葡萄资源叶片抗炭疽病鉴定及抗病基因的QTL定位
禹方方 张颖 姜建福 孙磊 刘崇怀 樊秀彩


摘 要:【目的】炭疽病是葡萄的主要病害之一,極易造成大规模的病害流行,严重影响葡萄产量及品质,筛选抗病种质及挖掘抗病基因有利于葡萄抗病机制研究及抗病育种的开展。【方法】利用室内离体叶片接种法,对60份中国野生葡萄种质、122份欧亚种质、76份欧美杂种以及美人指×刺葡萄0940的F1代杂交群体进行炭疽病抗性鉴定与评价,并利用SNP标记构建的遗传图谱对炭疽病抗性进行QTL定位。【结果】经抗性鉴定,共筛选出1份高抗、43份抗病和75份中抗种质,分别占总鉴定种质的0.39%、16.67%和29.07%。以筛选出的抗病种质刺葡萄0940和感病种质美人指的杂交后代为分离群体进行QTL定位,在第8号连锁群上检测到一个与抗炭疽病相关的QTL位点,可解释14.7%的表型变异。根据QTL定位区间基因注释结果,筛选出15个抗病相关基因,推测它们在葡萄抗炭疽病中发挥一定作用。【结论】明确了不同葡萄种质的炭疽病抗性水平,定位到1个抗炭疽病QTL位点并筛选出15个抗病相关基因。
关键词:葡萄炭疽病;离体叶片接种鉴定;QTL定位;候选基因
中图分类号:S663.1 A436.631 文献标志码:A 文章编号:1009-9980(2023)11-2325-15
Identification of resistance to anthracnose in leaves of grape resources and QTL localization of disease resistance genes
YU Fangfang, ZHANG Ying, JIANG Jianfu, SUN Lei, LIU Chonghuai, FAN Xiucai*
(Zhengzhou Fruit Research Institute, Chinese Academy of Agricultural Sciences, Zhengzhou 450009, Henan, China)
Abstract: 【Objective】 Grape anthracnose is one of the main diseases of grape, which mainly infects the fruits, young leaves and new branches of grape, causing fruit rot, shedding, or water loss and shrinkage into stiff fruits. Breeding disease-resistant grape varieties is the most economical, effective, and environmentally friendly long-term control strategy for the prevention of the anthracnose disease. There have been a few studies on the identification of the resistance to anthracnose and the QTL localization of relative disease resistant genes so far. The study aimed to identify the resistance to the anthracnose disease of different grape germplasms and seek for new QTL locus for the resistance and provide germplasm materials and basis for the breeding and research on the disease resistance mechanism. 【Methods】 The indoor ex vivo leaf inoculation method was used to identify and evaluate the resistance to anthracnose in 60 strains of Chinese wild grapes, 122 accessions of V. vinifera and 76 accessions of V. vinifera-V. labrusca, and the hybrid offsprings of Manicure Finger (susceptible)×Ciputao0940 (resistant), and the QTL localization of grape anthracnose resistance was carried out using the genetic map constructed by SNP markers. 【Results】 A total of 1 germplasm with high resistance, 43 germplasms with resistance and 75 germplasms with medium resistance were screened out, accounting for 0.39%, 16.67% and 29.07% of the total identified accessions, respectively. There were great differences in the resistance to anthracnose in different germplasm populations. 63.34% of the East Asian population 56.57% of the V. vinifera-V. labrusca population, and 31.15% of the Eurasian population had the resistance to anthracnose. In the identification of anthracnose resistance in Chinese wild grape germplasms, it was found that the resistance of different strains in the same population varied greatly, and the species with strong resistance on the whole included V. davidii, V. pseudoreticulata, V. amurensis, and V. piasezkii. The strong resistance of the strains include Shanputao♀, Longyuwanfuye1♀, Duolieye-yingyu♂, Shanputao-shanyang1807, Mianmaoputao-chayu1955, and Ciputao0940. The resistance of the hybrid offsprings of Manicure Finger (susceptible)×Ciputao0940 (resistant) were distributed in five grades. According to the normality test and single-sample Kolmogorov-Smirnov test based on the resistance level distribution of the F1 population, it was found that the hybrid offsprings of Finger (susceptible) × Ciputao0940 (resistant) showed continuous variation in anthracnose resistance, which was a typical quantitative trait controlled by polygens, and the phenotypic distribution of disease resistance identification results showed a trend of partial normal distribution, which could be analyzed by QTL localization. In the positioning of QTL related to grape resistance to anthracnose, the interval or site of LOD≥3.0 was used as the threshold value for QTL, and when the above conditions were met, the site corresponding to the highest LOD value in the interval was considered to be one QTL of grape anti-anthracnose. In this experiment, a QTL interval associated with grape anthracnose resistance was detected on the 8th linkage group, and the locus with the highest LOD value in this interval was located at 140.682 cM, and the tightly linked label was Maker1675910, which could explain the 14.7% of the phenotypic variation. Based on the gene annotation results of the QTL locus interval, 15 resistance-related genes were screened out, and they were hypothesized to play a role in grape anthracnose resistance. 【Conclusion】 The resistance level of different grape germplasms to anthracnose was clarified, one QTL site against anthracnose was located and 15 resistant-related genes were screened.
Key words: Grape anthracnose; Identification of ex vivo leaf inoculation; QTL localization; Candidate genes
收稿日期:2023-05-15 接受日期:2023-08-17
基金项目:国家园艺种质资源库(NHGRC2022-NH00-2);国家葡萄农业产业技术体系(CARS-29-yc-1);中国农业科学院科技创新工程专项(CAAS-ASTIP-2022-ZFRI)
作者简介:禹方方,在读硕士研究生,研究方向为葡萄遗传资源。E-mail:937456624@qq.com
*通信作者Author for correspondence. Tel:13676997369,E-mail:fanxiucai@caas.cn
葡萄炭疽病又名葡萄晚腐病,是葡萄真菌性病害之一,主要侵染葡萄的果实、幼叶和新枝,表现为局部出现病斑、果粒腐烂、脱落、或失水干缩为僵果,造成果实减产最高可达20%[1]。我国各地区引起葡萄炭疽病的致病菌种类并不完全相同,新疆、辽宁、云南、福建、贵州、浙江等葡萄产区的病原菌经鉴定为胶孢炭疽菌(Colletotrichum gloeosporioides)[2-8],广西、台湾等地分离菌株后发现病原菌为葡萄炭疽菌(C. viniferum)[9-10]。
抗病性鉴定是抗病育种的重要组成部分,从杂交亲本的选择到杂交后代的筛选均起着重要作用。葡萄真菌性病害的抗性鉴定方法主要包括自然鉴定和接种鉴定,自然鉴定即田间自然鉴定,而接种鉴定则分为田间接种鉴定和室内离体接种鉴定。田间自然鉴定和田间接种鉴定易受环境、时间、土地等因素影响,因此,为避免抗性鉴定试验受上述因素限制,导致鉴定效率较低,可采用室内离体接种鉴定法,该方法能精确控制接种环境、时间等试验条件,更加便捷地设置试验材料的对照组及重复组,确保试验结果的准确性,提高试验效率。
为深入挖掘葡萄种质对炭疽病的抗性基因,可利用QTL定位技术进行候选基因筛选。迄今为止,有关葡萄抗炭疽病的QTL定位研究较少,高晓铭等[11]以感病的欧亚种质里扎马特为母本、高抗的中国野生种质黑珍珠为父本进行杂交,鉴定F1代群体对炭疽病抗性并利用SSR和SRAP分子标记构建遗传图谱,最后采用区间作图法在12号染色体上检测到一个主效QTL,可解释37.07%的表型变异。Fu等[12]利用Cabernet Sauvignon和Shuang Hong杂交的F1代群体通过3 a(年)炭疽病抗性鉴定,最终在14号染色体上得到一个与抗病相关的QTL位点Cgr1,该位点解释了19.9%的表型变异,富含NBS-LRR基因,并鉴定出了一個与Cgr1的峰值密切相关的分子标记np19345。笔者通过对自然群体内不同葡萄种质的炭疽病抗性进行鉴定,筛选出抗炭疽病的优异种质,同时利用杂交群体抗炭疽病鉴定结果结合已构建的遗传图谱,开展葡萄抗炭疽病相关QTL分析,挖掘葡萄炭疽病抗性基因。
1 材料和方法
1.1 试验材料
自然群体:供试的258份葡萄种质均采自国家葡萄种质资源圃(郑州),包括60份中国野生葡萄种质、122份欧亚种质和76份欧美杂种。
杂交群体:母本美人指(V. vinifera,感病)和父本刺葡萄0940(V. davidii,抗病)种植于国家葡萄种质资源圃(郑州),于2015年在国家葡萄种质资源圃(郑州)进行杂交试验,同年10月从果实中收集种子,并采用层积法对种子进行保存。2016年春季将保存的种子进行催芽以及播种,随后将存活的101株实生苗定植于田间并进行管理。
1.2 病菌培养
试验所用葡萄炭疽病病原菌来自中国农业科学院郑州果树研究所新乡基地葡萄园,从表现炭疽病症状的葡萄果实上分离、纯化得到。通过测序,得到试验菌株的ACT、GAPDH和ITS区段碱基编码序列,将测序结果进行Blast比对,结果显示该病原菌为葡萄炭疽菌(C. viniferum),是炭疽菌属胶胞炭疽菌复合种(C. gloeosporioides species complex)。将供试菌株菌丝体挑至于马铃薯葡萄糖琼脂培养基(Potato Dextrose Agar,PDA)上,28 ℃黑暗条件下倒置培养5 d,待菌丝体布满平板后,使用5 mm打孔器在菌落边缘打孔备用。
1.3 叶片抗性鉴定
选取1年生枝条上第6至第9片成龄叶,用75%乙醇清洗1 min,再用10%次氯酸钠清洗1 min,最后用无菌水清洗3次进行表面灭菌。使用无菌针头对叶片的上下左右进行针刺以损伤叶片的上表皮层,随后将含有真菌菌丝体的PDA菌块(直径5 mm)放置在伤口上,无菌PDA块(直径5 mm)放置在被刺破的叶片上作为对照处理,最后将叶片放在培养皿内的湿滤纸上,在28 °C下进行培育。每份种质处理12枚叶片,每片叶根据面积大小接种2~4处,7 d后采用十字交叉法测量病斑大小。
1.4 统计及分析方法
病症等级根据病斑面积占叶面积的百分比分为0~5等级:0级,叶片无病斑;1级,0.1%~10.0%;2级,10.1%~30.0%;3级,30.1%~60.0%;4级,60.1%~80.0%;5级,80.1%~100.0%。叶面积用ImageJ计算,病症等级转化为病情指数(DI),计算公式如下:
根据病情指数对每个品种进行抗性分级,参考刘崇怀等[13]的分级标准,将抗性分为5级:高抗(HR),DI=0~5.0;抗(R),DI=5.1~20.0;中抗(MR),DI=20.1~30.0;感(S),DI=30.1~45.0;高感(HS),DI=45.1~100.0。
1.5 QTL定位
基于课题组已构建出的一张葡萄分子遗传图谱[14],结合杂交后代对葡萄炭疽病的抗性表型分析,选择MQM mapping进行葡萄抗炭疽病相关QTL定位分析。以图中LOD≥3.0的区间或位点作为QTL的入选临界值,当某区间LOD≥3.0时,认为该区间最高处对应的位点为该性状的1个QTL。利用定位区间所对应的物理位置,参考葡萄基因组PN40024(https://www.ncbi.nlm.nih.gov/genome/401)找到对应区间,结合基因功能注释筛选与炭疽病抗性相关的候选基因。
2 结果与分析
2.1 不同葡萄种质炭疽病抗性鉴定
在环境条件一致的情况下,采用离体叶片接种法,以欧美杂种红奥林和欧亚种里扎马特为对照种质,对122份欧亚种质、76份欧美杂种以及60份中国野生葡萄种质进行了抗炭疽病鉴定。鉴定结果如表1和图1所示,258份葡萄种质的病情指数在5.00~60.00之间,不同种质的病情指数差异较大。病情指数介于0~5.0之间的葡萄种质仅有1份,即山葡萄♀,表现为高抗,占供试种质的0.39%;病情指数介于5.1~20.0之间的种质有43份,表现为抗病,占供试种质的16.67%;病情指数介于20.1~30.0之间的种质有75份,表现为中抗,占供试种质的29.07%;病情指数介于30.1~45.0之间的种质有128份,表现为感病,占供试种质的49.61%;病情指数介于45.1~100.0之间的种质有11份,表现为高感,占供试种质的4.26%。
2.2 不同种群葡萄种质炭疽病抗性鉴定
不同种群葡萄种质的抗炭疽病鉴定结果见表2,东亚种群种质对炭疽病的整体抗性最强,在鉴定的60份种质中,抗病种质占比为63.34%;其次为欧美杂种,在鉴定的76份种质中,抗病种质占比为56.57%;欧亚种群种质的整体抗性最弱,在鉴定的122份种质中,抗病种质占比仅为31.15%。
2.3 中国野生葡萄种质炭疽病抗性鉴定结果
起源于中国、俄罗斯等国家的东亚种群葡萄常被用作育种的原始材料和砧木,本试验中供试的东亚种群葡萄均为起源于中国的野生葡萄。在对中国野生葡萄16个种60个株系进行抗炭疽病鉴定中发现,同一種群内不同种质间抗性差异较大(表3),像桑叶葡萄、变叶葡萄、华东葡萄等种内均存在抗病和感病的株系。但从整体上看,抗病性相对较强的种有刺葡萄、华东葡萄、山葡萄和变叶葡萄。经鉴定,筛选出抗性较强的种质有山葡萄♀、龙峪湾复叶1♀、多裂叶-蘡薁♂、山葡萄-山阳1807、绵毛葡萄-察隅1955、福安刺葡萄等。
2.4 QTL定位分析
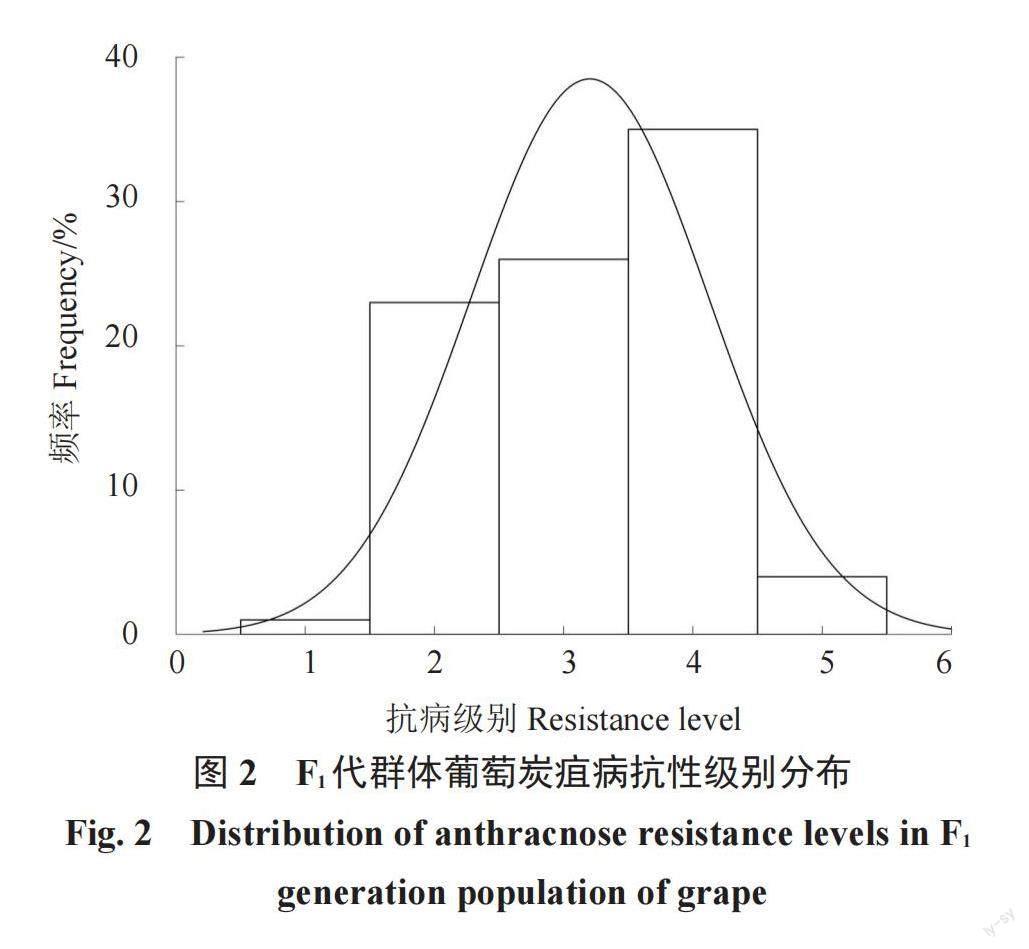
采用室内离体叶片接种法对父本刺葡萄0940与母本美人指及F1代分离群体进行抗病性鉴定,结果显示刺葡萄0940分布在2级,美人指分布在4级,子代在5个等级中均有分布。根据F1代群体的抗性级别分布进行正态检验(图2)和单样本Kolmogorov-Smirnov检验(表4),发现刺葡萄0940和美人指杂交后代在炭疽病抗性方面表现出了连续变异,是典型的多基因控制的数量性状,抗病鉴定结果的表型分布呈现偏正态分布的走势,可进行QTL定位分析。
利用整合的遗传图谱,结合F1代分离群体葡萄抗炭疽病鉴定结果,在第8号连锁群上检测到1个与葡萄抗炭疽病相关的QTL(表5),LOD值所对应的峰位为140.682 cM处,可解释14.7%的表型变异。
2.5 抗病候选基因筛选
根据QTL定位结果,在抗性区间内注释到了244个基因,结合基因功能注释,筛选出15个抗病候选基因(表6)。其中100243697和100256914为MYB(V-myb avian myeloblastosis viral)基因家族转录因子,100259062、100259037、104880030、100253914和100260239为转录因子,100852650、100248811、100247292、100243128、100258428、100248331均属于Leucine-rich repeat receptor-like kinases(LRR-RLKs)基因家族,109123048和100255013均属于WRKY基因家族,该家族是抗病基因家族中的一员。
3 讨 论
我国是葡萄属植物的起源地之一,野生葡萄种类丰富,近年来许多研究者开展了多种葡萄种质对炭疽病的抗性评价工作。基于多年来对葡萄炭疽病的抗性鉴定试验,发现葡萄属不同种群间、种群内的抗病性存在较大差异,其中,中国野生葡萄对炭疽病抗性普遍较强。贺普超等[15]在对我国野生葡萄炭疽病抗性研究中发现,山葡萄、刺葡萄和秦巴野葡萄不感炭疽病,秋葡萄、毛葡萄、复叶葡萄、华东葡萄和麦黄葡萄也表现出极强抗性,其中果实不感炭疽病的株系有毛葡萄泰山-12、山葡萄泰山-11、宁-6等9个株系。徐炎[16]田间自然鉴定发现刺葡萄、山葡萄、毛葡萄等8个野生种共32个株系对炭疽病均表现出极强抗病性。与野生种质相比,欧亚种质抗性普遍较弱。曾蓓[17]对野生种质刺葡萄和欧亚种质红地球分别进行炭疽病抗性鉴定,结果显示刺葡萄抗性明显强于红地球;郝雨[18]对野生变叶葡萄留坝-8和欧亚种质无核白进行炭疽病抗性鉴定,留坝-8表现为高抗,无核白为高感,变叶葡萄抗性强于欧亚种。笔者在本试验中基于离体叶片接种法对60份中国野生葡萄种质、122份欧亚种质和76份欧美杂种进行了炭疽病抗性鉴定,发现中国野生葡萄种质整体抗性最强,其次为欧美杂种,而欧亚种群的整体抗性最弱,与前人研究结果相符。
近年来,有关葡萄抗病性状的QTL定位研究迅速发展,已有多个抗病的QTL位点被报道。Merdinoglu等[19]通过区间作图发现了一个位于抗病亲本28-8-78的12号染色体上的抗霜霉病的QTL位点并将其命名为Rpv1,该位点可解释F1代73%的表型变异。在后续的研究中,又于4、5、6、7、8、9、10、11、12、14、15、18号染色体上鉴定出了27个与葡萄抗霜霉病有关的QTL位点,命名为Rpv2-28[20-30]。此外,Rpv1还被证明与抗白粉病的显性基因Run1紧密相连。Run或Ren均为葡萄抗白粉病的QTL位点,除位于12号染色体上的Rpv1外,还发现11个白粉病抗性QTL位点,分别被命名为Ren1-10、Run2.2和Run2.3,分布于2、13、14、15、16、18、19号染色体上[31-39]。为进一步研究与葡萄炭疽病抗性相关的基因,笔者利用课题组已构建的美人指与刺葡萄0940杂交群体遗传图谱,进行葡萄炭疽病抗性QTL定位,仅在8号连锁群上定位到一个与葡萄抗炭疽病相关的QTL位点,可解释14.7%的表型变异。目前,国内外有关葡萄对炭疽病抗性QTL定位的研究较少,仅在12和14两个连锁群上定位到有关葡萄抗炭疽病的QTL位点,而在其他连锁群上尚未明确报道。使用不同的群体往往会定位到不同的QTL位点,例如在葡萄抗霜霉病QTL定位中,亲本为Syrah和28-8-78时定位到了12号染色体10.3 Mb处,当亲本为Cabernet Sauvignon和Gloire De Montpellie时则定位到了9号染色体4.0 Mb处[40]。
迄今为止,研究者从植物中分离到许多具有抗病功能的基因,如WRKY、MYB、LRR-RLKs等。WRKY转录因子在葡萄抗生物胁迫、非生物胁迫等抗逆过程中发挥重要的转录调控作用,如启动子PR4b与转录因子WRKY40和WRKY75互作可提高葡萄对霜霉菌的防御能力[41],WRKY22和WRKY48与葡萄抗炭疽病有关[42]。笔者在本研究中共筛选到2个葡萄WRKY基因,分别为WRKY26和WRKY70。PyWRKY26为葡萄WRKY26的同源基因,已有研究证明,PyWRKY26可通过与PybHLH3相互作用参与调控梨花青苷的合成,从而提高梨对黑曲霉病原菌的抗性[43]。葡萄中VvWRKY26基因的启动子区域含有水杨酸途径的顺式作用元件,在外源SA处理后该基因的表达量显著提高,说明VvWRKY26与水杨酸信号通路有关,而SA途径与植物抗病免疫反应紧密相关,因此推测VvWRKY26可能与葡萄抗病有关[44-45]。此外,WRKY70在植物抗病反应中也发挥着重要作用,该基因位于SA和JA相互拮抗作用的交汇点,可正向调节SA途径、负向调节JA途径并平衡2种途径的关系[46]。MYB转录因子家族数量庞大,多种MYB转录因子都参与调控葡萄的抗病性,如VaMYB306在葡萄叶片中过表达可提高葡萄对灰霉病的抗性[47],VqMYB154可通过调控VqSTS9等靶基因增强葡萄对白粉病的抗病能力[48]。PHL7为MYB基因家族转录因子,是类黄酮途径中的重要组成部分,而类黄酮途径是植物抵御病原菌侵害的防卫反应之一,因此推测PHL7与植物抗病反应有关[49]。LRR-RLKs是RLKs基因家族中最大的亚家族,广泛地参与植物的发育过程和抗病反应[50]。拟南芥FLS2是最早发现的具有抗病功能的LRR-RLKs基因,该基因通过其胞外LRR结构域感知病原菌的鞭毛蛋白,从而察觉病原菌的侵染并激活免疫反应[51-52]。Xa21为水稻白叶枯病抗性基因,也是典型的LRR-RLKs基因,它可通过识别病原菌分泌物来触发寄主的免疫反应[53]。在定位区间内筛选出的100852650、100248811等6个基因均属于LRR-RLKs基因家族,根据其结构特性以及前人对此类基因的研究基础,推测这些基因与葡萄抗炭疽病有关。
4 结 论
笔者利用室内离体叶片接种法,对不同葡萄资源叶片抗炭疽病进行鉴定,共筛选出1份高抗、43份抗病和75份中抗葡萄种质;不同种群种质的炭疽病抗性存在较大差异,东亚种群对炭疽病的整体抗性最强,其次为欧美杂种,欧亚种群的整体抗性最弱。基于课题组已构建的一张葡萄分子遗传图谱,结合美人指和刺葡萄0940杂交后代群体对葡萄炭疽病的抗性鉴定结果,在第8号连锁群上检测到1个与葡萄抗炭疽病相关的QTL位点,可解释14.7%的表型变异。根据QTL定位区域内的基因注释结果,在抗性区间内筛选出15个抗病相关基因。
参考文献 References:
[1] 刘梅,JAYAWARDENA R S,刘阳,邢启凯,燕继晔,李兴红. 北京市葡萄炭疽病病原菌的分子鉴定[J]. 植物保护学报,2018,45(2):393-394.
LIU Mei,JAYAWARDENA R S,LIU Yang,XING Qikai,YAN Jiye,LI Xinghong. Molecular identification of the pathogen causing grape anthracnose in Beijing[J]. Journal of Plant Protection,2018,45(2):393-394.
[2] 雷百战,李国英. 新疆葡萄炭疽病病原的鉴定及其生物学特性的研究[J]. 石河子大学学报(自然科学版),2004,22(4):298-300.
LEI Baizhan,LI Guoying. Identification and biological characteristics of the pathogen of Colletotrichum gloeosporioides on grape in Xinjiang[J]. Journal of Shihezi University (Natural Science),2004,22(4):298-300.
[3] 李洋,劉长远,陈秀蓉,赵奎华,苗则彦,梁春浩,王辉. 辽宁省葡萄炭疽菌鉴定及对多菌灵敏感性研究[J]. 植物保护,2009,35(4):74-77.
LI Yang,LIU Changyuan,CHEN Xiurong,ZHAO Kuihua,MIAO Zeyan,LIANG Chunhao,WANG Hui. Identification of the grape anthracnose and its sensitivity to carbendazim in Liaoning[J]. Plant Protection,2009,35(4):74-77.
[4] 邓维萍,杨敏,杜飞,杨积忠,朱书生. 云南葡萄产区葡萄炭疽病病原鉴定及致病力分析[J]. 植物保护学报,2013,40(1):61-67.
DENG Weiping,YANG Min,DU Fei,YANG Jizhong,ZHU Shusheng. Identification of the pathogen causing grape anthracnose in Yunnan and its pathogenicity[J]. Journal of Plant Protection,2013,40(1):61-67.
[5] 雷龑,林雄杰,陈婷,刘鑫铭,蔡盛华,范国成. 福建葡萄炭疽病病原鉴定及致病性分析[J]. 果树学报,2014,31(6):1123-1127.
LEI Yan,LIN Xiongjie,CHEN Ting,LIU Xinming,CAI Shenghua,FAN Guocheng. Pathogen identification and pathogenicity analysis of grape ripe rot in Fujian[J]. Journal of Fruit Science,2014,31(6):1123-1127.
[6] 汪家胜,姜于兰,王德凤,杨亚曦,陈帝炉. 贵阳地区葡萄炭疽病病原菌鉴定及室内杀菌剂筛选[J]. 山地农业生物学报,2014,33(6):59-61.
WANG Jiasheng,JIANG Yulan,WANG Defeng,YANG Yaxi,CHEN Dilu. Pathogen identification of grape anthracnose in Guiyang area and fungicides screening in laboratory[J]. Journal of Mountain Agriculture and Biology,2014,33(6):59-61.
[7] 宗宇,吴志娟,朱友银,郭卫东,林佳佳,路梅. 葡萄炭疽病病原菌分离鉴定及其效用杀菌剂筛选[J]. 浙江师范大学学报(自然科学版),2017,40(4):435-440.
ZONG Yu,WU Zhijuan,ZHU Youyin,GUO Weidong,LIN Jiajia,LU Mei. Separation and identification of pathogen inducing grape anthracnose and screening of available fungicides in laboratory[J]. Journal of Zhejiang Normal University (Natural Sciences),2017,40(4):435-440.
[8] 刘丽,刘长远,王辉,关天舒,于舒怡,李柏宏. 葡萄炭疽病菌鉴定及同源性分析[J]. 中国农学通报,2017,33(35):132-136.
LIU Li,LIU Changyuan,WANG Hui,GUAN Tianshu,YU Shuyi,LI Baihong. Identification and homology analysis of Colletotrichum gloeosporioides in grape[J]. Chinese Agricultural Science Bulletin,2017,33(35):132-136.
[9] 谭海芸,吴林娜,张玮,周咏梅,李兴红. 广西南宁避雨栽培葡萄炭疽病的病原菌鉴定[J]. 植物保护,2022,48(5):269-273.
TAN Haiyun,WU Linna,ZHANG Wei,ZHOU Yongmei,LI Xinghong. Identification of the pathogen of grape anthracnose in Nanning,Guangxi[J]. Plant Protection,2022,48(5):269-273.
[10] DUAN C H,CHEN G Y. First report of Colletotrichum viniferum causing ripe rot of grape berry in Taiwan [J]. Plant Disease,2022,106(2):764.
[11] 高晓铭,刘崇怀,张国海,张颖,姜建福,孙海生,樊秀彩. 葡萄抗炭疽病QTL的初步定位分析[J]. 园艺学报,2016,43(12):2442-2450.
GAO Xiaoming,LIU Chonghuai,ZHANG Guohai,ZHANG Ying,JIANG Jianfu,SUN Haisheng,FAN Xiucai. Preliminary analysis of grape anthracnose resistance QTL[J]. Acta Horticulturae Sinica,2016,43(12):2442-2450.
[12] FU P N,TIAN Q Y,LAI G T,LI R F,SONG S R,LU J. Cgr1,a ripe rot resistance QTL in Vitis amurensis ‘Shuang Hong grapevine[J]. Horticulture Research,2019,6:67.
[13] 劉崇怀,沈育杰,陈俊. 葡萄种质资源描述规范和数据标准[M]. 北京:中国农业出版社,2006.
LIU Chonghuai,SHEN Yujie,CHEN Jun. Descriptors and data standard for grape (Vitis L.)[M]. Beijing:China Agriculture Press,2006.
[14] LI P,TAN X B,LIU R T,RAHMAN F U,JIANG J F,SUN L,FAN X C,LIU J H,LIU C H,ZHANG Y. QTL detection and candidate gene analysis of grape white rot resistance by interspecific grape (Vitis vinifera L. × Vitis davidii Foex.) crossing[J]. Horticulture Research,2023,10(5):uhad063.
[15] 贺普超,任治邦. 我国葡萄属野生种对炭疽病抗性的研究[J]. 果树科学,1990,7(1):7-12.
HE Puchao,REN Zhibang. Study on the resistances of wild vitis species native to China to grape ripe rot[J]. Journal of Fruit Science,1990,7(1):7-12.
[16] 徐炎. 中國野生葡萄抗白腐病、炭疽病基因RAPD标记及其克隆[D]. 杨凌:西北农林科技大学,2000.
XU Yan. Clones and their RAPD Markers of the resistance to white rot & ripe rot in Chinese wild Vitis[D]. Yangling:Northwest A & F University,2000.
[17] 曾蓓. 刺葡萄对常见真菌性病害的抗性鉴定及诱变筛选抗霜霉病资源的研究[D]. 长沙:湖南农业大学,2017.
ZENG Bei. Identifying the resistance of Vitis davidii Fo?x to common fungal diseases and studying the mutation screening of resistance resources[D]. Changsha:Hunan Agricultural University,2017.
[18] 郝雨. 葡萄炭疽菌与抗感葡萄种质互作中的侵染特性及寄主防卫反应研究[D]. 杨凌:西北农林科技大学,2022.
HAO Yu. Study on infection characteristics and host defense response in interactions between Colletotrichum on grape and grapes resistant and susceptible accessions[D]. Yangling:Northwest A & F University,2022.
[19] MERDINOGLU D,WIEDEMAN-MERDINOGLU S,COSTE P,DUMAS V,HAETTY S,BUTTERLIN G,GREIF C. Genetic analysis of downy mildew resistance derived from muscadinia rotundifolia[J]. Acta Horticulturae,2003,603:451-456.
[20] MARGUERIT E,BOURY C,MANICKI A,DONNART M,BUTTERLIN G,N?MORIN A,WIEDEMANN-MERDINOGLU S,MERDINOGLU D,OLLAT N,DECROOCQ S. Genetic dissection of sex determinism,inflorescence morphology and downy mildew resistance in grapevine[J]. Theoretical and Applied Genetics,2009,118(7):1261-1278.
[21] BELLIN D,PERESSOTTI E,MERDINOGLU D,WIEDEMANN-MERDINOGLU S,ADAM-BLONDON A F,CIPRIANI G,MORGANTE M,TESTOLIN R,DI GASPERO G. Resistance to Plasmopara viticola in grapevine ‘Bianca is controlled by a major dominant gene causing localised necrosis at the infection site[J]. Theoretical and Applied Genetics,2009,120(1):163-176.
[22] MOREIRA F M,MADINI A,MARINO R,ZULINI L,STEFANINI M,VELASCO R,KOZMA P,GRANDO M S. Genetic linkage maps of two interspecific grape crosses (Vitis spp.) used to localize quantitative trait loci for downy mildew resistance[J]. Tree Genetics & Genomes,2011,7:153-167.
[23] BLASI P,BLANC S,WIEDEMANN-MERDINOGLU S,PRADO E,R?HL E H,MESTRE P,MERDINOGLU D. Construction of a reference linkage map of Vitis amurensis and genetic mapping of Rpv8,a locus conferring resistance to grapevine downy mildew[J]. Theoretical and Applied Genetics,2011,123(1):43-53.
[24] SCHWANDER F,EIBACH R,FECHTER I,HAUSMANN L,ZYPRIAN E,T?PFER R. Rpv10:a new locus from the Asian Vitis gene pool for pyramiding downy mildew resistance loci in grapevine[J]. Theoretical and Applied Genetics,2012,124(1):163-176.
[25] VENUTI S,COPETTI D,FORIA S,FALGINELLA L,HOFFMANN S,BELLIN D,CINDRI? P,KOZMA P,SCALABRIN S,MORGANTE M,TESTOLIN R,DI GASPERO G. Historical introgression of the downy mildew resistance gene Rpv12 from the Asian species Vitis amurensis into grapevine varieties[J]. PLoS One,2013,8(4):e61228.
[26] OCHSSNER I,HAUSMANN L,T?PFER R. Rpv14,a new genetic source for Plasmopara viticola resistance conferred by Vitis cinerea[J]. Vitis,2016,55(2):79-81.
[27] DIVILOV K,BARBA P,CADLE-DAVIDSON L,REISCH B I. Single and multiple phenotype QTL analyses of downy mildew resistance in interspecific grapevines[J]. Theoretical and Applied Genetics,2018,131(5):1133-1143.
[28] SAPKOTA S,CHEN L L,YANG S S,HYMA K E,CADLE-DAVIDSON L,HWANG C F. Construction of a high-density linkage map and QTL detection of downy mildew resistance in Vitis aestivalis-derived ‘Norton[J]. Theoretical and Applied Genetics,2019,132(1):137-147.
[29] LIN H,LENG H,GUO Y S,KONDO S,ZHAO Y H,SHI G L,GUO X W. QTLs and candidate genes for downy mildew resistance conferred by interspecific grape (V. vinifera L. ×V. amurensis Rupr.) crossing[J]. Scientia Horticulturae,2019,244:200-207.
[30] BHATTARAI G,FENNELL A,LONDO J P,COLEMAN C,KOVACS L G. A novel grape downy mildew resistance locus from Vitis rupestris[J]. American Journal of Enology and Viticulture,2021,72(1):12-20.
[31] DALBO? M A,YE G N,WEEDEN N F,WILCOX W F,REISCH B I. Marker-assisted selection for powdery mildew resistance in grapes[J]. Journal of the American Society for Horticultural Science,2001,126(1):83-89.
[32] BARKER C L,DONALD T,PAUQUET J,RATNAPARKHE M B,BOUQUET A,ADAM-BLONDON A F,THOMAS M R,DRY I. Genetic and physical mapping of the grapevine powdery mildew resistance gene,Run1,using a bacterial artificial chromosome library[J]. Theoretical and Applied Genetics,2005,111(2):370-377.
[33] RIAZ S,TENSCHER A C,RAMMING D W,WALKER M A. Using a limited mapping strategy to identify major QTLs for resistance to grapevine powdery mildew (Erysiphe necator) and their use in marker-assisted breeding[J]. Theoretical and Applied Genetics,2011,122(6):1059-1073.
[34] BLANC S,WIEDEMANN-MERDINOGLU S,DUMAS V,MESTRE P,MERDINOGLU D. A reference genetic map of Muscadinia rotundifolia and identification of Ren5,a new major locus for resistance to grapevine powdery mildew[J]. Theoretical and Applied Genetics,2012,125(8):1663-1675.
[35] VAN HEERDEN C J,BURGER P,VERMEULEN A,PRINS R. Detection of downy and powdery mildew resistance QTL in a ‘Regent × ‘Red Globe population[J]. Euphytica,2014,200(2):281-295.
[36] PAP D,RIAZ S,DRY I B,JERMAKOW A,TENSCHER A C,CANTU D,OL?H R,WALKER M A. Identification of two novel powdery mildew resistance loci,Ren6 and Ren7,from the wild Chinese grape species Vitis piasezkii[J]. BMC Plant Biology,2016,16(1):170.
[37] ZYPRIAN E,OCH?NER I,SCHWANDER F,?IMON S,HAUSMANN L,BONOW-REX M,MORENO-SANZ P,GRANDO M S,WIEDEMANN-MERDINOGLU S,MERDINOGLU D,EIBACH R,T?PFER R. Quantitative trait loci affecting pathogen resistance and ripening of grapevines[J]. Molecular Genetics and Genomics,2016,291(4):1573-1594.
[38] ZENDLER D,SCHNEIDER P,T?PFER R,ZYPRIAN E. Fine mapping of Ren3 reveals two loci mediating hypersensitive response against Erysiphe necator in grapevine[J]. Euphytica,2017,213(3):68.
[39] TEH S L,FRESNEDO-RAM?REZ J,CLARK M D,GADOURY D M,SUN Q,CADLE-DAVIDSON L,LUBY J J. Genetic dissection of powdery mildew resistance in interspecific half-sib grapevine families using SNP-based maps[J]. Molecular Breeding,2017,37(1):1-16.
[40] 李鵬,刘锐涛,谭西北,张颖,刘崇怀. 葡萄遗传图谱构建与抗病QTL定位研究进展[J]. 果树学报,2023,40(6):1245-1254.
LI Peng,LIU Ruitao,TAN Xibei,ZHANG Ying,LIU Chonghuai. Research progress in genetic map construction and QTL mapping for disease resistance in grapevine[J]. Journal of Fruit Science,2023,40(6):1245-1254.
[41] 刘兵,李梦媛,张娜,尚博兴,刘国甜,徐炎. 中国野生葡萄抗霜霉病相关基因VpPR4b及其启动子的克隆和功能分析[J]. 园艺学报,2021,48(2):265-275.
LIU Bing,LI Mengyuan,ZHANG Na,SHANG Boxing,LIU Guotian,XU Yan. Cloning and functional analysis of the CDS and promoter of VpPR4b gene response to downy mildew in Chinese wild grape[J]. Acta Horticulturae Sinica,2021,48(2):265-275.
[42] 沈才琦,孙磊,张颖,姜建福,刘崇怀,樊秀彩. 胶孢炭疽菌侵染不同抗性葡萄叶片的转录组学分析[J]. 果树学报,2022,39(5):730-742.
SHEN Caiqi,SUN Lei,ZHANG Ying,JIANG Jianfu,LIU Chonghuai,FAN Xiucai. Transcriptomic analysis of different resistant leaves infected by Colletotrichum gloeosporioides in grape[J]. Journal of Fruit Science,2022,39(5):730-742.
[43] 李闯. WRKY26-bHLH3共促进红皮梨花青苷合成及花青苷抑制黑曲霉侵染的分子机制研究[D]. 合肥:合肥工业大学,2020.
LI Chuang. Molecular mechanism of WRKY26-bHLH3 co-promoting red pear anthocyanin synthesis and anthocyanin inhibition of Aspergillus niger infection[D]. Hefei:Hefei University of Technology,2020.
[44] 于和平. 葡萄WRKY26、WRKY27基因启动子克隆及功能分析[D]. 大连:辽宁师范大学,2013.
YU Heping. Cloning and functional analysis of Vitis vinifera WRKY26 and WRKY27 Promoters[D]. Dalian:Liaoning Normal University,2013.
[45] 张国蓉,梁长梅,郭建勇,张泽宁,张鹏飞,赵旗峰,梁晋军,温鹏飞. 葡萄VvWRKY26基因克隆与表达分析[J]. 农业生物技术学报,2023,31(3):475-487.
ZHANG Guorong,LIANG Changmei,GUO Jianyong,ZHANG Zening,ZHANG Pengfei,ZHAO Qifeng,LIANG Jinjun,WEN Pengfei. Clone and expression analysis of VvWRKY26 gene in grape (Vitis vinifera)[J]. Journal of Agricultural Biotechnology,2023,31(3):475-487.
[46] 何樂. WRKY70与抗性相关转录因子的相互作用研究[D]. 长沙:湖南农业大学,2015.
HE Le. Interaction between WRKY70 and plant resistance relative transcription factors[D]. Changsha:Hunan Agricultural University,2015.
[47] ZHU Y X,ZHANG X M,ZHANG Q H,CHAI S Y,YIN W C,GAO M,LI Z,WANG X P. The transcription factors VaERF16 and VaMYB306 interact to enhance resistance of grapevine to Botrytis cinerea infection[J]. Molecular Plant Pathology,2022,23(10):1415-1432.
[48] 姜长岳. 中国野生毛葡萄VqMYB154转录因子调控抗白粉病机理研究[D]. 杨凌:西北农林科技大学,2021.
JIANG Changyue. Mechanism analysis of transcription factor VqMYB154 regulating the resistance to powdery mildew in Chinese wild Vitis quinquangularis[D]. Yangling:Northwest A & F University,2021.
[49] ZHANG B D,ZHANG M Y,JIA X J,HU G J,REN F,FAN X D,DONG Y F. Integrated transcriptome and metabolome dissecting interaction between Vitis vinifera L. and grapevine fabavirus[J]. International Journal of Molecular Sciences,2023,24(4):3247.
[50] 王琪,吴允哲,刘学英,孙丽莉,廖红,傅向东. 类受体激酶调控水稻生长发育和环境适应研究进展[J]. 植物学报,2023,58(2):199-213.
WANG Qi,WU Yunzhe,LIU Xueying,SUN Lili,LIAO Hong,FU Xiangdong. The rice receptor-like kinases function as key regulators of plant development and adaptation to the environment[J]. Chinese Bulletin of Botany,2023,58(2):199-213.
[51] G?MEZ-G?MEZ L,BOLLER T. FLS2:an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis[J]. Molecular Cell,2000,5(6):1003-1011.
[52] IMKAMPE J,HALTER T,HUANG S H,SCHULZE S,MAZZOTTA S,SCHMIDT N,MANSTRETTA R,POSTEL S,WIERZBA M,YANG Y,VAN DONGEN W M A M,STAHL M,ZIPFEL C,GOSHE M B,CLOUSE S,DE VRIES S C,TAX F,WANG X F,KEMMERLING B. The Arabidopsis leucine-rich repeat receptor kinase BIR3 negatively regulates BAK1 receptor complex formation and stabilizes BAK1[J]. The Plant Cell,2017,29(9):2285-2303.
[53] 林悦龙,王昱澎,朱永生,郑燕梅,蔡秋华,何炜,陈丽萍,谢鸿光,魏毅东,谢华安,张建福. 粳稻云引稻瘟病抗性近等基因系与免疫应答相关的LRR-RLKs差异基因分析[J]. 科学通报,2021,66(30):3873-3885.
LIN Yuelong,WANG Yupeng,ZHU Yongsheng,ZHENG Yanmei,CAI Qiuhua,HE Wei,CHEN Liping,XIE Hongguang,WEI Yidong, XIE Huaan,ZHANG Jianfu. Analysis of immune response-related LRR-RLKs differential genes in the rice blast resistance near-isogenic lines of Japonica variety Yunyin[J]. Chinese Science Bulletin,2021,66(30):3873-3885.

