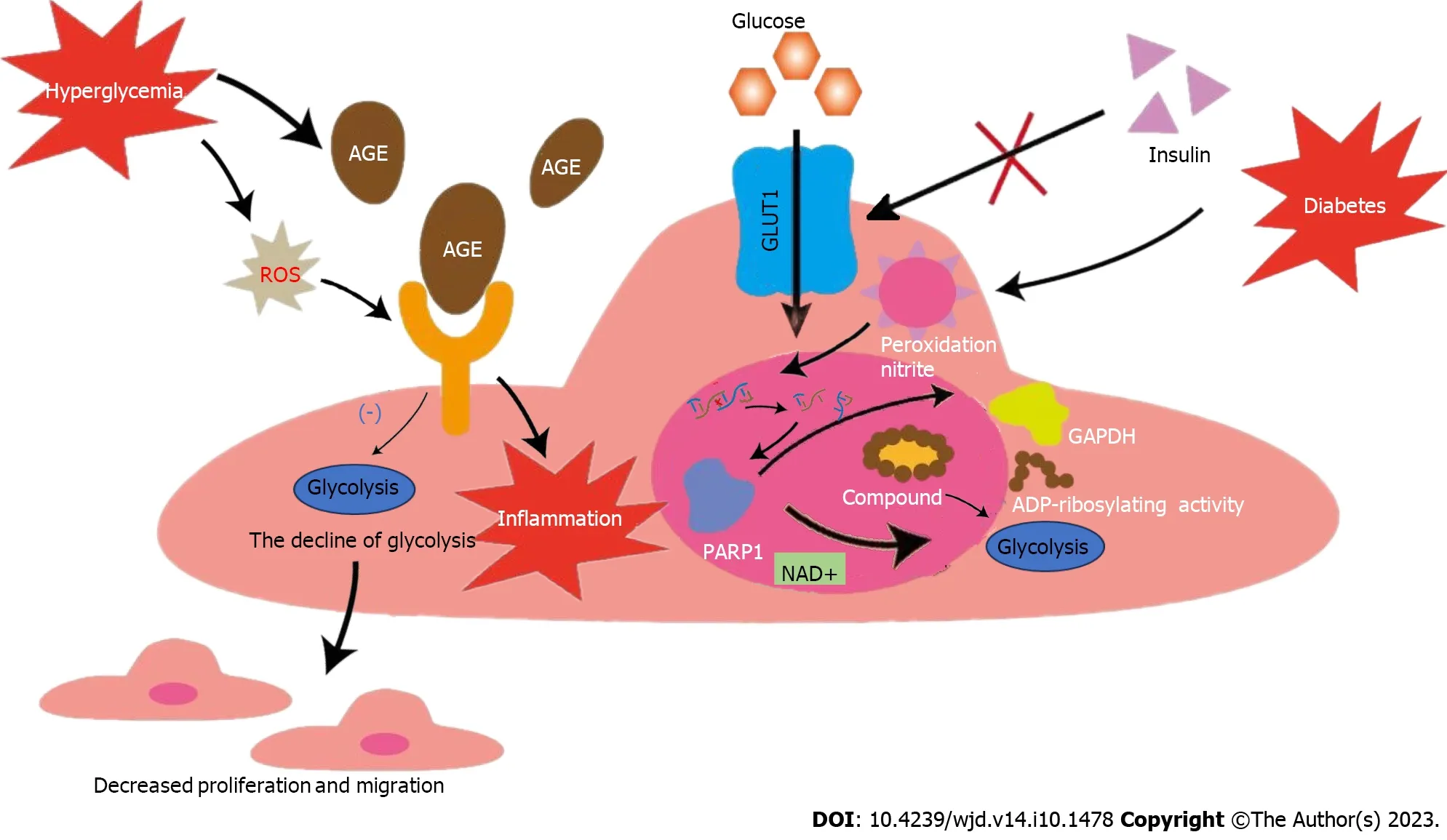
Figure 1 Effects of glycolysis on endothelial cells in hyperglycemic environment. The activity of glucose transporter 1 is regulated by extracellular glucose concentration,independent of insulin,making endothelial cells in patients with diabetes more vulnerable.Nitrosation stress caused by diabetes can damage DNA and activate poly polymerase 1 (PARP1).PARP1 can not only promote the consumption of NAD+,but also reduce the activity of GAPDH after adenosine diphosphate ribosylation.Both pathways inhibit glycolysis and lead to dysfunction of ECs.Diabetes can promote the accumulation of advanced glycosylation endproducts (AGEs),and diabetes-induced reactive oxygen species can increase the expression of receptor for AGE and pro-inflammatory endogenous ligands.The above process leads to the down-regulation of ECs glycolysis and the intensification of inflammation,causing decreased migration and proliferation of ECs.GLUT1: Glucose transporter 1;EC: Endothelial cell;AGE: Advanced glycosylation end-products;ROS: Reactive oxygen species;PARP-1: Poly polymerase 1;ADP: Adenosine diphosphate;GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Macrophages
Macrophage polarization is a typical phenomenon associated with AS[53].M1 and M2 constitute the common macrophage phenotypes.M1 macrophages show a pro-inflammatory phenotype and play a major role in unstable plaques[54],they are more commonly observed in the shoulder area of unstable and vulnerable-to-rupture plaques[55].M2 macrophages show an anti-inflammatory phenotype and are more commonly expressed in stable plaques[56].In contrast,M2 cells play a key role in the occurrence and progression of AS and maintain the stability of AS plaques.Notably,15-lipoxygenase expressed by M2 macrophages is closely associated with the formation of foam cells and actively promotes AS plaque formation and progression.Simultaneously,M2 macrophages secrete various anti-inflammatory factors,such as transforming growth factor-β,which protects against AS[57].Usually,the ratio of M1 to M2 cells plays an important role in AS plaque progression[58].Diabetes-induced HG can enhance glucose metabolism and inflammatory response in macrophages[59].ROS produced by macrophages under oxidative stress conditions can injure mitochondrial DNA,leading to mitochondrial dysfunction and inhibiting oxidative phosphorylation.Simultaneously,ROS induces sustained expression of hypoxia-inducible factor 1α (HIF-1α),consequently promoting glycolysis[60].The combination of the aforementioned factors eventually stimulates M1 and suppresses M2 activation[61],which favors plaque instability.Moreover,local intraplaque hypoxia can trigger a similar responseviathe HIF-1α pathway[62].These changes in macrophage energy metabolism interfere with stable lipid metabolism and significantly increase the intracellular lipid content[63],which promotes transformation of macrophages into foam cells that participate in AS plaque formation.The production of large amounts of AGE is a typical feature of patients with diabetes.HIF-1α is upregulated in AGE-bovine serum albumin (BAS)-induced M1 polarization,and HIF-1α knockdown reduces AGE-BASinduced M1 polarizationviaregulation of pyruvate dehydrogenase kinase 4[64].This study further highlighted the association between glucose energy metabolism in macrophages and diabetes-related inflammation.A recent study comparing macrophages in diabetic and non-diabetic mice found that glucose uptake and glycolysis were not increased but rather decreased in the former group[65].Although it is premature to conclude whether these results were coincidental or influenced by some interfering factors,this finding challenges the notion that diabetes-induced HG directly promotes glucose metabolism in macrophages.Recently,other researchers have suggested that HG is not the only,or even the most important,factor in diabetes-related cardiovascular disease[66].Diabetes is an extremely complex background,so the conditions to prove this point are very demanding.However,we still believe that this finding is worthy of further verification,which has important significance for us to understand the specific mechanism of glycolysis in diabetic AS and explore related treatment methods.Nevertheless,changes in glycolysis significantly affect macrophage energy metabolism and polarization in diabetic AS and therefore plays a crucial role in the development of diabetic AS and plaque stabilization (Figure 2).

Figure 2 Effects of glycolysis on macrophages in hyperglycemic environment. Reactive oxygen species (ROS) induced by hyperglycemia can damage mitochondrial DNA and inhibit oxidative phosphorylation.Simultaneously,ROS can induce the expression of hypoxia-inducible factor 1α and promote glycolysis.Under the above two conditions,the ratio of M1 and M2 changes.M2 has the ability to secrete 15-lipoxygenase to promote foam cell formation and atherosclerotic protective factors such as transforming growth factor-beta,which plays an important role in maintaining plaque stability.The increase of M1 and the decrease of M2 ultimately aggravate the instability of diabetic atherosclerotic plaques. OXPHOS: Oxidative phosphorylation;AGE: Advanced glycosylation endproducts;BAS: Bovine serum albumin;TGF-β: Transforming growth factor-beta;HIF-1α: Hypoxia-inducible factor 1α.
Vascular SMCs
Proliferation and subintimal migration of VSMCs is a key event in AS formation[67].VSMCs,which are viewed as plaque stabilizers,proliferate and migrate to plaque fibrous caps and produce collagen and extracellular matrix,which contribute to plaque stability[68,69].Enhanced glycolysis in VSMCs is important for platelet-derived growth factorinduced VSMC proliferation and migration,which is an important contributor to AS[70,71].In the context of diabetes,VSMC proliferation secondary to oxidative injury is a primary process that accelerates AS progression[72].Intracellular transportation of low-density lipoprotein (LDL),followed by subintimal oxidation,results in the formation of oxidized LDL (ox-LDL)[73].Ox-LDL significantly influences VSMC proliferation and migration and,through this effect,contributes to plaque vulnerability and AS progression[74,75].Pyruvate kinase subtype M2 (PKM2) is a key rate-limiting enzyme involved in glycolysis.Ox-LDL up-regulates PKM2-dependent glycolysis to trigger VSMC proliferation and migration and eventually promotes AS[76].Additionally,AS vascular remodeling is accompanied by a shift in the VSMC phenotype from a contractile phenotype under normal physiological conditions to a synthetic proliferative phenotype under pathological conditions[77].Furthermore,inflammatory stimulation in a diabetic environment with high glucose levels may also promote such phenotypic transformation of VSMCs[78].A significant percentage of enzymes involved in glycolysis are crotonylated and ubiquitinated.Some researchers have suggested that the crosstalk between crotonylation and ubiquitination in glycolysis may be a potential mechanism underlying the phenotypic remodeling of VSMCs[79].The results of a study on SMC-specific PKM2-deficient mouse model support the hypothesis that SMC-derived PKM2 promotes injury-induced neointimal hyperplasiaviaenhanced phenotypic conversion,proliferation,and migration from a genetic perspective[80].However,the specific role of glycolysis-induced metabolic mechanisms in phenotypic switching of VSMCs remains unclear and requires further investigation.Nonetheless,glycolysis significantly affects VSMC participation in diabetic AS development (Figure 3).

Figure 3 Effects of glycolysis on vascular smooth muscle cells in hyperglycemic environment. The crosstalk between crotonylation and ubiquitination in glycolysis related enzymes may be a potential mechanism underlying the phenotypic remodeling of vascular smooth muscle cells (VSMCs).Ox-lowdensity lipoprotein promotes proliferation and migration of VSMC by up-regulating PKM2-related glycolysis.Oxidative damage in the context of diabetes plays an important role in these processes,causing the development of diabetic atherosclerosis.PKM: Pyruvate kinase subtype;LDL: Low-density lipoprotein.
TRAlNED lMMUNlTY,GLYCOLYSlS,AND AS
Earlier researchers held the belief that only adaptive immunity can establish immune memory.However,following extensive experimental studies have contradicted this perspective[81].Innate immune cells produce immune memory following antigenic stimulation and tend to respond more strongly to reinfection in a nonspecific manner,a phenomenon termed as trained immunity[82].Although the long-term activation of trained immune system strengthens the body's ability to fight infection,it also negatively affects chronic inflammation[83].
AS is a typical chronic low-grade vascular inflammatory disease;therefore,trained immunity has a major role in this disorder.β-glucan-induced trained immunity is a typical model[84].In vitro studies using monocytes trained with dextran have shown that AKt-mTOR-HIF-1α signaling pathway is the metabolic basis underlying trained immunity[85].Another study has also shown that the transition from oxidative phosphorylation to a significant increase in aerobic glycolysis was the driving force for the development of trained immunophenotype[86].These changes in cellular metabolism observed during the induction of trained immunity constitute metabolic reprogramming,which can induce epigenetic remodeling and chromatin structure changes (such as methylation or an increase in mRNA of the key enzymes involved in glycolysis)[81].Ultimately,these changes increase transcription of inflammatory genes,triggering the immune response (these genes encode not only cytokines and chemokines associated with AS but also proteins associated with foam cells and plaque vulnerability)[87].Another study observed that shear stress at AS sites throughout the vasculature shows similar effects of glycolytic up-regulation of histone modifications and signaling pathways[88],which reiterates the tight association between trained immunity and AS.Moreover,the HG environment in patients with diabetes induces long-term epigenetic regulation of inflammatory genes[89].Corresponding research suggests that hypoglycemic therapy for patients with diabetes is likely to be affected by these findings,potentially reducing its efficacy and subsequently,increasing the risk of AS[90].Glycolysis provides the energy for trained immunity,serving as its dynamic foundation.Thus,changes in glycolysis profoundly influence the role of trained immunity on diabetic AS.
Glycolysis forms the metabolic basis of both ECs and trained immunity,thereby implying a plausible correlation between the two.Atherogenic factors induce trained immunity in ECsviaoxidative phosphorylation,glycolytic metabolic transformation,epigenetic modification of pro-inflammatory genes,and activation of the Akt-mTOR-HIF-1a signaling pathway[91].Ox-LDL,a lipid that promotes AS,drives the production of innate immune cells and trained immune phenotypes in ECs[92].Reportedly,training monocytes with this substance can also induce trained immunity,accompanied by a significant increase in glycolysis[93].Additionally,trained immune phenotype is reversed after treatment with mammalian target of rapamycin (mTOR) inhibitors,glycolytic inhibitor 3,or the HIF-1a inhibitor following Ox-LDL exposure[85].Therefore,it is conceivable to speculate that the reversal of trained immunity using glycolysis inhibitors or genes involved in glycolysis knockout may be a potential therapeutic option for AS.
To summarize,the relationship between trained immunity and glycolysis provides a new perspective on the treatment of diabetic AS.However,most current studies on trained immunity have been performedinvitroor in animal experimental models.Available and clinical data are insufficient to establish definitive conclusions[84].Therefore,the clinical efficacy of these methods remains a potential research topic requiring further investigation.
MlCRORNA,GLYCOLYSlS,AND AS
MicroRNAs (miRNAs) are a class of non-coding single-stranded RNA encoded by endogenous genes,approximately 22 nucleotides in length[94].Their role as regulators of gene expression has long been of interest to researchers.An increasing number of recent studies have highlighted the role of miRNAs in AS and its progression.The NF-KB/miRNA-425-5P/MCT4 signaling axis can down-regulate the expression of monocarboxylate transporters (MCT4) within HUVECs derived from patients with diabetes,as well as HUVECs subjected to high glucose levels and interleukin (IL)-1β.This process leads to impaired lactate transport,thereby inducing EC dysfunction and even apoptosis[95].The study discussed the mechanism underlying endothelial vascular injury in diabetes mellitus from a new perspective of glycolysis and lactate transport disorders.Therefore,inhibition of miRNA-425-5P expression and improvement in ECs may be a potential strategy for diabetic AS treatment.Another study on HUVECs showed that miR-143 inhibited glycolysis by directly targeting hexokinase 2 (HK2),leading to endothelial dysfunction and an increased risk of AS[96].Therefore,down-regulation of the miR-143 Level to restore HK2 expression and restoration of the balance of glycolysis in EC are necessary for the prevention and treatment of AS plaques.miR-638 can inhibit proliferation,migration,and glycolysis of VSMCs by targeting lactate dehydrogenase A (LDHA)[97].It is plausible to speculate that miR-638 can effectively inhibit the development and progression of AS plaques by targeting LDHA and inhibiting glycolysis in VSMCs.M1-type macrophages use aerobic glycolysis to rapidly generate energy[98].miR-33 regulates the inflammatory phenotypic polarization program of macrophagesviaalteration of the balance between cellular fatty acid oxidation and glycolysis,which affects AS plaque progression.Anti-miR-33 was also found to exert a protective effect against AS[99],suggesting the potential value of therapeutically silencing miR-33 in the context of AS.Through the regulation of gene expression,such as down-regulating the amount of certain glycolytic enzymes or inhibiting their activity,miRNA significantly affects glycolysis,playing a non-negligible role in the occurrence and development of diabetic AS.Thus,targeting miRNA to regulate glycolysis,may potentially be an important therapeutic approach for treating diabetic AS.
GM AND AS
GM refers to the diverse microorganisms,including bacteria and fungi that colonize the gastrointestinal tract of humans and other animals including insects.Microbiota participate in the synthesis of various bioactive substances,which play key roles in human health and diseases[100,101].GM-derived metabolites transmit signals for effective communication between the host and microbiota and are indispensable mediators in several important reactions[102].Several metabolic diseases including diabetes are attributable to a dysfunctional gut microbiome[103].Bacterial DNA is shared between the gut and AS plaques[104].With regard to the microbial composition of unstable and stable plaques,the feces of patients with unstable plaques show a decrease in the Roseburiam species[105].AS plaque and its stability may be closely associated with the human GM.Additionally,transplantation of GM affects the host's susceptibility to AS[106].In conclusion,various phenomena suggest that GM and its metabolites are intricately and closely associated with diabetic AS.
Trimethylamine-N-oxide (TMAO) is mainly derived from the metabolism of methylamine-rich nutrients by the GM.TMA,produced and processed in the liver in the presence of flavin monooxygenase,results in the generation of TMAO[107].Experimental and clinical studies have reported the role of TMAO in the etiology of diabetes[108],and TMAO has also gained increasing attention as a contributor to AS.High blood TMAO concentrations can promote cholesterol transport in macrophages for the formation of foam cells,eventually causing AS[109].The amount of Bacteroides in the human intestine was positively correlated with plasma TMA concentrations[110].
Metagenomic analysis of the GM has revealed that the percentage of Bacteroidetes was significantly higher in patients with symptomatic AS than in controls[111].Notably,plaques in patients with high levels of TMAO tend to show thinner fibrous caps and a greater number of microvessels[112].The association between elevated TMAO plasma levels and unstable AS plaques is readily evident.This notion has been supported by an animal study[113],and further research underscores that high TMAO plasma levels can increase pro-inflammatory gene expression,consequently leading to a marked increase in inflammatory cytokines levels,adhesion molecules,and chemokines[114].In summary,TMAO produces adverse effects in AS.The NLP3 is a multiprotein complex formed by pattern recognition receptor activation[115].Studies performed using carotid ECs in mice with partially ligated carotid arteries have reported TMAO-induced NLP3 inflammasome activation,which increased IL-1B levels,caspase-1 activity,and cell permeability,subsequently leading to EC injury in AS[116].Among these,caspase-1 activation was shown to play a vital role in mitochondrial injury and proteolytic cleavage of glycolytic enzymes[117].Some studies have also shown that glycolytic changes are closely associated with NLRP3 activation[118].Therefore,we hypothesize that intervening in glycolysis may,to some extent,counteract the adverse effects of TMAO in diabetic AS.
Butyrate,a short-chain fatty acid (SCFA),is another product of the GM[119] that plays a key role in the regulation of cellular energy metabolism,particularly glycolysis.For example,butyrate can cross-link with T cell receptors to switch cells from mitochondrial respiration to glycolysis during T cell activation[120];butyrate can inhibit glucose transport and glycolysis by reducing GLUT1 and cytoplasmic glucose-6-phosphate dehydrogenase abundance regulated by GPR109AAKT signaling in colorectal cancer cells[121].Patients with diabetes have lower levels of butyrate-producing bacteria than those without diabetes[122].Some researchers argue that the effects of butyrate-producing bacteria on AS are attributed to the metabolic effect of butyrate in the intestines.They showed that gut-associated butyrate-producing bacteria interact with dietary plant polysaccharides and affect gene expression of distal intestinal cells with a switch from glycolytic metabolism toward fatty acid utilization,which consequently reduces systemic inflammation and improves AS[123].In addition,studies have shown that the use of live B.vulgatus and B.dorei can also help suppress the pro-inflammatory immune response to prevent coronary AS.However,the specific mechanism and whether glycolysis is involved have not been clarified,which also deserves further study[124].As a result,the concept of supplementing anti-atherosclerotic bacteria for patients with diabetes hold promise as a topic worthy of study.This approach may contribute to the treatment of diabetic AS through glycolysis regulation.
Many studies have reported good efficacy of berberine (BBR),an isoquinoline alkaloid extracted from herbs,including Coptis coptidis,in the treatment of metabolic and cardiovascular diseases[125,126].BBR can simultaneously regulate insulin signaling,inhibit A-glucosidase,induce glycolysis,and inhibit gluconeogenesis;therefore,it plays a significant role in reducing blood glucose levels in diabetes[127,128].Following the upsurge in GM research,the effect of BBR on GM has attracted widespread attention.BBR can cause changes in more than 20 genera in DB/DB mice (C57BLKS/JNju,an animal model of type 2 diabetes).Notably,significant alterations were observed in the expression of seven operational taxonomic units,including increased prevalence of a series of SCFA-producing bacteria[129].For example,Butyricimonas promotes glycolysis of branched-chain amino acids to produce SCFA as a source of energy[130].Effects of BBR on AS are also closely associated with GM activity.A comparison between BBR-treated and untreated mice fed the same high-fat diet showed significant differences in the abundance of Firmicutes and Verrucobacteria;the BBR-treated mice showed reduced expression of inflammatory factors and a lower incidence of AS[131].Other studies also support the role of BBR in inhibition or destruction of some harmful intestinal bacteria and in increasing the numbers of bacteria that reduce TMAO concentration and AS plaque size[132].In conclusion,BBR not only has a significant effect on the treatment of diabetes through inducing glycolysis but also the capacity for the prevention and treatment of AS by affecting the GM.
Hence,it is reasonable to conclude that glycolysis metabolism-related therapies,such as regulating GM through probiotic supplements or fecal transplantation[133],have a huge potential for future treatments of diabetes AS.
DRUGS,GLYCOLYSlS,AND AS
Drugs constitute an important component of the therapeutic arsenal against diabetic AS.Many researchers have emphasized the rapid development of drugs from the perspective of glycolysis.Ethyl pyruvate is a stable pyruvate derivative[134],that has been shown to provide energy for the differentiation of regulatory T cells (Tregs) and enhance their proliferationviathe up-regulation of glycolysis,which increases the number and function of Tregs and improves the clinical symptoms of type 1 diabetes in mice[135].Furthermore,that aspalathin and its associated compounds can specifically inhibit sirtuin 6 at a certain concentration,improve insulin-mediated activation of AKT,enhance glycolysis,and inhibit gluconeogenesis through the activation of non-repressor protein 5 and peroxisome proliferator-activated receptor-γ coactivator.Maintaining glucose homeostasis is important in patients with type 2 diabetes mellitus[136].Diabetes is the root cause of a more severe and complex symptoms of diabetic AS;therefore,improving diabetes is fundamental to the treatment of diabetic AS.
Rapamycin,a classic glycolysis inhibitor,has pharmacological effects that can inhibit the mTOR pathway and suppress cell glycolysis in addition to its anti-inflammatory and antiproliferative actions;it can also effectively inhibit AS progression[137].Polylactic acid glycolic acid copolymers coated with rapamycin can target AS plaques in mice and may locally deliver glycolysis inhibitors.This method has been shown to be safeinvitro[138].Therefore,it can be concluded that nanoparticles coated with glycolytic inhibitors on macrophage membrane coating may be useful for precise treatment through targeted inhibition of AS.Several studies have shown that glutamine antagonists down-regulate mTORC1 activity and attenuate the up-regulation of glycolysis in response to growth factor stimulation and effectively control vascular restenosis caused by excessive VSCM proliferation[139,140].Therefore,glutamine antagonists may be useful for the treatment of AS and AS-induced vascular occlusive disease.Recent studies have demonstrated a positive correlation between PFKFB3 expression and unstable plaque phenotypes in human coronary and carotid arteries.Mice in which the glycolysis inhibitor,PFK158,repressed PFKFB3 showed improved AS plaque stability and fibrous cap thickening[141].AS plaque progression is closely associated with the influx of specific immune cells[142];therefore,inhibiting PFKFB3 also reduces the glycolysis flux of immune cells,effectively inhibits their metabolism and further minimizes AS progression[141].Therefore,PFK158 may be important for AS plaque stabilization and reducing the risk of AS development.
In conclusion,targeting both diabetes and AS can effectively reduce the risk of acute cardiovascular events secondary to diabetic AS.Furthermore,this approach can also counteract the role of a major risk factor for diabetic AS in the long term,which can facilitate better treatment of diabetic AS (Figure 4).
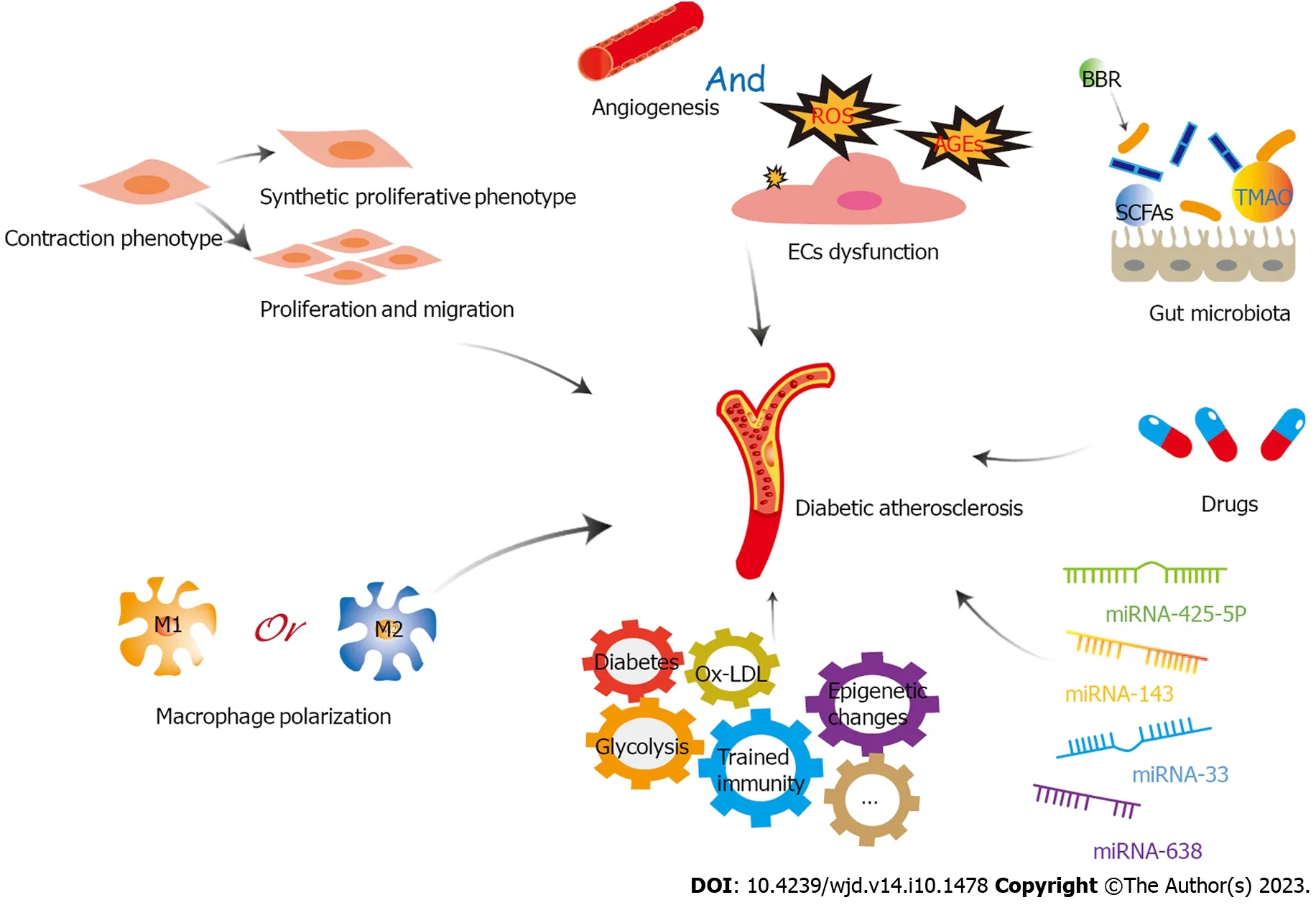
Figure 4 A variety of factors play important roles in diabetic atherosclerosis through glycolysis,including endothelial cells dysfunction,proliferation,migration and phenotypic transformation of vascular smooth muscle cells,macrophage polarization,trained immunity,microRNAs,gut microbiota,and drugs. EC: Endothelial cell;LDL: Low-density lipoprotein;ROS: Reactive oxygen species;TMAO: Trimethylamine-N-oxide;BBR: Berberine;miRNA: microRNA;SCFAs: short-chain fatty acids.
CONCLUSlON
With the global incidence of diabetes increasing year by year,diabetic AS is a growing threat to society.The mechanisms of diabetic AS are highly intricate,and this paper aims to stimulate interest in the emerging field hat delves into the roles of glycolysis in the occurrence and development of diabetic AS.Glycolysis is the metabolic basis of main cells involved in the development of diabetic AS,including ECs,macrophages,and VSMCs.Glycolysis plays an important role in diabetic AS by influencing the functional status of ECs,polarization of macrophages,and proliferation,migration and phenotypic transformation of VSMCs.Therefore,glycolysis should be considered as a potential target for treating diabetic AS.However,it must be acknowledged that,in comparison with the study of glycolysis in cancer treatment,the study concerning the role of glycolysis in diabetic AS does not go that far.Nonetheless,as the significant metabolic mechanism shared by cancer and AS cells,the role of glycolysis in cancer cells also has a considerable reference significance AS cells.As mentioned above,butyrate produced by intestinal flora can inhibit glycolysis in colon cancer cells,providing an important foundation for research on the role of butyrate-producing bacteria in AS.Therefore,in-depth exploration of GM,trained immunity,miRNA,and drugs,which play important roles in the regulation of tumor glycolysis,is also expected to significantly improve our research on diabetic AS glycolysis.HG is the main reason for the abnormal function of vascular ECs and the polarization of macrophages due to the change of energy metabolism,causing AS.However,many scholars argue that HG is not a single factor that affects glycolysis in diabetic AS.We believe that diabetes products such as AGEs and the inflammatory environment caused by diabetes may be involved in this effect.However,the specific influencing mechanism requires further research.In addition,most studies on the role of glycolysis in diabetic AS remain in animal or cellular models at present.Thus,there is a need to bridge the gap between results of research and their clinical applications.Nevertheless,we firmly believe that the that regulation of glycolysis is a potentially promising therapeutic strategy for diabetic AS in the future.
FOOTNOTES
Author contributions:Liu QJ,Yuan W,Yang P,and Shao C contributed to conceptualization and writing-review and editing;and all authors have read and agreed to the published version of the manuscript.
Supported bythe Cardiovascular Disease Clinical Research Center of Zhenjiang,No.SS2 018008;Social Development Foundation of Jiangsu,No.BE2 021694;and Jiangsu Provincial 333 Talent Project,No.BRA2020.
Conflict-of-interest statement:The authors declare no conflict of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Ping Yang 0000-0003-3555-847X;Chen Shao 0000-0002-0676-1295.
S-Editor:Chen YL
L-Editor:A
P-Editor:Chen YL
 World Journal of Diabetes2023年10期
World Journal of Diabetes2023年10期
- World Journal of Diabetes的其它文章
- lndirect comparison of efficacy and safety of chiglitazar and thiazolidinedione in patients with type 2 diabetes: A meta-analysis
- Characteristics of glucose change in diabetes mellitus generalized through continuous wavelet transform processing: A preliminary study
- Analysis of influencing factors and interaction of body weight and disease outcome in patients with prediabetes
- Establishment and evaluation of a risk prediction model for gestational diabetes mellitus
- Effects of insulin aspart and metformin on gestational diabetes mellitus and inflammatory markers
- Effects of vitamin D supplementation on glucose and lipid metabolism in patients with type 2 diabetes mellitus and risk factors for insulin resistance
